Exploration of Synergistic Regulation Mechanisms of Cerebral Ganglion and Muscle in Eriocheir sinensis Activated in Response to Alkalinity Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Alkalinity Stress
2.2. Proteomics Analysis of the Cerebral Ganglion of E. sinensis
2.2.1. Sample Collection
2.2.2. Proteomics Analysis
Protein Extraction, Separation, Enzymolysis, and Desalination
Liquid Chromatography–Tandem Mass Spectrometry/Mass Spectrometry (LC-MS/MS) Analysis and Database Query
Gene Ontology (GO) Annotation on Proteomics Analysis
Enrichment Analysis on Differentially Expressed Proteins (DEPs)
2.3. Metabolomic Analysis of the Muscle of E. sinensis
2.3.1. Sample Collection
2.3.2. Metabolomics Analysis
Extraction of Metabolites from the Muscle of E. sinensis and Ultra Performance Liquid Chromatography/Mass Chromatography (UPLC-MS) Analysis
Principal Component Analysis (PCA) and Partial Least Squares Discrimination Analysis (PLS-DA) on Differential Expressed Metabolites (DEMs)
KEGG Enrichment Analysis on DEMs
2.4. Combined Proteomic and Metabolomic Analyses on Cerebral Ganglion and Muscle
3. Results
3.1. Proteomics Analysis
3.1.1. Basic Statistics and Annotation of Cerebral Ganglion Proteome
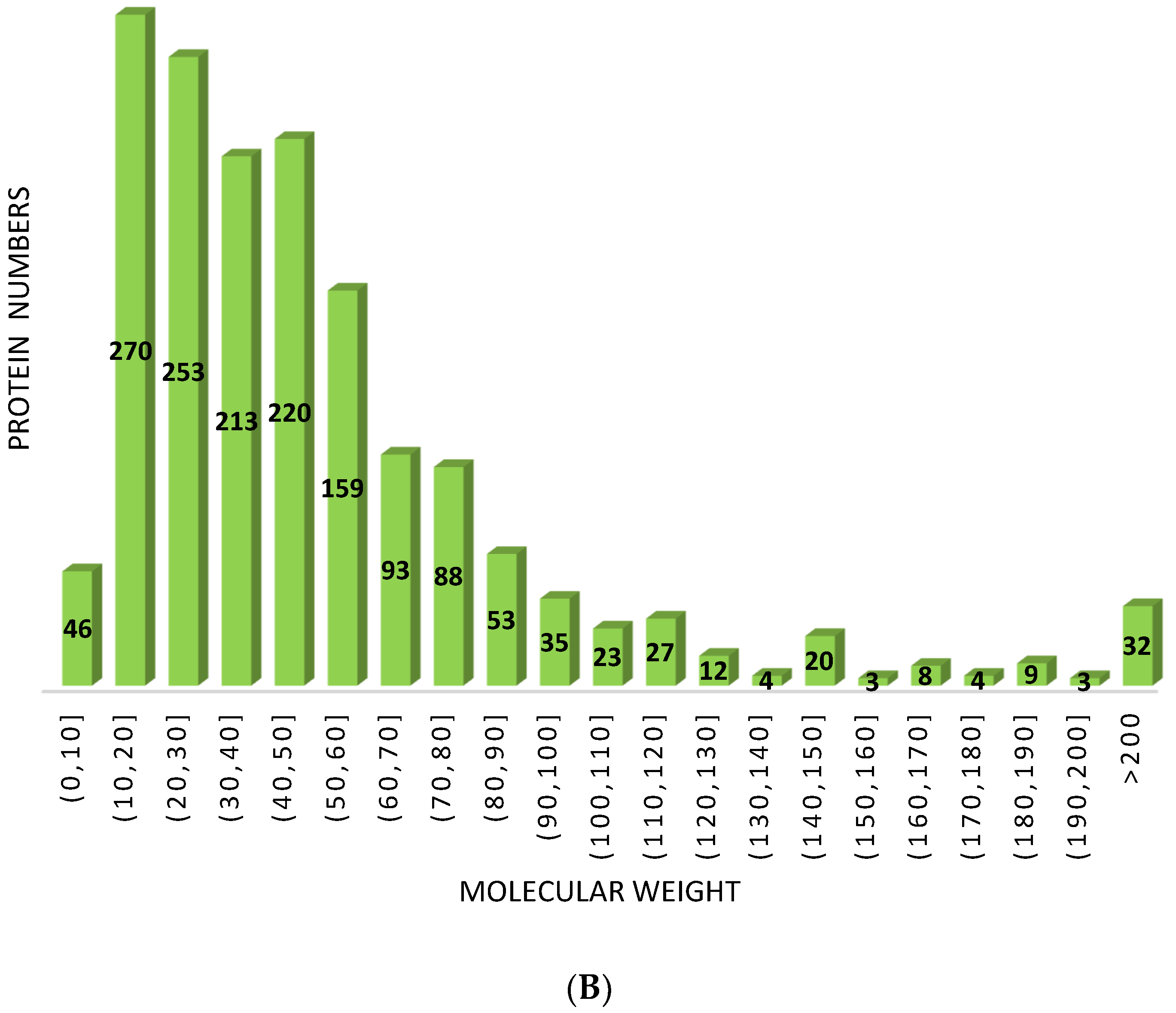
Peptide Length and Molecular Weight Distribution
GO Annotation of Proteome
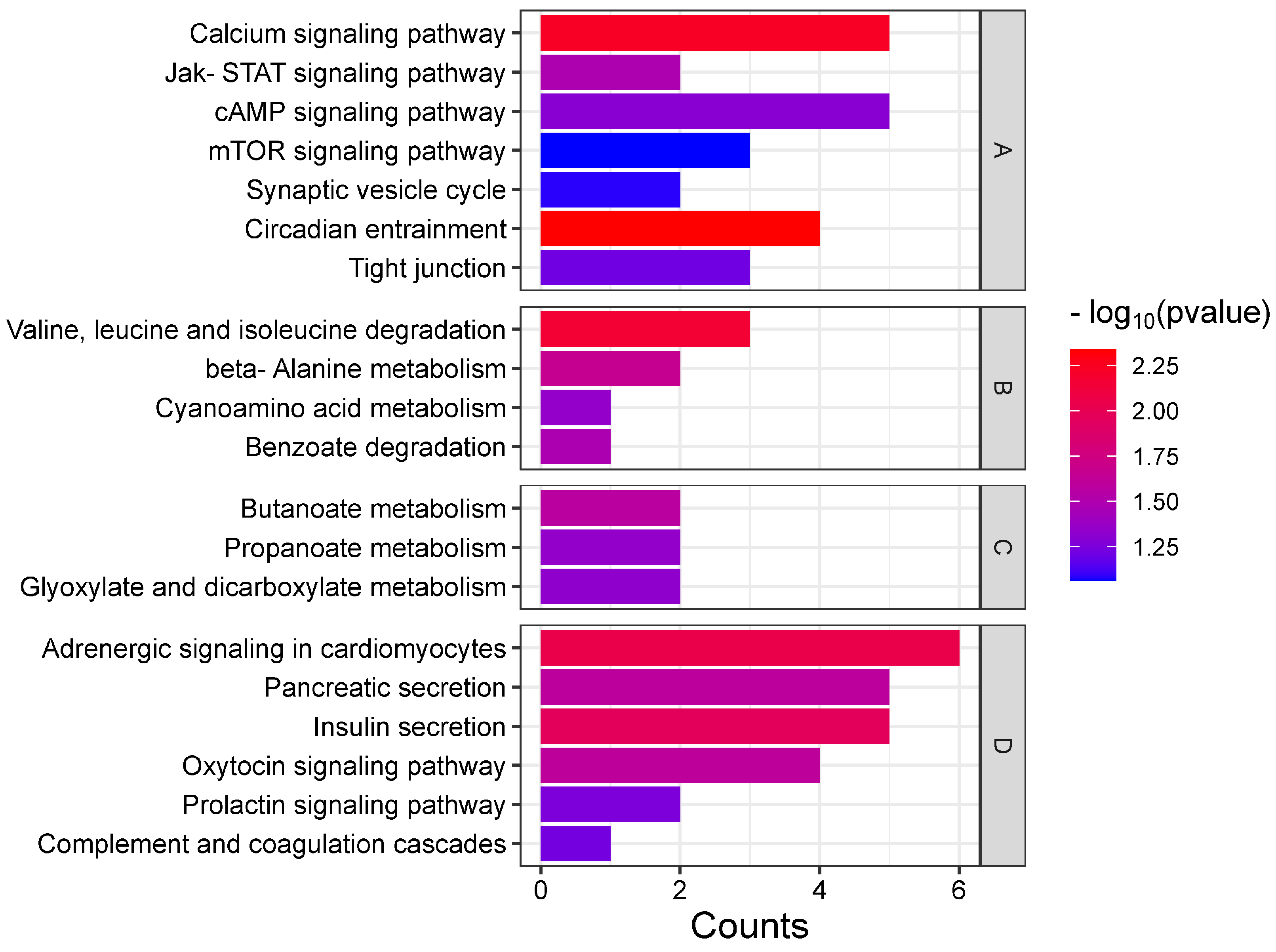
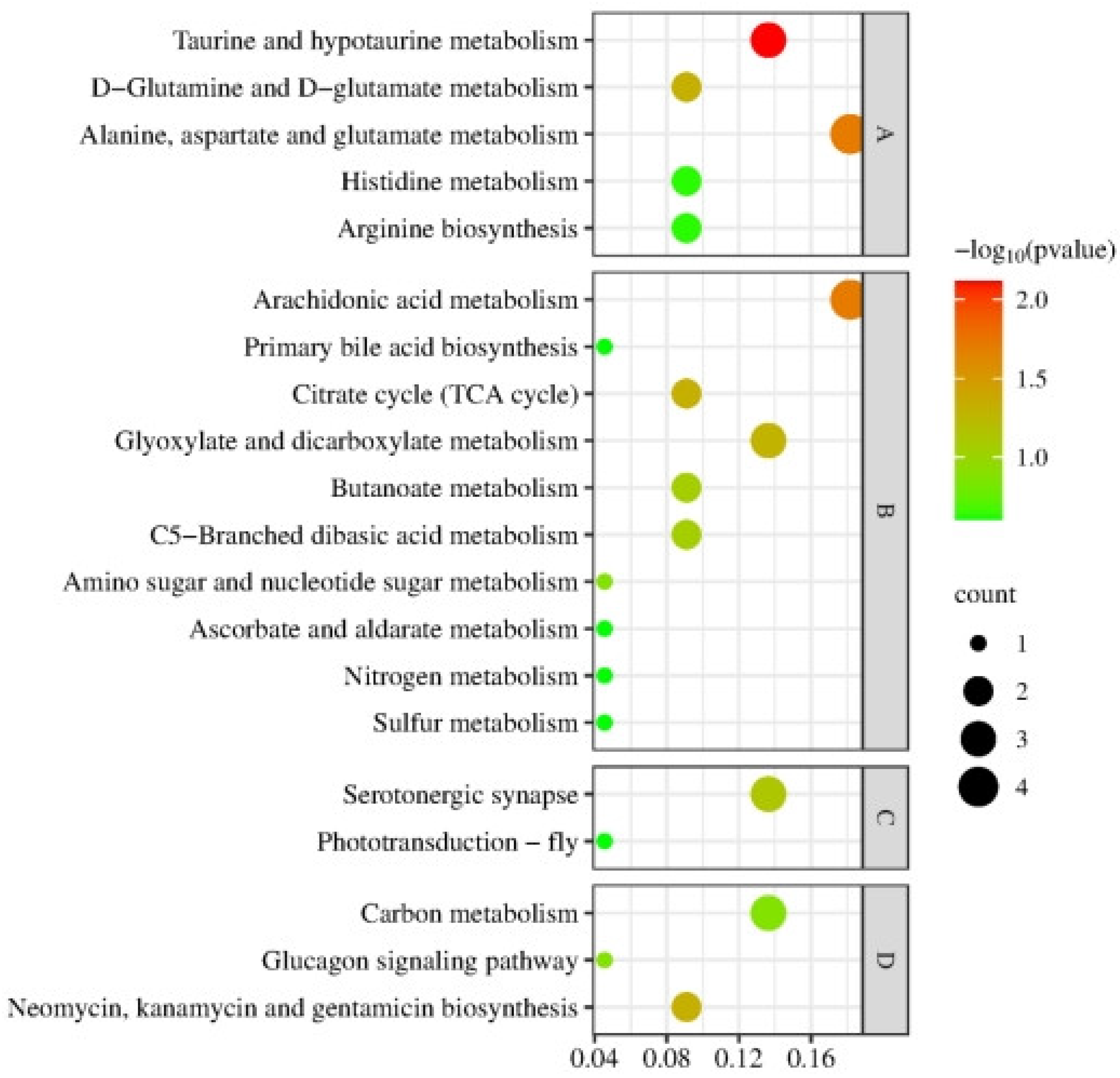
3.1.2. Enrichment Analysis of the Top 20 KEGG Pathways
3.2. Metabolomics Analysis
3.2.1. Multivariate Statistics of Differentially Expressed Metabolites
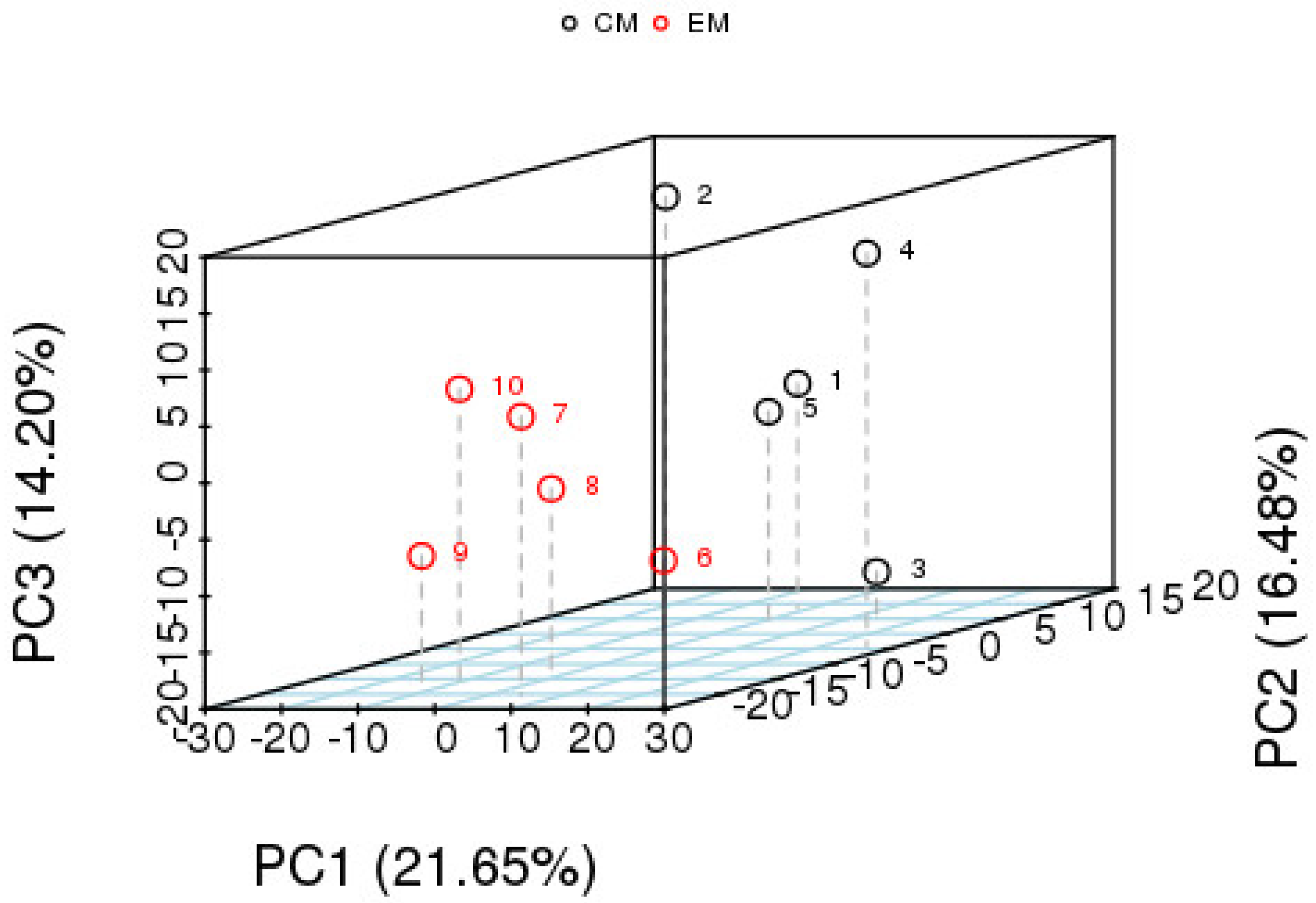
PCA Analysis of Differential Metabolites
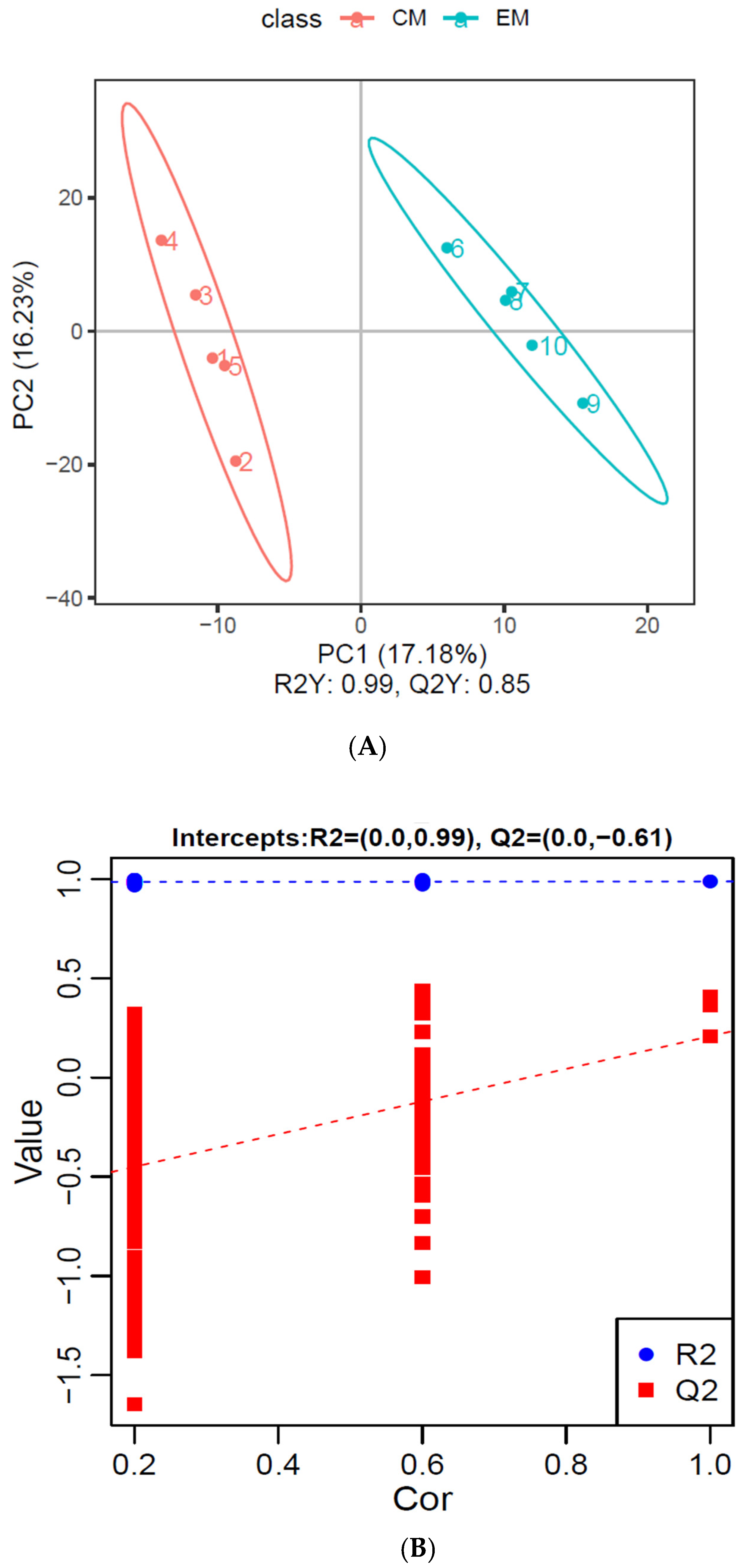
PLS-DA Analysis of Differential Metabolites
3.2.2. Enrichment Analysis on Top 20 KEGG Pathways
3.3. Combined Proteomics and Metabolomics Analyses of Cerebral Ganglion and Muscle of E. sinensis under Acute Alkalinity Stress
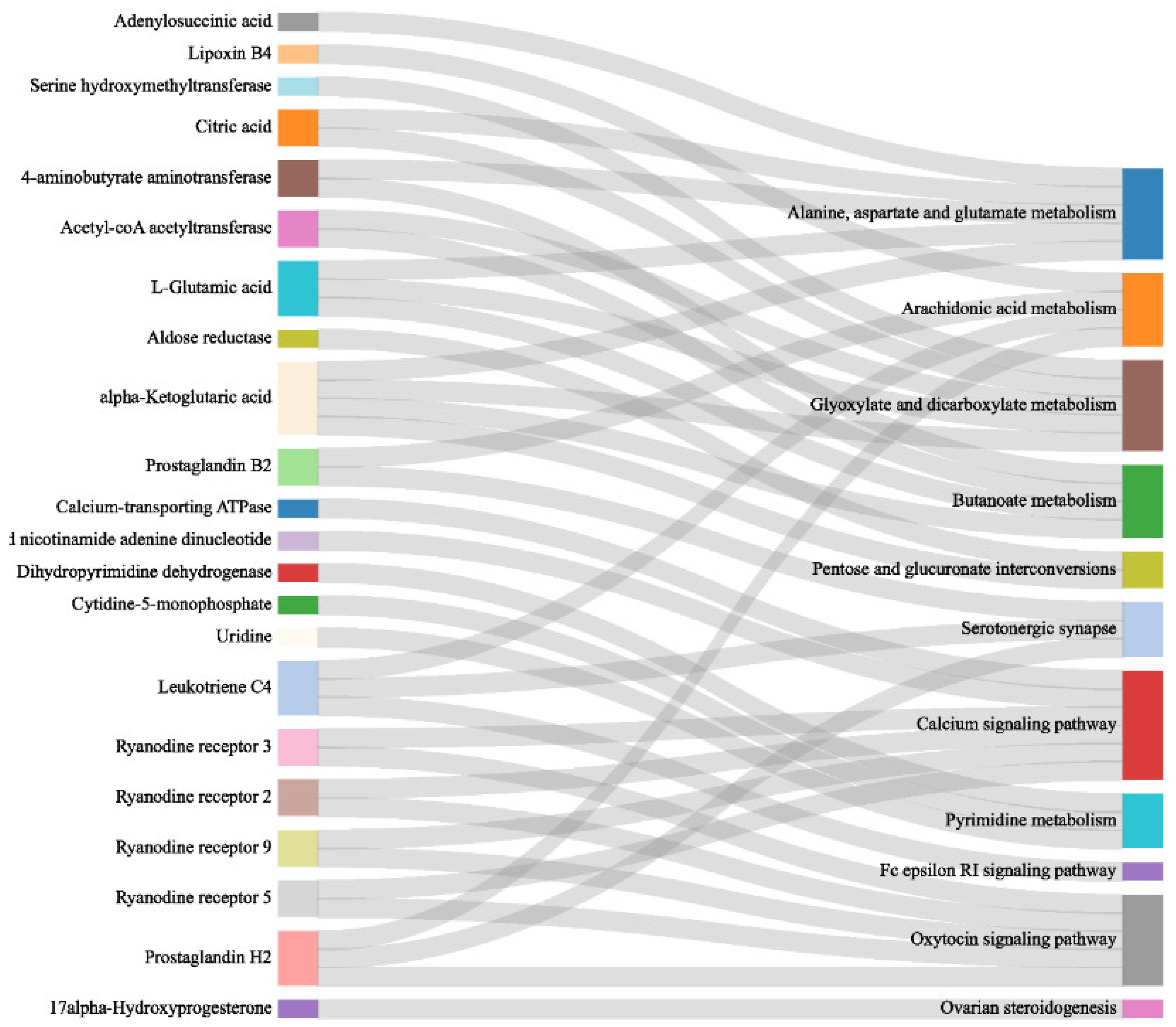
3.3.1. Co-Enrichment Pathways of Cerebral Ganglion Proteome and Muscle Metabolome
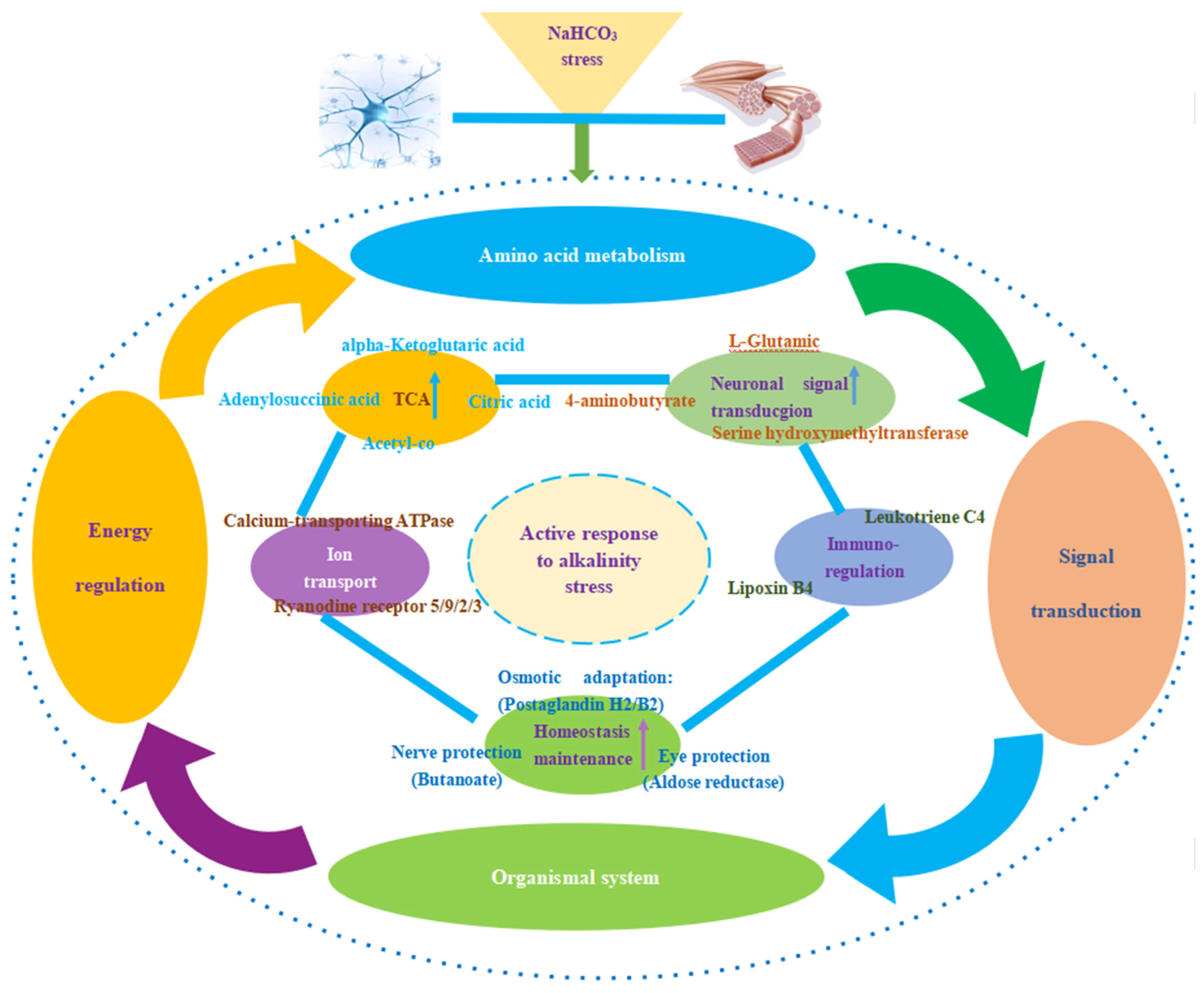
3.3.2. Synergistic Regulation Mechanisms of Cerebral Ganglion and Muscle of E. sinensis under Alkalinity Stress
4. Discussion
4.1. Synergistic Regulation of Amino Acid Metabolism in Cerebral Ganglion and Muscle of E. sinensis under Alkalinity Stress
4.2. Synergistic Regulation of Energy Metabolism under Alkalinity Stress
4.3. Synergistic Regulation of Signal Transduction and Immunoregulation under Alkalinity Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bednarsek, N.; Ambrose, R.; Calosi, P.; Childers, R.K.; Feely, R.A.; Litvin, S.Y.; Long, W.C.; Spicer, J.I.; Strus, J.; Taylor, J.; et al. Synthesis of thresholds of ocean acidification impacts on decapods. Front. Mar. Sci. 2021, 8, 651102. [Google Scholar] [CrossRef]
- Long, W.C.; Swiney, K.M.; Foy, R.J. Effects of ocean acidification on young-of-the-year golden king crab (Lithodes aequispinus) survival and growth. Mar. Biol. 2021, 168, 126. [Google Scholar] [CrossRef]
- Dickinson, G.H.; Bejerano, S.; Salvador, T.; Makdisi, C.; Patel, S.; Long, W.C.; Swiney, K.M.; Foy, R.J.; Steffel, B.V.; Smith, K.E.; et al. Ocean acidification alters properties of the exoskeleton in adult Tanner crabs, Chionoecetes bairdi. J. Exp. Biol. 2021, 224, jeb232819. [Google Scholar] [CrossRef] [PubMed]
- Bednarsek, N.; Feely, R.A.; Beck, M.W.; Alin, S.R.; Siedlecki, S.A.; Calosi, P.; Norton, E.L.; Saenger, C.; Strus, J.; Greeley, D.; et al. Exoskeleton dissolution with mechanoreceptor damage in larval Dungeness crab related to severity of present-day ocean acidification vertical gradients. Sci. Total Environ. 2020, 716, 136610. [Google Scholar] [CrossRef]
- FAO. Global Map of Salt Affected Soils Version 1.0. 2021. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils/en/ (accessed on 27 October 2021).
- Wang, W.; Chen, X.; Wang, L.; Zhang, H.; Yin, G.; Zhang, Y. Approaching the Truth of the Missing Carbon Sink. Pol. J. Environ. Stud. 2016, 25, 1799–1802. [Google Scholar] [CrossRef]
- Pellegrin, L.; Nitz, L.F.; Maltez, L.C.; Copatti, C.E.; Garcia, L. Alkaline water improves the growth and antioxidant responses of pacu juveniles (Piaractus mesopotamicus). Aquaculture 2020, 519, 734713. [Google Scholar] [CrossRef]
- Peng, M.; Li, Z.; Liu, X.; Niu, D.; Li, J. Inland alkaline brackish water aquaculture of juvenile razor clam, Survival, growth, physiology and immune responses. Aquacult. Rep. 2020, 18, 100463. [Google Scholar] [CrossRef]
- Fishery Administration Bureau of the Ministry of Agriculture. Fishery Statistics Yearbook of China in 2016; Agriculture Press: Beijing, China, 2016; pp. 19–21.
- Song, Q.H.; Zhao, Y.F. Analysis on status and standard for Eriocheir sinensis breeding industry. Sci. Fish Farm. 2018, 10, 13–15. [Google Scholar]
- Zilius, M.; Bonaglia, S.; Broman, E.; Chiozzini, V.G.; Samuiloviene, A.; Nascimento, F.J.A.; Cardini, U.; Bartoli, M. N2 fixation dominates nitrogen cycling in a mangrove fiddler crab holobiont. Sci. Rep. 2020, 10, 13966. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Metabolomics analysis reveals the response mechanism to carbonate alkalinity toxicity in the gills of Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109487. [Google Scholar] [CrossRef]
- Wang, S.; Guo, K.; Luo, L.; Zhang, R.; Xu, W.; Song, Y.; Zhao, Z. Fattening in Saline and Alkaline Water Improves the Color, Nutritional and taste quality of adult Chinese mitten crab Eriocheir sinensis. Foods 2022, 11, 2573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Z.; Wang, C.; Qi, C.; Gu, Z.; Li, E.; Qin, J.G.; Chen, L. A Comparative Study on Growth and Metabolism of Eriocheir sinensis juveniles under chronically low and high pH stress. Front. Physiol. 2020, 11, 885. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Effects of saline-alkaline stress on the tissue structure, antioxidation, immunocompetence and metabolomics of Eriocheir sinensis. Sci. Total Environ. 2023, 871, 162109. [Google Scholar] [CrossRef]
- Cripps, G.; Widdicombe, S.; Spicer, J.I.; Findlay, H.S. Biological impacts of enhanced alkalinity in Carcinus maenas. Mar. Pollut. Bull. 2013, 71, 190–198. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, K.; Liang, G.; Li, X.; Niu, M.; Wang, H.; Wang, C.; Mu, C.; Zhu, R. Comparative study on non-volatile flavor substances of Scyllaparamamosain cultured in inland low saline-alkaline water. J. Food Compos. Anal. 2023, 118, 105157. [Google Scholar] [CrossRef]
- Niu, M.; Li, X.; Chen, Y.; Qin, K.; Liang, G.; Hu, Y.; Jiang, X.; Wang, H.; Zhu, R.; Wang, C.; et al. Response of intestinal microbiota to saline-alkaline water in mud crab (Scylla paramamosain) based on multiple low salinity culture modes. Front. Mar. Sci. 2023, 10, 1153326. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Wang, C.; Li, W.; Ge, Q.; Qin, Z.; Li, J.; Li, J. Effects of long-term high carbonate alkalinity stress on the ovarian development in Exopalaemon carinicauda. Water 2022, 14, 3690. [Google Scholar] [CrossRef]
- Liang, G.; Qin, K.; Chen, Y.; Niu, M.; Wang, H.; Wang, C.; Mu, C.; Chen, L.; Wang, F.; Su, Q.; et al. Transcriptomic analysis of adaptive mechanisms in response to inland saline-alkaline water in the mud crab, Scylla paramamosain. Front. Mar. Sci. 2022, 9, 974501. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Xiong, K.; Zhang, C.; Fang, J.K.H.; Song, J.; Tai, Z.; Zhu, Q.; Hu, M.; Wang, Y. Effects of ocean acidification on molting, oxidative stress, and gut microbiota in juvenile horseshoe crab Tachypleus tridentatus. Front. Physiol. 2022, 12, 813582. [Google Scholar] [CrossRef]
- Thangal, S.H.; Muralisankar, T.; Anandhan, K.; Gayathri, V.; Yogeshwaran, A. Effect of CO2 driven ocean acidification on the mud crab Scylla serrata instars. Environ. Pollut. 2022, 312, 119995. [Google Scholar] [CrossRef]
- Sreelekshmi, S.; Manish, K.; Peter, M.C.S.; Inbaraj, R.M. Analysis of neuroendocrine factors in response to conditional stress in zebrafish Danio rerio (Cypriniformes, Cyprinidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 252, 109242. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, L.J.; Wu, M.X.; Peng, G.F.; Zhang, Y.L. Aquaporins1 and 3 in the tissues of Paramisgurnus dabryanus and their expression profiles in response to ammonia and drought. Front. Mar. Sci. 2022, 9, 1009679. [Google Scholar] [CrossRef]
- Heigrujam, E.; Ali, I.; Bhargava, S. NPY up-regulation in the tadpole brain of Euphlyctis cyanophlyctis during osmotic stress. Gen. Comp. Endocrinol. 2017, 251, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Gaytan, M.F.; Gomez-Jimenez, S.; Gamez-Alejo, L.A.; Rosas-Rodriguez, J.A.; Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Effect of salinity on the synthesis and concentration of glycine betaine in osmoregulatory tissues from juvenile shrimps Litopenaeus vannamei. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 240, 110628. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Panche, A.N. Application of proteomics in shrimp and shrimp aquaculture. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101015. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pedrouso, M.; Varela, Z.; Franco, D.; Fernandez, J.A.; Aboal, J.R. Can proteomics contribute to biomonitoring of aquatic pollution? A critical review. Environ. Pollut. 2020, 267, 115473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Qian, L.; Ding, L.Y.; Wang, L.; Wong, M.H.; Tao, H.C. Ecological and toxicological assessments of anthropogenic contaminants based on environmental metabolomics. Environ. Sci. Ecotechnol. 2021, 5, 100081. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S. Blast2GO, A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics. 2008, 2008, 619832. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, F.; Ding, S.; Sun, L.; Liu, Z.; Ding, K.; Lu, J. Label-free quantitative proteomic analysis reveals potential biomarkers and pathways in renal cell carcinoma. Tumor Biol. 2015, 36, 939–951. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Boulesteix, A.L.; Strimmer, K. Partial least squares, a versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinf. 2007, 8, 32–44. [Google Scholar] [CrossRef]
- Wang, J.B.; Pu, S.B.; Sun, Y.; Li, Z.F.; Niu, M.; Yan, X.Z.; Zhao, Y.L.; Wang, L.F.; Qin, X.M.; Ma, Z.J.; et al. Metabolomic Profiling of Autoimmune Hepatitis, The diagnostic utility of nuclear magnetic resonance spectroscopy. J. Prot. Res. 2014, 13, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Bostwick, A.; Pepper, H.; Snyder, N.W. Alanine Modulates lactyl-coa abundance in HEPG2 cells. FASEB J. 2022, 36, R4457. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhong, H.T.; Cao, T.; Huang, Y.F.; Ji, X.Y.; Fan, G.C.; Peng, T.Q. Gamma-aminobutyrate transaminase protects against lipid overload-triggered cardiac injury in mice. Int. J. Mol. Sci. 2022, 23, 2182. [Google Scholar] [CrossRef]
- Si, Y.F.; Wen, H.S.; Li, Y.; He, F.; Li, J.F.; Li, S.P.; He, H.W. Liver transcriptome analysis reveals extensive transcriptional plasticity during acclimation to low salinity in Cynoglossus semilaevis. BMC Genom. 2018, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Abraham, W.C. Glutamate receptors and synaptic plasticity, The impact of Evans and Watkins. Neuropharmacology 2022, 206, 108922. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Zhou, Z.; Wang, L.L.; Li, M.J.; Wang, W.L.; Yi, Q.L.; Huang, S.; Song, L.S. Dopamine and serotonin modulate free amino acids production and Na+/K+ pump activity in Chinese mitten crab Eriocheir sinensis under acute salinity stress. Front. Physiol. 2018, 9, 1080. [Google Scholar] [CrossRef]
- Meng, X.Q.; Liu, H.Q.; Peng, L.X.; He, W.G.; Li, S.Y. Potential clinical applications of alpha-ketoglutaric acid in diseases. Mol. Med. Rep. 2022, 25, 151. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, Q.F.; Yu, H.; Liu, D.Z.; Dong, S.L.; Zhou, Y.E.; Yang, W.Z.; Xue, N.; Bao, H.C.; Yu, Y.N. Dynamic transcriptome and LC-MS/MS analysis revealed the important roles of taurine and glutamine metabolism in response to environmental salinity changes in gills of rainbow trout (Oncorhynchus mykiss). Int. J. Biol. Macro. 2022, 221, 1545–1557. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, Q.; Zhang, X.N.; Gao, G.; Niu, M.M.; Wang, H.; Chen, L.Z.; Wang, C.L.; Mu, C.K.; Wang, F.F. Metabolic response in the gill of Portunus trituberculatus under short-term low salinity stress based on GC-MS technique. Front. Mar. Sci. 2022, 9, 881016. [Google Scholar] [CrossRef]
- Rybalka, E.; Goodman, C.A.; Campelj, D.G.; Hayes, A.; Timpani, C.A. Adenylosuccinic acid, a novel inducer of the cytoprotectant Nrf2 with efficacy in Duchenne muscular dystrophy. Curr. Med. Res. Opin. 2021, 37, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Timpani, C.A.; Goodman, C.A.; Stathis, C.G.; White, J.D.; Mamchaoui, K.; Butler-Browne, G.; Gueven, N.; Hayes, A.; Rybalka, E. Adenylosuccinic acid therapy ameliorates murine Duchenne Muscular Dystrophy. Sci. Rep. 2020, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Lund, I.; Rodriguez, C.; Izquierdo, M.S.; El Kertaoui, N.; Kestemont, P.; Reis, D.B.; Dominguez, D.; Perez, J.A. Influence of salinity and linoleic or alpha-linolenic acid based diets on ontogenetic development and metabolism of unsaturated fatty acids in pike perch larvae (Sander lucioperca). Aquaculture 2019, 500, 550–561. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Delisle, L.; Petton, B.; Corporeau, C.; Pernet, F. Metabolism of the Pacific oyster, Crassostrea gigas, is influenced by salinity and modulates survival to the Ostreid herpesvirus OsHV. Biol. Open 2018, 7, bio028134. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, C.A.; Bushnell, E.A.C.; Gauld, J.W.; Boyd, R.J. The catalytic formation of leukotriene C-4, a critical step in inflammatory processes. Phys. Chem. Chem. Phys. 2014, 16, 16284–16289. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Fujita, K.; Tavajo, S.; Sotak, M.; Gu, Y.; Adler, E.; Chen, J.; Borgeson, E.; Lange, S. Investigating the therapeutic potential of lipoxins in attenuating muscle inflammation and cardiac fibrosis. J. Mol. Cel. Cardioll. 2022, 173, S126. [Google Scholar] [CrossRef]
- Sun, Y.C.; Han, S.C.; Yao, M.Z.; Liu, H.B.; Wang, Y.M. Exploring the metabolic biomarkers and pathway changes in crucian under carbonate alkalinity exposure using high-throughput metabolomics analysis based on UPLC-ESI-QTOF-MS. Rsc Adv. 2020, 10, 1552–1571. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Jain, A.; Takabe, T.; Tanaka, Y.; Negi, M.; Singh, N.; Jain, N.; Mishra, V.; Maniraj, R.; Krishnamurthr, S.L.; et al. Heterologous expression of serine hydroxymethyltransferase-3 from rice confers tolerance to salinity stress in E-coli and Arabidopsis. Front. Plant Sci. 2019, 10, 217. [Google Scholar] [CrossRef]
- Harned, T.C.; Stan, R.V.; Cao, Z.; Chakrabarti, R.; Higgs, H.N.; Chang, C.C.Y.; Chang, T.Y. Acute ACAT1/SOAT1 blockade increases MAM cholesterol and strengthens er-mitochondria connectivity. Int. J. Mol. Sci. 2023, 24, 5525. [Google Scholar] [CrossRef]
- He, X.; Zhang, T.; Zeng, Y.; Pei, P.; Liu, Y.; Jia, W.; Zhao, H.; Bi, M.; Wang, S. Sodium butyrate mediates histone crotonylation and alleviated neonatal rats hypoxic-ischemic brain injury through gut-brain axis. Front. Microbiol. 2022, 13, 993146. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG, new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Liu, X.Y.; Peng, J.; He, F.; Tursun, X.; Li, S.P.; Xin, X.L.; Aisa, H.A. Shabyar Ameliorates high glucose induced retinal pigment epithelium injury through suppressing aldose reductase and AMPK/mTOR/ULK1 autophagy pathway. Front. Pharmacol. 2022, 13, 852945. [Google Scholar] [CrossRef]
- Tang, H.H.; Zhang, Y.F.; Yang, L.L.; Hong, C.; Chen, K.X.; Li, Y.M.; Wu, H.L.L. Serotonin/5-HT7 receptor provides an adaptive signal to enhance pigmentation response to environmental stressors through cAMP-PKA-MAPK, Rab27a/RhoA, and PI3K/AKT signaling pathways. FASEB J. 2023, 37, e22893. [Google Scholar] [CrossRef]
- Servili, A.; Leveque, E.; Mouchel, O.; Devergne, J.; Lebigre, C.; Roussel, S.; Mazurais, D.; Zambonino-Infante, J.L. Ocean acidification alters the acute stress response of a marine fish. Sci. Total Environ. 2023, 858, 159804. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Hussain, S.; Ahmar, S.; Naz, M.; Zafar, H.; Pandey, S.; Chauhan, J.; Siddiqui, M.H.; Pinghua, C. Calcium homeostasis and potential roles to combat environmental stresses in plants. S. Afr. J. Bot. 2022, 148, 683–693. [Google Scholar] [CrossRef]
- Khaitin, A.; Rudkovskii, M.; Uzdensky, A. Ca2+ mediates axotomy-induced necrosis and apoptosis of satellite glial cells remote from the transection site in the isolated crayfish mechanoreceptor. Mol. Cell. Neurosci. 2018, 88, 7–15. [Google Scholar] [CrossRef]
- Forsythe, P. Mast cells in neuroimmune interactions. Trends Neurosci. 2019, 42, 43–55. [Google Scholar] [CrossRef] [PubMed]


| Name | Classification | Log2FC | p-Value | Regulation |
|---|---|---|---|---|
| 4-aminobutyrate aminotransferase | DEP | inf | 2.94 × 10−6 | up |
| alpha-Ketoglutaric acid | DEM | 1.81 | 4.90 × 10−3 | up |
| Citric acid | DEM | 0.73 | 4.28 × 10−2 | up |
| Adenylosuccinic acid | DEM | 0.99 | 2.95 × 10−2 | up |
| L-Glutamic acid | DEM | 0.71 | 3.63 × 10−2 | up |
| Prostaglandin H2 | DEM | 1.51 | 3.79 × 10−2 | up |
| Prostaglandin B2 | DEM | 1.67 | 1.29 × 10−2 | up |
| Leukotriene C4 | DEM | 1.197 | 4.09 × 10−2 | up |
| Lipoxin B4 | DEM | 1.52 | 1.50 × 10−3 | up |
| ABAT | DEP | inf | 2.94 × 10−6 | up |
| Aldose reductase | DEP | 0.2 | 4.00 × 10−4 | up |
| Calcium-transporting ATPase | DEP | −0.39 | 2.87 × 10−2 | down |
| Ryanodine receptor 5 | DEP | −3.07 | 1.50 × 10−2 | down |
| Ryanodine receptor 9 | DEP | 0.12 | 2.77 × 10−3 | up |
| Ryanodine receptor 2 | DEP | 0.35 | 1.12 × 10−3 | up |
| Ryanodine receptor 3 | DEP | 0.21 | 3.75 × 10−4 | up |
| Reduced nicotinamide adenine dinucleotide | DEM | −5.25 | 5.60 × 10−3 | down |
| Uridine | DEM | −0.54 | 1.04 × 10−2 | down |
| Cytidine-5′-monophosphate | −4.81 | 8.90 × 10−3 | down | |
| 17alpha-Hydroxyprogesterone | DEM | 0.32 | 3.02 × 10−3 | up |
| Serine hydroxymethyltransferase | DEP | 0.63 | 5.72 × 10−4 | up |
| Acetyl-coA acetyltransferase | DEP | 0.98 | 1.07 × 10−4 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhou, J.; Ge, J.; Tang, Y.; Xu, G. Exploration of Synergistic Regulation Mechanisms of Cerebral Ganglion and Muscle in Eriocheir sinensis Activated in Response to Alkalinity Stress. Animals 2024, 14, 2374. https://doi.org/10.3390/ani14162374
Wang M, Zhou J, Ge J, Tang Y, Xu G. Exploration of Synergistic Regulation Mechanisms of Cerebral Ganglion and Muscle in Eriocheir sinensis Activated in Response to Alkalinity Stress. Animals. 2024; 14(16):2374. https://doi.org/10.3390/ani14162374
Chicago/Turabian StyleWang, Meiyao, Jun Zhou, Jiachun Ge, Yongkai Tang, and Gangchun Xu. 2024. "Exploration of Synergistic Regulation Mechanisms of Cerebral Ganglion and Muscle in Eriocheir sinensis Activated in Response to Alkalinity Stress" Animals 14, no. 16: 2374. https://doi.org/10.3390/ani14162374
APA StyleWang, M., Zhou, J., Ge, J., Tang, Y., & Xu, G. (2024). Exploration of Synergistic Regulation Mechanisms of Cerebral Ganglion and Muscle in Eriocheir sinensis Activated in Response to Alkalinity Stress. Animals, 14(16), 2374. https://doi.org/10.3390/ani14162374







