Metabolomics Profiling of Serum and Urine from Chuanzang Black Pigs with Different Residual Feed Intake
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collections
2.3. Analysis of Serum and Urine Metabolites
2.4. Statistical Analyses
3. Results
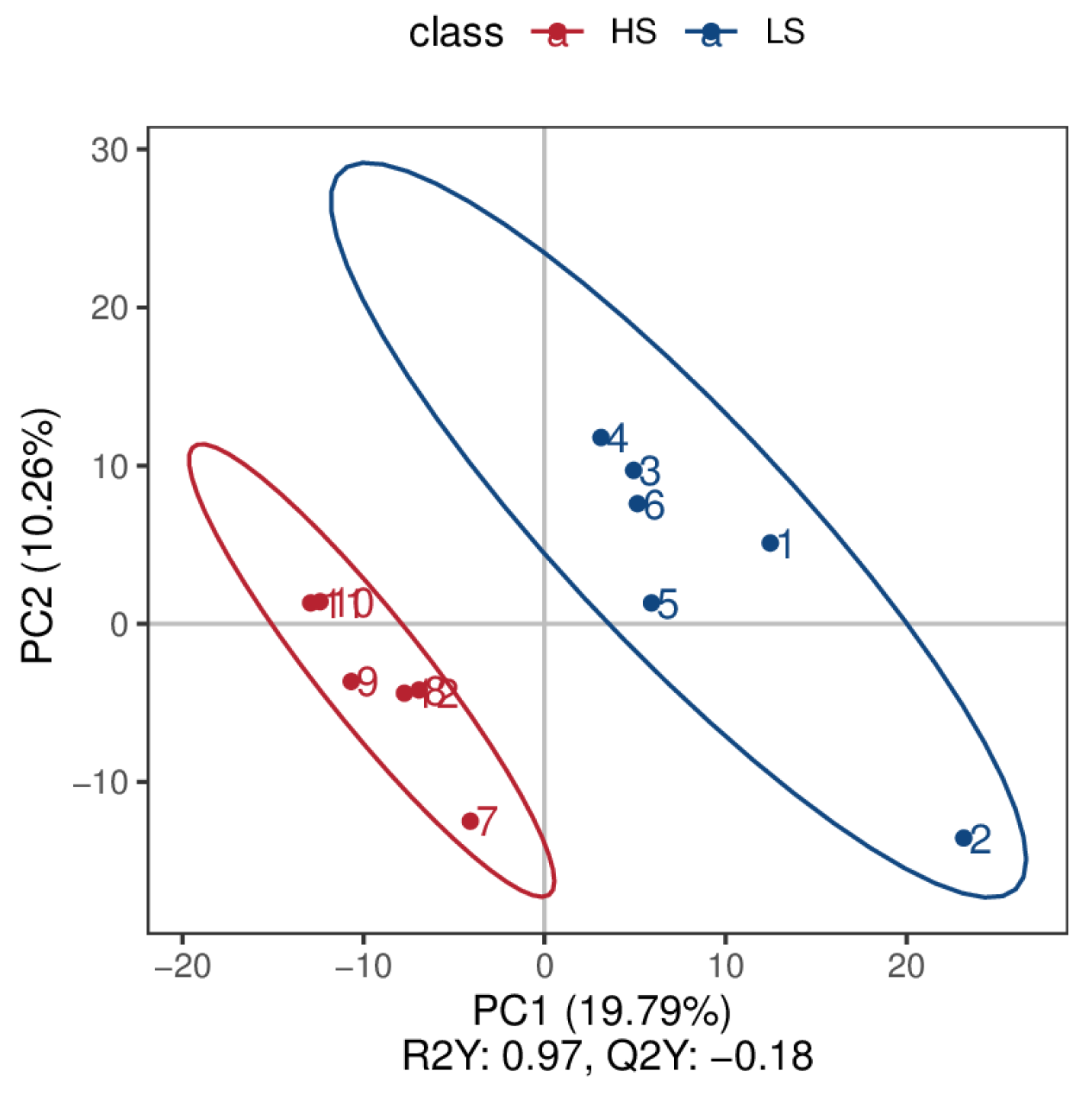
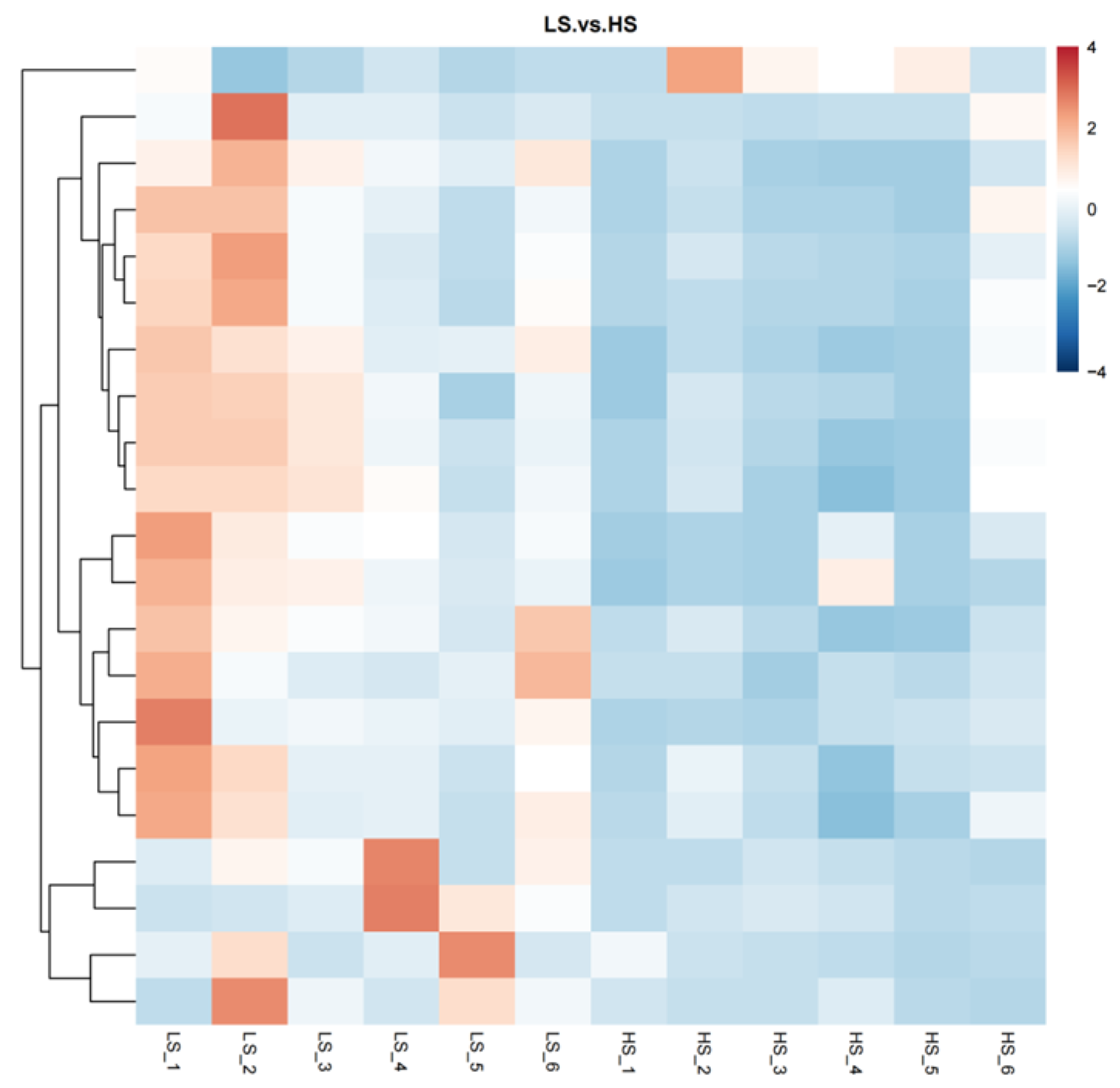
3.1. Serum Metabolome Profling
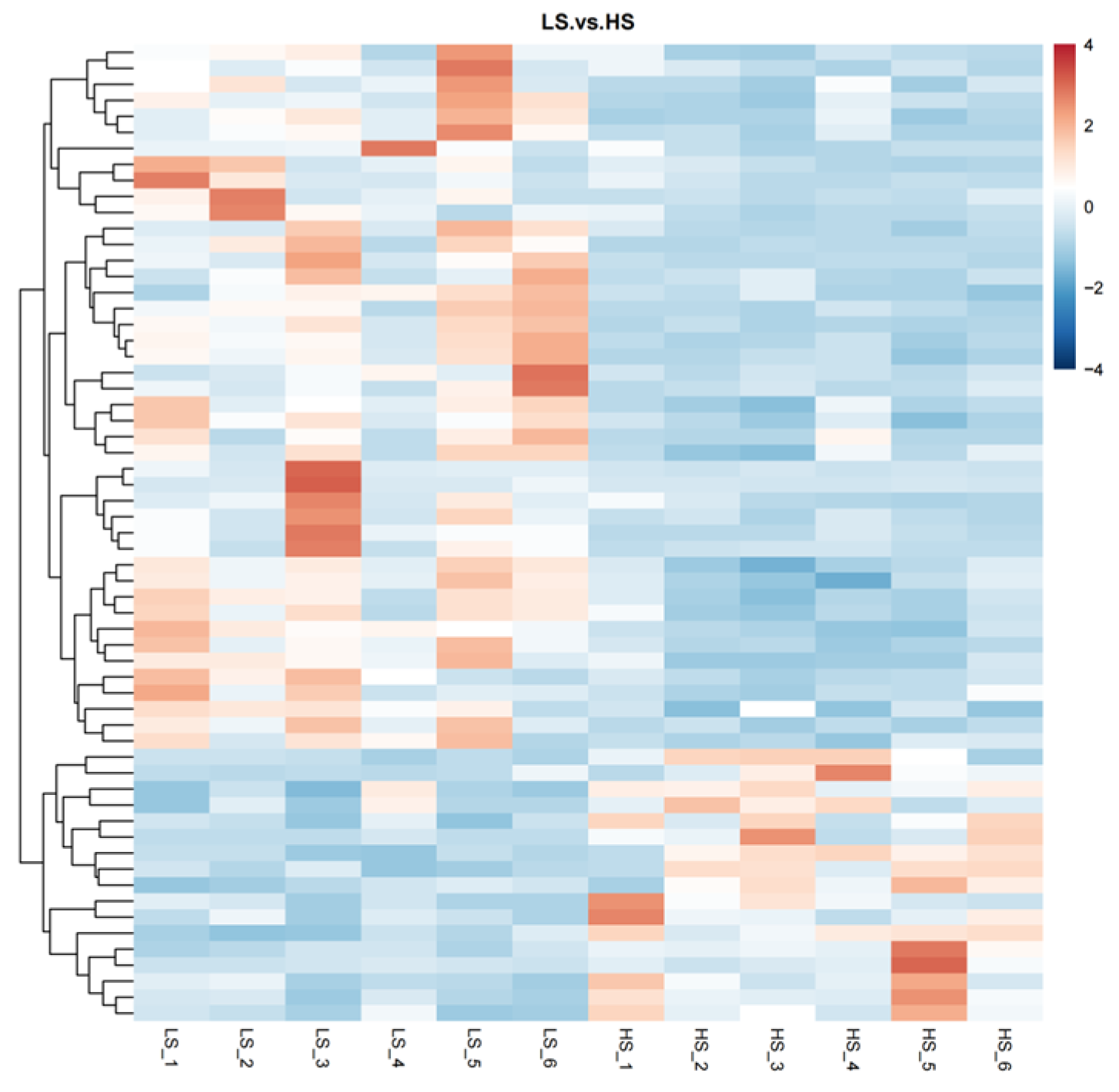
3.2. Urine Metabolome Profling
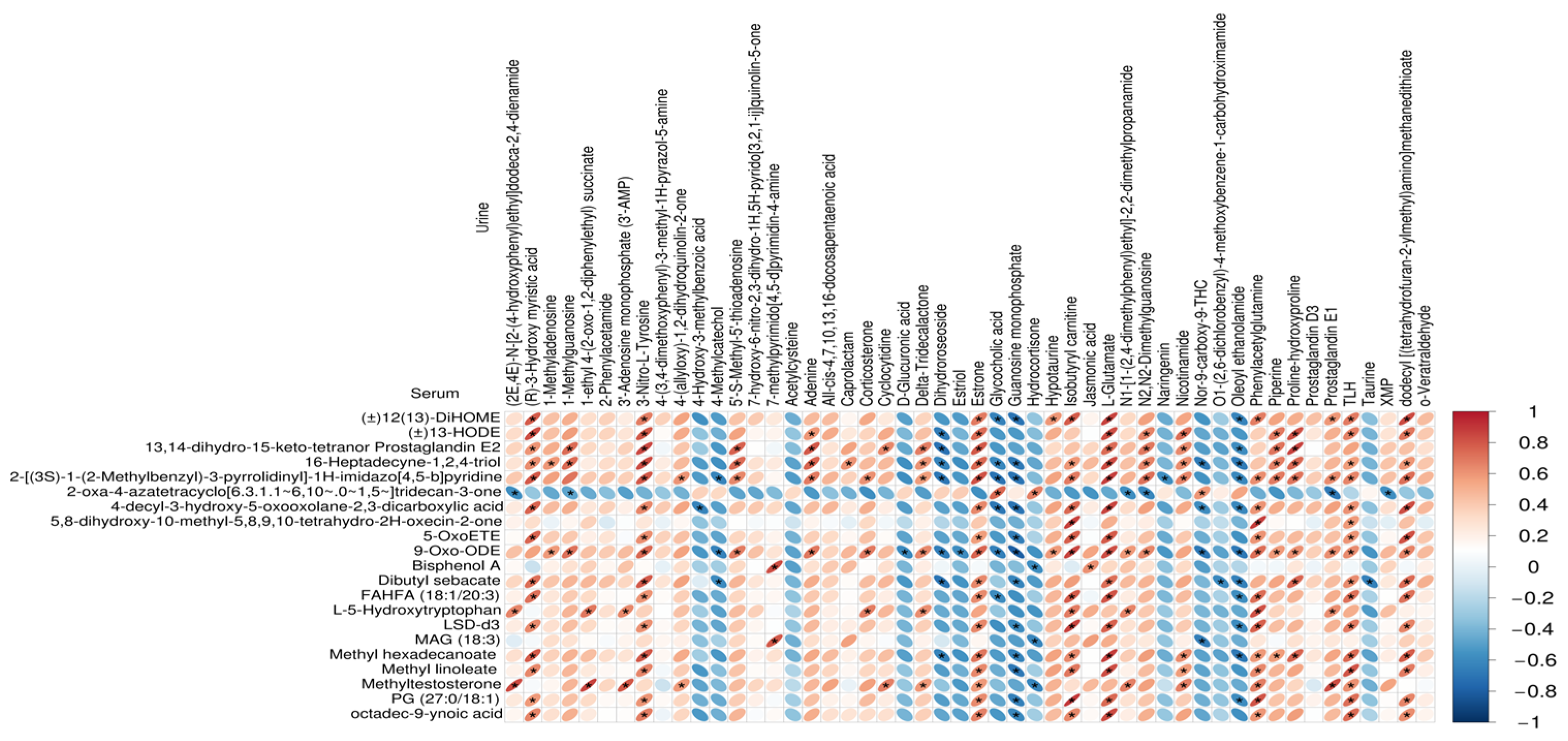
3.3. Correlations between Serum Metabolites and Urine Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, Z.; Wang, Z.; Xie, X.; Tian, S.; Wang, F.; Wang, Q.; Ni, S.; Pan, Y.; Xiao, Q. Evaluation of the genetic diversity, population structure and selection signatures of three native chinese pig populations. Animals 2023, 13, 2010. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.; Bidanel, J.P.; Billon, Y.; Lagant, H.; Guillouet, P.; Sellier, P.; Noblet, J.; Hermesch, S. Correlated responses in sow appetite, residual feed intake, body composition, and reproduction after divergent selection for residual feed intake in the growing pig. J. Anim. Sci. 2012, 90, 1097–1108. [Google Scholar] [CrossRef]
- Koch, R.M.; Swiger, L.A.; Chambers, D.; Gregory, K.E. Efficiency of feed use in beef cattle. J. Anim. Sci. 1963, 22, 486–494. [Google Scholar] [CrossRef]
- Zhang, H.; Elolimy, A.A.; Akbar, H.; Thanh, L.P.; Yang, Z.; Loor, J.J. Association of residual feed intake with peripartal ruminal microbiome and milk fatty acid composition during early lactation in Holstein dairy cows. J. Dairy Sci. 2022, 105, 4971–4986. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, C.; Brossard, L.; Dourmad, J.Y. Improvement of feed and nutrient efficiency in pig production through precision feeding. Anim. Feed. Sci. Technol. 2020, 268, 114611. [Google Scholar] [CrossRef]
- Wu, J.; Ye, Y.; Quan, J.; Ding, R.; Wang, X.; Zhuang, Z.; Zhou, S.; Geng, Q.; Xu, C.; Hong, L.; et al. Using nontargeted LC-MS metabolomics to identify the Association of Biomarkers in pig feces with feed efficiency. Porc. Health Manag. 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Liaubet, L.; Guilmineau, C.; Lefort, G.; Billon, Y.; Reigner, S.; Bailly, J.; Marty-Gasset, N.; Gress, L.; Servien, R.; Bonnet, A.; et al. Plasma 1H-NMR metabolic and amino acid profiles of newborn piglets from two lines divergently selected for residual feed intake. Sci. Rep. 2023, 13, 7127. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, J.K.; Dekkers, J.C.M.; Huff-Lonergan, E.; Tuggle, C.K.; Lonergan, S.M. Identification of potential serum biomarkers to predict feed efficiency in young pigs. J. Anim. Sci. 2016, 94, 1482–1492. [Google Scholar] [CrossRef]
- Do, D.N.; Ostersen, T.; Strathe, A.B.; Mark, T.; Jensen, J.; Kadarmideen, H.N. Genome-wide association and systems genetic analyses of residual feed intake, daily feed consumption, backfat and weight gain in pigs. BMC Genet. 2014, 15, 27. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef]
- Barri, T.; Dragsted, L.O. UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: Effect of experimental artefacts and anticoagulant. Anal. Chim. Acta 2013, 768, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.J.; Kellner, T.A.; Shurson, G.C. Characteristics of lipids and their feeding value in swine diets. J. Anim. Sci. Biotechnol. 2015, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Pastorelli, G.; Cannata, S.; Corino, C. Recent advances in the use of fatty acids as supplements in pig diets A review. Anim. Feed. Sci. Technol. 2010, 162, 1–11. [Google Scholar] [CrossRef]
- Bruininx, E.; Van Den Borne, J.; van Heugten, E.; Van Milgen, J.; Verstegen, M.; Gerrits, W. Oxidation of dietary stearic, oleic, and linoleic acids in growing pigs follows a biphasic pattern. J. Nutr. 2011, 141, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Di Sessa, A.; Caprio, S.; Chalasani, N.; Liu, W.; Liang, T.; Graf, J.; Herzog, R.I.; Johnson, C.D.; Umano, G.R.; et al. Oxidized derivatives of linoleic acid in pediatric metabolic syndrome: Is their pathogenic role modulated by the genetic background and the gut microbiota? Antioxid. Redox Signal. 2019, 30, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Karisa, B.; Moore, S.; Plastow, G. Analysis of biological networks and biological pathways associated with residual feed intake in beef cattle. Anim. Sci. J. 2014, 85, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, Y.; Liu, F.; Liu, A.; Jing, L.; Zhao, C.; Luan, Y.; Miao, Y.; Zhao, S.; Li, X. Transcriptome analysis reveals that vitamin a metabolism in the liver affects feed efficiency in pigs. G3 Genes Genomes Genet. 2016, 6, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Faraut, T.; Prunier, A. Pathways and genes involved in steroid hormone metabolism in male pigs: A review and update. J. Steroid Biochem. Mol. Biol. 2014, 140, 44–55. [Google Scholar] [CrossRef]
- Kiezun, M.; Dobrzyn, K.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Szeszko, K.; Zaobidna, E.; Bors, K.; Wyrebek, J.; Mykytiyk, A.; et al. The effect of estrone and estradiol on the expression of the orexin/hypocretin system in the porcine uterus during early pregnancy. Domest. Anim. Endocrinol. 2019, 68, 11–24. [Google Scholar] [CrossRef]
- Prakashan AD, K.; Muthukumar, S.P.; Martin, A. Taurine activates SIRT1/AMPK/FOXO1 signaling pathways to favorably regulate lipid metabolism in C57BL6 obese mice. Mol. Nutr. Food Res. 2024, 68, e2200660. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Han, J.; Xu, X.; Wang, Y.; Zhang, M.; Zhu, J.; He, Y. Taurine-mediated gene transcription and cell membrane permeability reinforced co-production of bioethanol and monascus azaphilone pigments for a newly isolated monascus purpureus. Biotechnol. Biofuels Bioprod. 2024, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, X.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, J.; Chen, D. Excessive dietary taurine supplementation reduces growth performance, liver and intestinal health of weaned pigs. Livest. Sci. 2014, 168, 109–119. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, L.; Chu, Y.; Feng, F.; Tang, W.; Chen, C.; Qiu, Y.; Hu, Z.; Diao, H.; Tang, Z. Dietary taurine improves growth performance and intestine health via the GSH/GSSG antioxidant system and Nrf2/ARE signaling pathway in weaned piglets. Antioxidants 2023, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Q.; Wu, G.Y. L-Glutamate nutrition and metabolism in swine. Amino Acids 2018, 50, 1497–1510. [Google Scholar] [CrossRef]
- Zhu, M.; Qin, Y.C.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. L-Glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway. Food Funct. 2020, 11, 2714–2724. [Google Scholar] [CrossRef]
- He, W.; Furukawa, K.; Toyomizu, M.; Nochi, T.; Bailey, C.A.; Wu, G. Interorgan metabolism, nutritional impacts, and safety of dietary L-Glutamate and L-Glutamine in poultry. Adv. Exp. Med. Biol. 2021, 1332, 107–128. [Google Scholar] [PubMed]
- Kirchgessner, M.; Roth, F.X.; Paulicks, B.R. Effects of adding glutamic acid to low protein diets for fattening pigs on criteria of growth and carcass composition. Agribiol. Res. 1993, 46, 346–358. [Google Scholar]
- Kong, X.F.; Zhou, X.L.; Feng, Z.M.; Li, F.N.; Ji, Y.J.; Tan, B.E.; Liu, Y.Y.; Geng, M.M.; Wu, G.Y.; Blachier, F.; et al. Dietary supplementation with monosodium L-glutamate modifies lipid composition and gene expression related to lipid metabolism in growing pigs fed a normal- or high-fat diet. Livest. Sci. 2015, 180, 247–252. [Google Scholar] [CrossRef]
- Hu, C.J.; Jiang, Q.Y.; Zhang, T.; Yin, Y.L.; Li, F.N.; Deng, J.P.; Wu, G.Y.; Kong, F. Dietary supplementation with arginine and glutamic acid modifies growth performance, carcass traits, and meat quality in growing-finishing pigs. J. Anim. Sci. 2017, 95, 2680–2689. [Google Scholar] [CrossRef]
- Mosinska, P.; Fichna, J.; Storr, M. Inhibition of ileal bile acid transporter: An emerging therapeutic strategy for chronic idiopathic constipation. World J. Gastroenterol. 2015, 21, 7436–7442. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.M.; Albers, S.; Trautwein, C. Hepatology Snapshot: Role of bile acids in the gut-liver axis. J. Hepatol. 2018, 68, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wei, W.; Hu, L.; Zhang, Y.; Zhang, H.; Liu, J. Reduced feeding frequency improves feed efficiency associated with altered fecal microbiota and bile acid composition in pigs. Front. Microbiol. 2021, 12, 761210. [Google Scholar] [CrossRef] [PubMed]



| Ingredient and Nutritive Level | 40–75 kg | 75–105 kg |
|---|---|---|
| Ingredient (%) | ||
| Corn | 32.40 | 37.20 |
| Wheat | 10.00 | 10.00 |
| Crumbs | 10.00 | 12.00 |
| Wheat bran | 12.00 | 7.30 |
| paddy | 10.00 | 10.00 |
| soya bean meal | 5.80 | 3.80 |
| corn DDGS | 5.00 | 5.00 |
| Corn germ meal | 8.00 | 8.00 |
| Alfalfa grass particles | 3.00 | 3.00 |
| Limestone | 0.90 | 1.00 |
| CaHPO4 | 1.00 | 0.73 |
| NaCl | 0.40 | 0.40 |
| L-Lysine-HCl (70%) | 0.60 | 0.61 |
| DL-Methionine (99%) | 0.00 | 0.01 |
| L-Threonine | 0.10 | 0.11 |
| L-Tryptophan (25%) | 0.10 | 0.14 |
| choline chloride | 0.10 | 0.10 |
| Premix 1 | 0.60 | 0.60 |
| Total | 100.00 | 100.00 |
| Nutritive levels (%) | ||
| Crude protein | 13.5 | 12.50 |
| Corase fiber | 5.01 | 4.68 |
| Crude ash | 5.75 | 4.96 |
| Digestible energy, MJ/kg | 13.27 | 12.72 |
| Metabolic energy, MJ/kg | 12.52 | 12.44 |
| Calcium | 0.70 | 0.65 |
| Total phosphorus | 0.63 | 0.56 |
| Digestible Lys | 0.70 | 0.66 |
| Digestible Met | 0.21 | 0.20 |
| Digestible Thr | 0.43 | 0.41 |
| Digestible Try | 0.13 | 0.13 |
| Items | LS | HS | SEM | p-Value |
|---|---|---|---|---|
| Initial body weight (kg) | 43.385 | 42.897 | 0.752 | 0.76 |
| Final body weight (kg) | 106.570 | 105.770 | 2.203 | 0.86 |
| Average daily gain (kg/day) | 0.786 | 0.776 | 0.023 | 0.83 |
| Average daily feed intake (kg/day) | 2.871 | 3.203 | 0.087 | 0.06 |
| Feed conversion ratio | 3.665 | 4.137 | 0.071 | <0.01 |
| RFI | −0.195 | 0.180 | 0.049 | <0.01 |
| Metabolites | FC 1 | VIP 2 | p |
|---|---|---|---|
| 9-Oxo-ODE | 3.93 | 2.21 | <0.01 |
| (±)13-HODE | 3.70 | 2.12 | <0.01 |
| 2-[(3S)-1-(2-Methylbenzyl)-3-pyrrolidinyl]-1H-imidazo[4,5-b]pyridine | 2.14 | 2.02 | <0.01 |
| Methyl hexadecanoate | 2.10 | 1.97 | <0.01 |
| 16-Heptadecyne-1,2,4-triol | 2.74 | 1.95 | <0.01 |
| MAG (18:3) | 5.33 | 2.03 | <0.01 |
| 13,14-dihydro-15-keto-tetranor Prostaglandin E2 | 2.25 | 1.91 | 0.01 |
| Dibutyl sebacate | 2.10 | 1.88 | 0.01 |
| (±)12(13)-DiHOME | 2.50 | 1.79 | 0.01 |
| PG (27:0/18:1) | 3.83 | 1.68 | 0.01 |
| LSD-d3 | 2.63 | 1.67 | 0.02 |
| FAHFA (18:1/20:3) | 2.67 | 1.65 | 0.02 |
| 4-decyl-3-hydroxy-5-oxooxolane-2,3-dicarboxylic acid | 2.59 | 1.70 | 0.02 |
| Methyl linoleate | 2.21 | 1.62 | 0.02 |
| Methyltestosterone | 2.76 | 1.56 | 0.03 |
| Bisphenol A | 3.35 | 1.98 | 0.03 |
| octadec-9-ynoic acid | 2.30 | 1.53 | 0.04 |
| L-5-Hydroxytryptophan | 2.41 | 1.53 | 0.04 |
| 5,8-dihydroxy-10-methyl-5,8,9,10-tetrahydro-2H-oxecin-2-one | 2.67 | 1.48 | 0.04 |
| 5-OxoETE | 2.52 | 1.44 | 0.05 |
| 2-oxa-4-azatetracyclo[6.3.1.1~6,10~.0~1,5~]tridecan-3-one | 0.45 | 1.44 | 0.05 |
| Metabolites | FC 1 | VIP 2 | p |
|---|---|---|---|
| 1-Methylguanosine | 4.95 | 2.11 | <0.01 |
| L-Glutamate | 2.19 | 2.09 | <0.01 |
| Nicotinamide | 2.93 | 1.98 | <0.01 |
| Adenine | 2.62 | 2.00 | <0.01 |
| 5′-S-Methyl-5′-thioadenosine | 3.37 | 1.96 | <0.01 |
| 4-(allyloxy)-1,2-dihydroquinolin-2-one | 2.62 | 1.94 | <0.01 |
| N1-[1-(2,4-dimethylphenyl)ethyl]-2,2-dimethylpropanamide | 2.61 | 1.93 | <0.01 |
| 3′-Adenosine monophosphate (3′-AMP) | 3.08 | 1.89 | <0.01 |
| Guanosine monophosphate | 0.35 | 1.97 | <0.01 |
| o-Veratraldehyde | 10.15 | 1.89 | <0.01 |
| Nor-9-carboxy-δ9-THC | 0.28 | 1.88 | <0.01 |
| Proline-hydroxyproline | 2.30 | 1.82 | <0.01 |
| XMP | 3.01 | 1.80 | <0.01 |
| Estrone | 2.34 | 1.78 | <0.01 |
| Prostaglandin E1 | 2.70 | 1.83 | <0.01 |
| Estriol | 0.44 | 1.83 | <0.01 |
| 1-Methyladenosine | 2.60 | 1.89 | <0.01 |
| Delta-Tridecalactone | 3.20 | 1.82 | <0.01 |
| Cyclocytidine | 2.18 | 1.73 | <0.01 |
| N2,N2-Dimethylguanosine | 2.05 | 1.80 | <0.01 |
| Hydrocortisone | 0.48 | 1.76 | 0.01 |
| Corticosterone | 4.20 | 1.89 | 0.01 |
| D-Glucuronic acid | 0.40 | 1.69 | 0.01 |
| 3-Nitro-L-Tyrosine | 3.01 | 1.76 | 0.01 |
| Piperine | 2.69 | 1.66 | 0.01 |
| 4-Methylcatechol | 0.35 | 1.63 | 0.01 |
| Prostaglandin D3 | 4.50 | 1.69 | 0.01 |
| Naringenin | 0.33 | 1.56 | 0.02 |
| 2-Phenylacetamide | 2.30 | 1.63 | 0.02 |
| Jasmonic acid | 2.75 | 1.60 | 0.02 |
| Dihydroroseoside | 0.44 | 1.65 | 0.02 |
| 1-ethyl 4-(2-oxo-1,2-diphenylethyl) succinate | 2.56 | 1.55 | 0.02 |
| 7-hydroxy-6-nitro-2,3-dihydro-1H,5H-pyrido[3,2,1-ij]quinolin-5-one | 2.20 | 1.52 | 0.02 |
| Hypotaurine | 2.29 | 1.65 | 0.02 |
| Glycocholic acid | 0.35 | 1.61 | 0.02 |
| (2E,4E)-N-[2-(4-hydroxyphenyl)ethyl]dodeca-2,4-dienamide | 2.52 | 1.54 | 0.02 |
| Oleoyl ethanolamide | 0.49 | 1.52 | 0.02 |
| Caprolactam | 2.99 | 1.59 | 0.02 |
| Phenylacetylglutamine | 3.32 | 1.58 | 0.02 |
| Acetylcysteine | 0.41 | 1.51 | 0.02 |
| Taurine | 0.38 | 1.52 | 0.02 |
| TLH | 3.51 | 1.53 | 0.02 |
| dodecyl [(tetrahydrofuran-2-ylmethyl)amino]methanedithioate | 2.46 | 1.53 | 0.02 |
| 7-methylpyrimido[4,5-d]pyrimidin-4-amine | 2.03 | 1.52 | 0.03 |
| Isobutyryl carnitine | 2.17 | 1.54 | 0.03 |
| (R)-3-Hydroxy myristic acid | 2.06 | 1.46 | 0.03 |
| 4-(3,4-dimethoxyphenyl)-3-methyl-1H-pyrazol-5-amine | 11.06 | 1.57 | 0.03 |
| O1-(2,6-dichlorobenzyl)-4-methoxybenzene-1-carbohydroximamide | 0.10 | 1.52 | 0.03 |
| 4-Hydroxy-3-methylbenzoic acid | 0.30 | 1.49 | 0.03 |
| All-cis-4,7,10,13,16-docosapentaenoic acid | 2.64 | 1.46 | 0.03 |
| Artesunate | 2.87 | 1.46 | 0.03 |
| Hydroxyproline | 3.92 | 1.43 | 0.03 |
| 2-[(carboxymethyl)(methyl)amino]-5-methoxybenzoic acid | 2.04 | 1.50 | 0.03 |
| cis-4-Hydroxy-D-proline | 2.08 | 1.46 | 0.03 |
| Kanosamine | 2.40 | 1.52 | 0.04 |
| Dehydroepiandrosterone | 2.48 | 1.43 | 0.04 |
| N-Acetyl-L-aspartic acid | 2.33 | 1.50 | 0.04 |
| Catechol | 0.49 | 1.39 | 0.04 |
| N1-[3,5-di(trifluoromethyl)phenyl]-2-cyclopentyl-2-phenylacetamide | 0.18 | 1.49 | 0.04 |
| N-(9-oxodecyl)acetamide | 0.44 | 1.36 | 0.04 |
| Deisopropylatrazine | 2.12 | 1.35 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Li, C.; He, Z.; Liu, H.; Wang, M.; He, J. Metabolomics Profiling of Serum and Urine from Chuanzang Black Pigs with Different Residual Feed Intake. Animals 2024, 14, 2323. https://doi.org/10.3390/ani14162323
Zhou X, Li C, He Z, Liu H, Wang M, He J. Metabolomics Profiling of Serum and Urine from Chuanzang Black Pigs with Different Residual Feed Intake. Animals. 2024; 14(16):2323. https://doi.org/10.3390/ani14162323
Chicago/Turabian StyleZhou, Xiang, Chongying Li, Zongze He, Hongwei Liu, Man Wang, and Jian He. 2024. "Metabolomics Profiling of Serum and Urine from Chuanzang Black Pigs with Different Residual Feed Intake" Animals 14, no. 16: 2323. https://doi.org/10.3390/ani14162323





