LEP Gene Promotes Milk Fat Synthesis via the JAK2-STAT3 and mTOR Signaling Pathways in Buffalo Mammary Epithelial Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
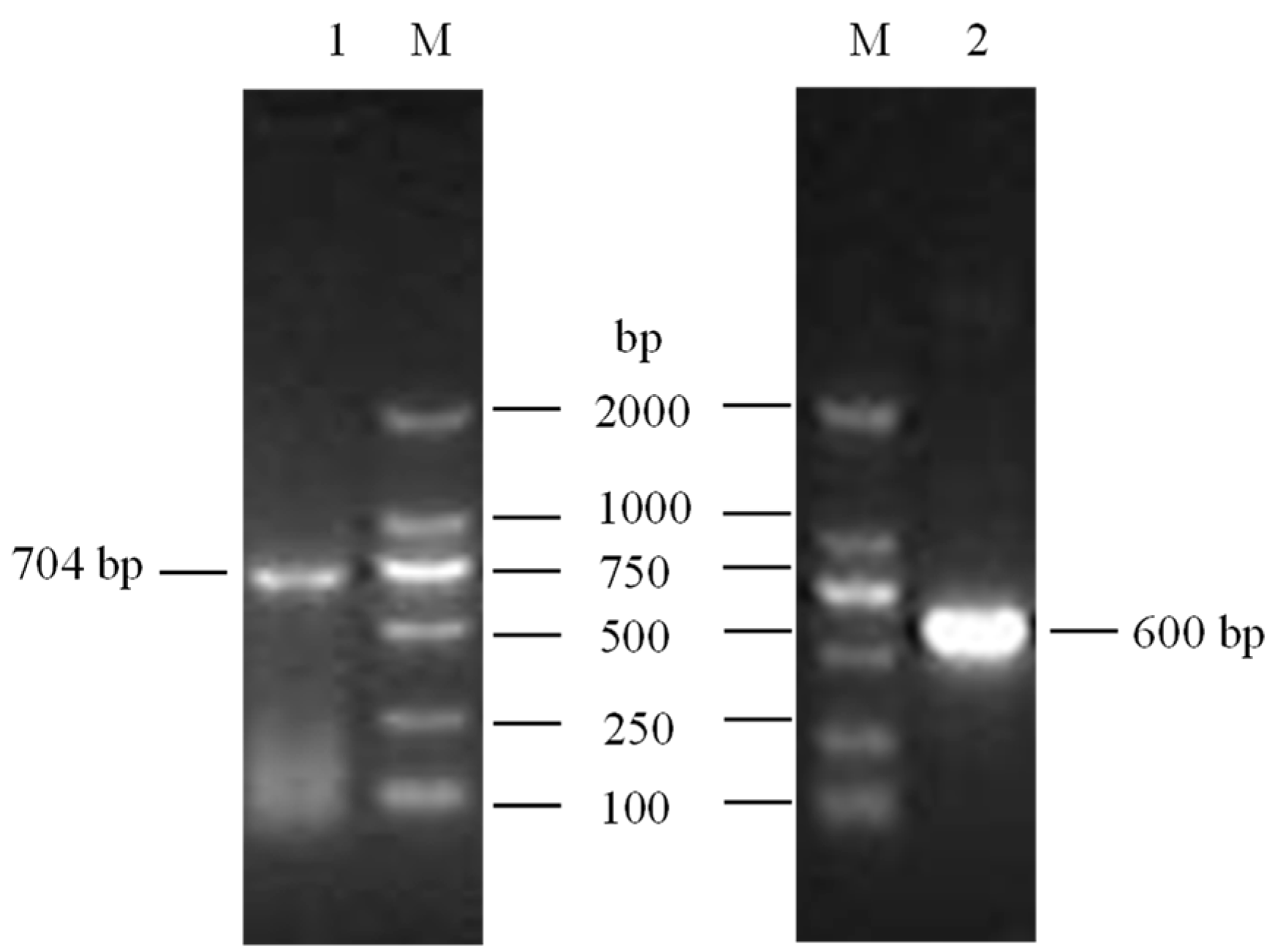
2.2. Gene Cloning and Identification
2.3. Bioinformatics Analysis
2.4. Overexpression Vector Construction
2.5. Cell Culture and Transduction
2.6. Gene Expression Detection and Analysis
2.7. TG Test in BuMECs
2.8. Detection and Analysis of LEP Polymorphisms
2.9. Data Statistics
3. Results
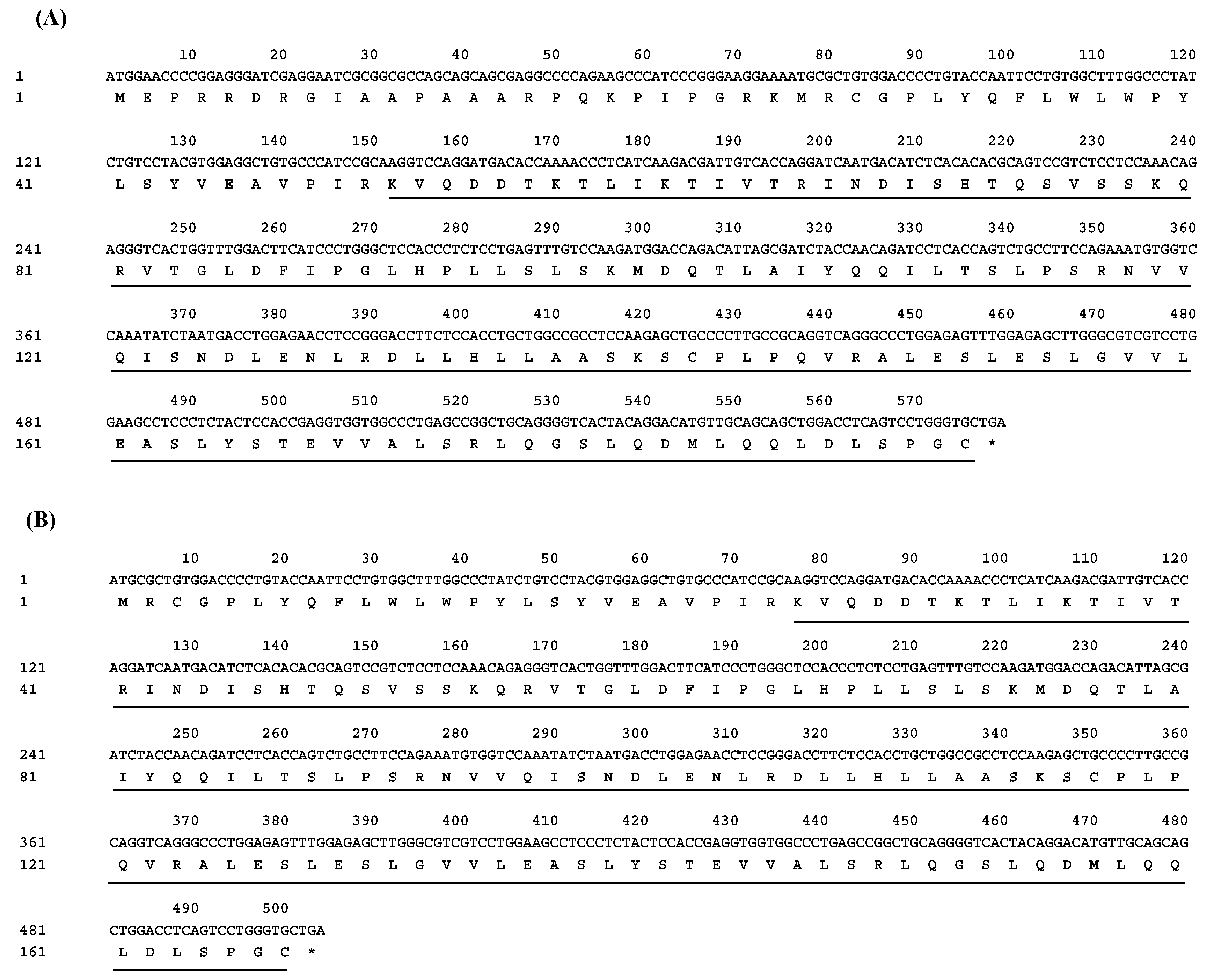
3.1. Cloning and Identification of Buffalo LEP
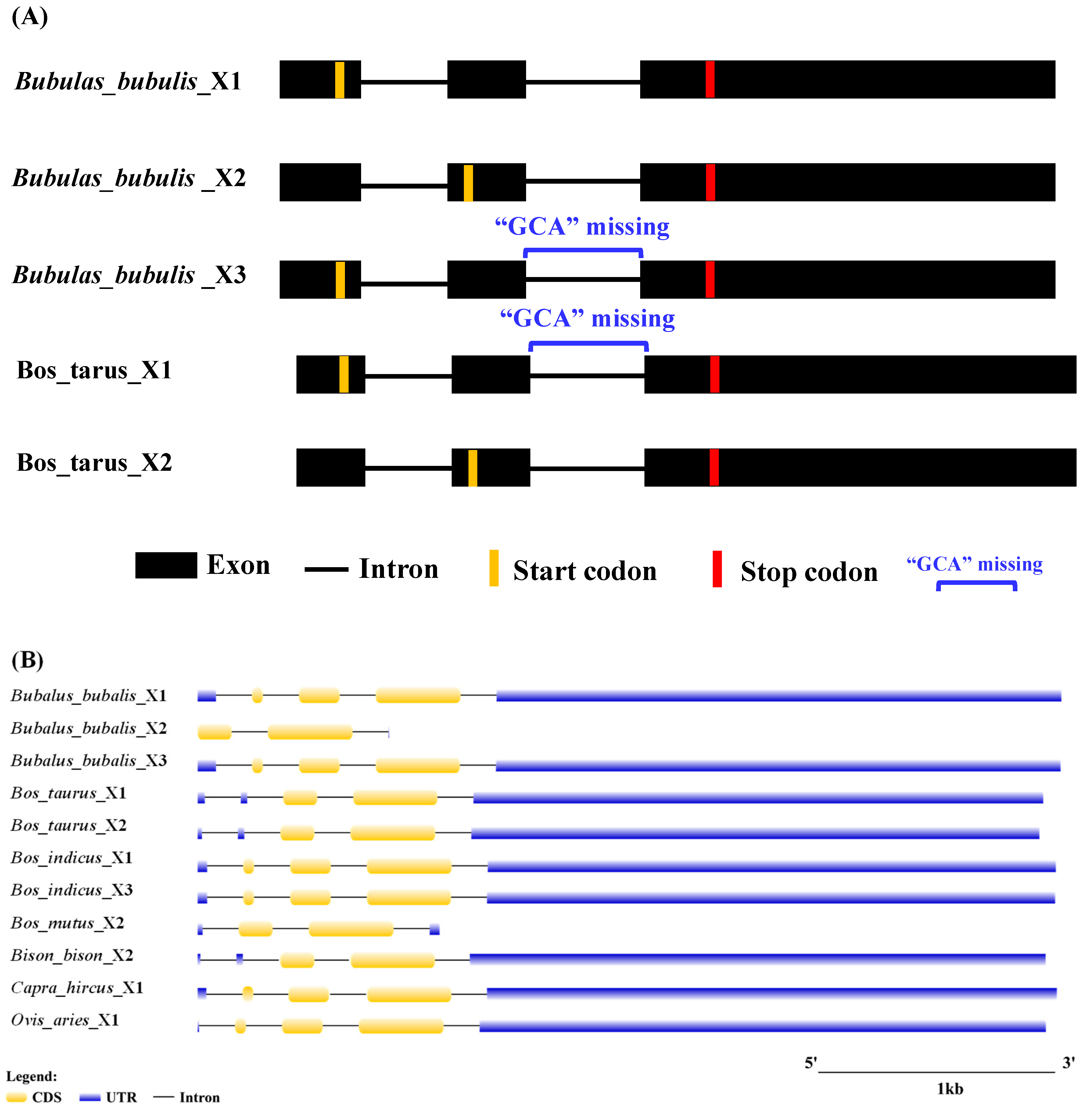
3.2. Transcriptional Region Structure of LEP
3.3. Identity of Amino Acid Sequences and Phylogenetic Tree of LEP
3.4. Structures and Physicochemical Characteristics of LEP Protein
3.5. Signal Peptide and Transmembrane Region
3.6. Biological Process, Molecular Function, and Functional Modification Site
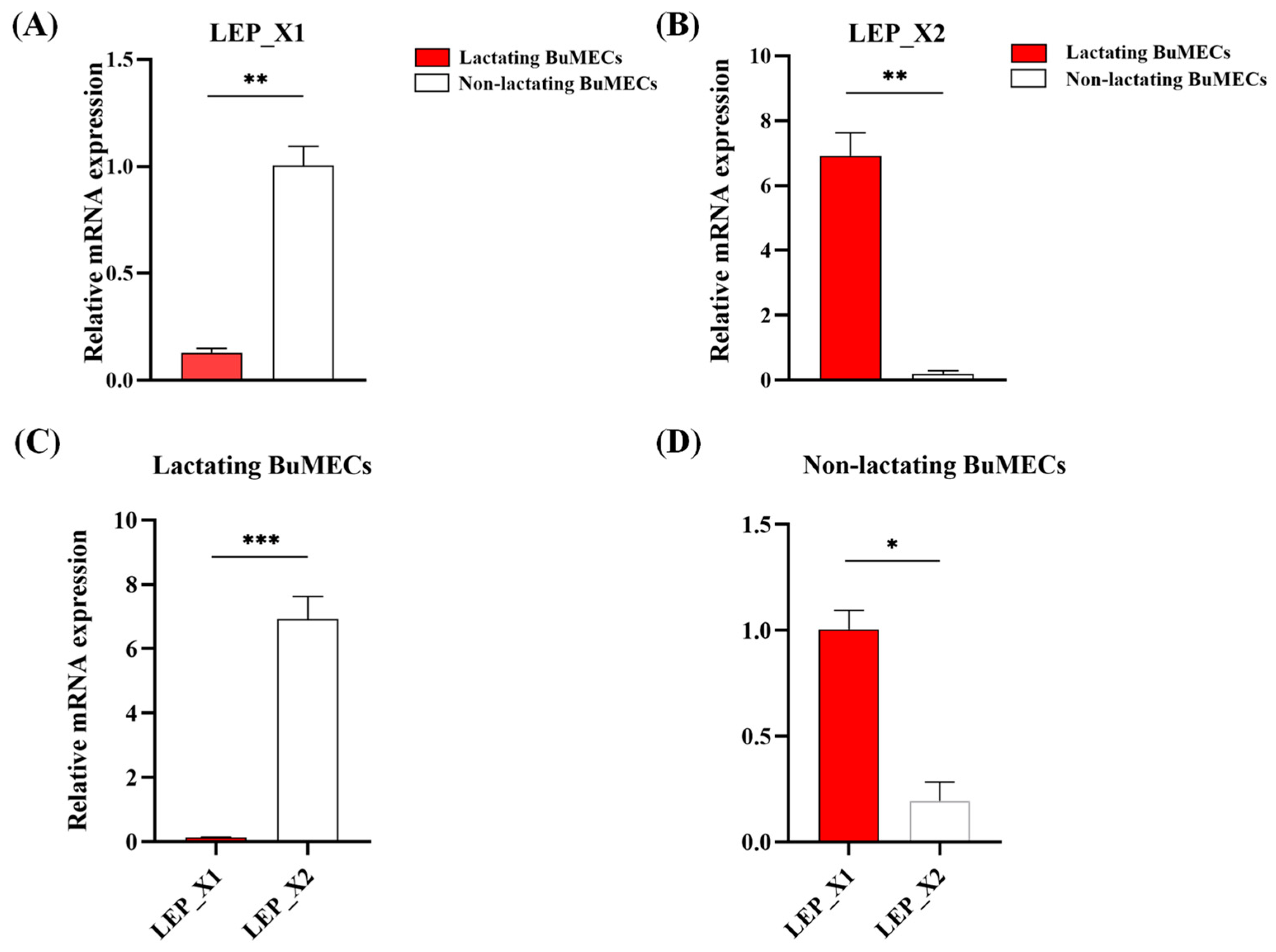
3.7. Differential Expression of LEP
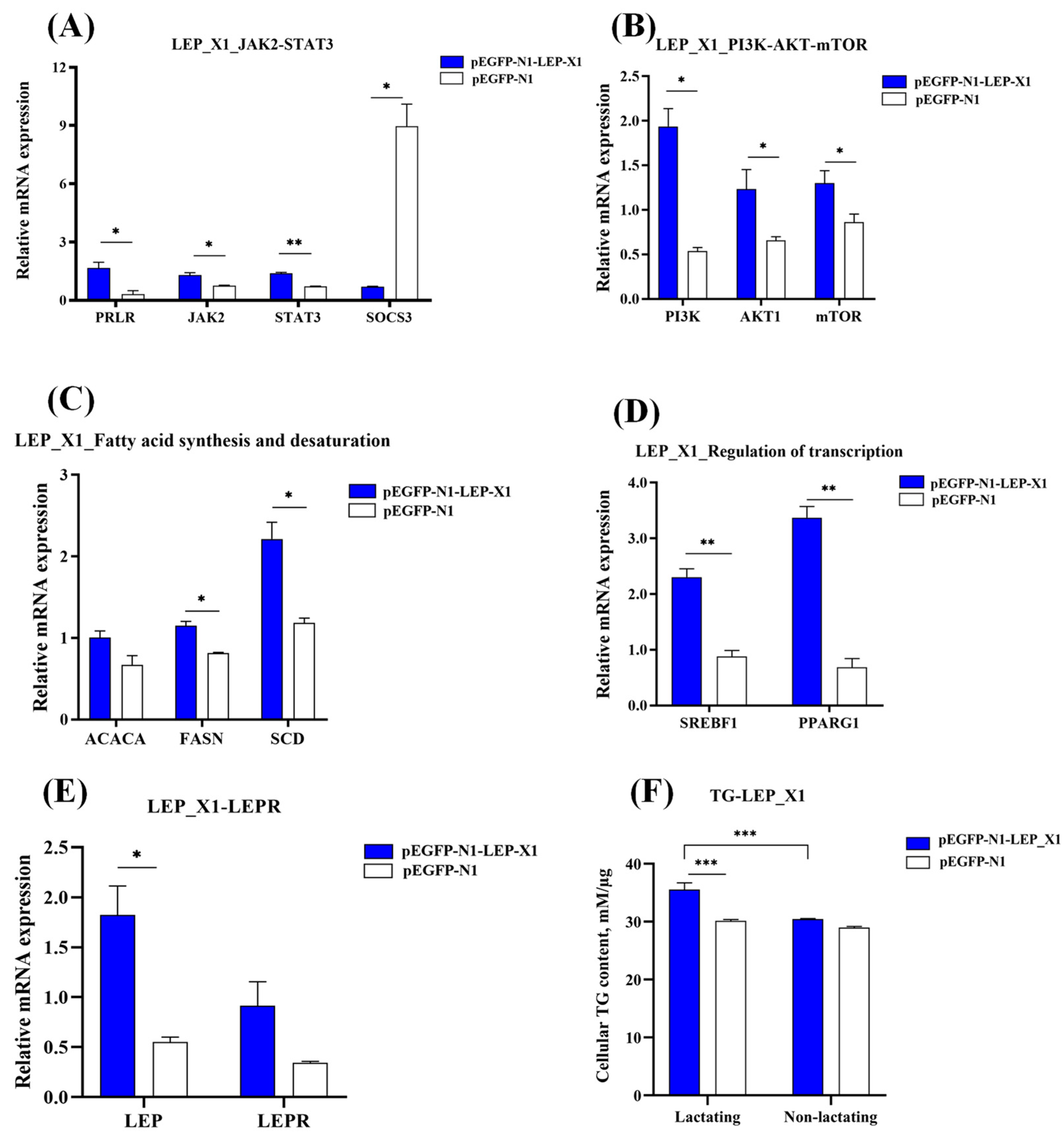
3.8. Overexpression of LEP_X1 Upregulates Genes Related to Milk Fat Synthesis in BuMECs and Increases TG Concentration
3.9. Overexpression of LEP_X2 Upregulates the Genes Related to Milk Fat Synthesis in BuMECs and Increases TG Concentration
3.10. Antagonism Interaction between LEP_X1 and LEP_X2
3.11. Population Variation Analysis
3.12. Sequence Differences Among Bovidae Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Ricardo, P.; Margherita, M.; Marisa, B.; Lori, L.; Jeffrey, M.F. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Delavaud, C.; Bonnet, M. Leptin expression in ruminants: Nutritional and physiological regulations in relation with energy metabolism. Domest. Anim. Endocrinol. 2005, 29, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, A.; Sláma, P.; Havlíček, Z.; Filipčík, R. Metabolic parameters concentrations in blood serum of Czech Pied bulls depending on single nucleotide polymorphism of leptin gene. J. Microbiol. Biotechnol. Food Sci. 2014, 2021, 145–147. [Google Scholar]
- Knyazev, S.; Safina, N.; Sharafutdinov, G.; Akhmetov, T.; Ravilov, R.; Vafin, F.; Loretts, O.; Kukhar, V.; Panfilova, O.; Tsoy, M. Association of LEP gene polymorphism with biochemical parameters of lipid metabolism and milk productivity of Holstein cattle. E3S Web Conf. 2021, 254, 01007. [Google Scholar] [CrossRef]
- Bonnet, M.; Gourdou, I.; Leroux, C.; Chilliard, Y.; Djiane, J. Leptin expression in the ovine mammary gland: Putative sequential involvement of adipose epithelial and myoepithelial cells during pregnancy and lactation. J. Anim. Sci. 2002, 80, 723–728. [Google Scholar] [CrossRef]
- Tanpure, T.; Dubey, P.K.; Singh, K.P.; Kathiravan, P.; Mishra, B.P.; Niranjan, S.K.; Kataria, R.S. PCR-SSCP analysis of leptin gene and its association with milk production traits in river buffalo (Bubalus bubalis). Trop. Anim. Health Prod. 2012, 44, 1587–1592. [Google Scholar] [CrossRef]
- Komisarek, J.; Antkowiak, I. The relationship between leptin gene polymorphisms and reproductive traits in Jersey cows. Pol. J. Vet. Sci. 2007, 10, 193–197. [Google Scholar]
- Clempson, A.M.; Pollott, G.E.; Brickell, J.S.; Bourne, N.E.; Munce, N.; Wathes, D.C. Evidence that leptin genotype is associated with fertility, growth, and milk production in Holstein cows. J. Dairy Sci. 2011, 94, 3618–3628. [Google Scholar] [CrossRef]
- Mahrous, K.F.; Aboelenin, M.M.; Rashed, M.A.; Sallam, M.A.; Rushdi, H.E. Detection of polymorphism within leptin gene in Egyptian river buffalo and predict its effects on different molecular levels. J. Genet. Eng. Biotechnol. 2020, 18, 6–17. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef]
- Reidy, S.P.; Weber, J.-M. Leptin: An essential regulator of lipid metabolism. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 125, 285–298. [Google Scholar] [CrossRef] [PubMed]
- El Nahas, S.M.; Bibars, M.A.; Taha, D.A. Genetic characterization of Egyptian buffalo CSN3 gene. J. Genet. Eng. Biotechnol. 2013, 11, 123–127. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, W.; Yuan, Z.; Wang, X.; Huang, A. Anti-inflammatory effect of two novel peptides derived from Binglangjiang buffalo whey protein in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Chem. 2023, 429, 136804. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Li, D.; Miao, Y. A survey on the genetic resource of Binlangjiang water buffalo. J. Yunnan Agric. Univ. 2008, 23, 265–269. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, Y.; Teng, X.; Miao, Y. Identification, molecular characteristics, and tissue differential expression of DGAT2 full-CDS cDNA sequence in Binglangjiang buffalo (Bubalus bubalis). Arch. Anim. Breed. 2020, 63, 81–90. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, S.; Leng, J.; Xu, S.; Tang, S.; Liu, C.; Gao, Y.; Mao, H. Impacts of shade on physiological and behavioural pattern of Dehong buffalo calves under high temperature. Appl. Anim. Behav. Sci. 2016, 177, 1–5. [Google Scholar] [CrossRef]
- Wani, N.A.; Wani, G.M.; Khan, M.Z.; Salahudin, S. Effect of oocyte harvesting techniques on in vitro maturation and in vitro fertilization in sheep. Small Rumin. Res. 2000, 36, 63–67. [Google Scholar] [CrossRef]
- Lalitha, S. Primer Premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. In Bioinformatics Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000; pp. 71–91. [Google Scholar] [CrossRef]
- Peden, J.F. Analysis of Codon Usage. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2000. [Google Scholar]
- Walker, J.M. The Proteomics Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Nielsen, H. Predicting Secretory Proteins with SignalP. In Protein Function Prediction: Methods and Protocols; Kihara, D., Ed.; Springer: New York, NY, USA, 2017; pp. 59–73. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, Y. Predicting the topology of transmembrane helical proteins using mean burial propensity and a hidden-Markov-model-based method. Protein Sci. 2009, 12, 1547–1555. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Clewley, J.P.; Arnold, C. MEGALIGN. In Sequence Data Analysis Guidebook; Swindell, S.R., Ed.; Springer: Totowa, NJ, USA, 1997; pp. 119–129. [Google Scholar] [CrossRef]
- Mulder, N.; Apweiler, R. InterPro and InterProScan. In Comparative Genomics; Bergman, N.H., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 59–70. [Google Scholar] [CrossRef]
- von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2004, 33, D433–D437. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Fan, X.; Qiu, L.; Teng, X.; Zhang, Y.; Miao, Y. Effect of INSIG1 on the milk fat synthesis of buffalo mammary epithelial cells. J. Dairy Res. 2020, 87, 349–355. [Google Scholar] [CrossRef]
- Zhou, F.; Fan, X.; Miao, Y. LPIN1 promotes triglycerides synthesis and is transcriptionally regulated by PPARG in buffalo mammary epithelial cells. Sci. Rep. 2022, 12, 2390. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, W.; Qiu, L.; Zhang, G.; Zhang, Y.; Miao, Y. Elongase of very long chain fatty acids 6 (ELOVL6) promotes lipid synthesis in buffalo mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. 2021, 106, 1–11. [Google Scholar] [CrossRef]
- Zhou, F.; Ouyang, Y.; Miao, Y. Peroxisome proliferator-activated receptor gamma regulates genes involved in milk fat synthesis in mammary epithelial cells of water buffalo. Anim. Sci. J. 2021, 92, e13537. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Vandesompele, J.; Shipley, G.L.; Pfaffl, M.W.; Nolan, T.; Mueller, R.; Kubista, M.; Huggett, J.; Hellemans, J.; Garson, J.A.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Di Pietro, F.; Ortenzi, F.; Tilio, M.; Concetti, F.; Napolioni, V. Genomic DNA extraction from whole blood stored from 15- to 30-years at −20 °C by rapid phenol–chloroform protocol: A useful tool for genetic epidemiology studies. Mol. Cell. Probes 2011, 25, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S. Composition and physico-chemical characteristics of buffalo milk with particular emphasis on lipids, proteins, minerals, enzymes and vitamins. J. Anim. Plant Sci. 2013, 23, 62–74. [Google Scholar]
- Feuermann, Y.; Mabjeesh, S.J.; Shamay, A. Leptin affects prolactin action on milk protein and fat synthesis in the bovine mammary gland. J. Dairy Sci. 2004, 87, 2941–2946. [Google Scholar] [CrossRef]
- Bartha, T.; Sayed-Ahmed, A.; Rudas, P. Expression of leptin and its receptors in various tissues of ruminants. Domest. Anim. Endocrinol. 2005, 29, 193–202. [Google Scholar] [CrossRef]
- Uversky, V.N. Natively unfolded proteins: A point where biology waits for physics. Protein Sci. 2009, 11, 739–756. [Google Scholar] [CrossRef]
- Invernizzi, G.; Thering, B.J.; McGuire, M.A.; Savoini, G.; Loor, J.J. Sustained upregulation of stearoyl-CoA desaturase in bovine mammary tissue with contrasting changes in milk fat synthesis and lipogenic gene networks caused by lipid supplements. Funct. Integr. Genom. 2010, 10, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.H. The Jak–STAT pathway: Specific signal transduction from the cell membrane to the nucleus. Eur. J. Clin. Investig. 1996, 26, 1–12. [Google Scholar] [CrossRef]
- Richard, A.J.; Stephens, J.M. The role of JAK-STAT signaling in adipose tissue function. Biochim. Biophys. Acta 2014, 1842, 431–439. [Google Scholar] [CrossRef]
- Wunderlich, C.M.; Hovelmeyer, N.; Wunderlich, F.T. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAK-STAT 2013, 2, e23878. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Xiao, J.; Ma, Y.; Ma, J.; Gao, J.; Cao, Z. Role of the JAK-STAT Pathway in Bovine Mastitis and Milk Production. Animals 2020, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef]
- Fazolini, N.P.; Cruz, A.L.; Werneck, M.B.; Viola, J.P.; Maya-Monteiro, C.M.; Bozza, P.T. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle 2015, 14, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. An Emerging Role of mTOR in Lipid Biosynthesis. Curr. Biol. 2009, 19, R1046–R1052. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, B.; Hausman, D.B.; Harris, R.B.S. Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R1557–R1564. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.P. Molecular characterization and novel genetic variability in leptin (obese) gene of mithun (Bos frontalis). Afr. J. Biotechnol. 2012, 11, 9315–9322. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.; Han, N.; Shou, J.; Hou, R.; Zhang, Z.; Bian, H.; Zhu, M. Characterization and Expression of Ailuropoda melanoleuca Leptin (obgene). Zool. Sci. 2010, 27, 41–46. [Google Scholar] [CrossRef]
- Bartek, J.; Bartos, J.; Galuska, J.; Galuskova, D.; Stejskal, D.; Ivo, K.; Ehrmann, J.; Ehrman Jr, J.; Chlup, R. Expression of ob gene coding the production of the hormone leptin in hepatocytes of liver with steatosis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2001, 145, 15–20. [Google Scholar] [CrossRef]
- Roy, S.; Kornberg, T.B. Paracrine signaling mediated at cell–cell contacts. BioEssays 2014, 37, 25–33. [Google Scholar] [CrossRef]
- Zabeau, L.; Defeau, D.; Van der Heyden, J.; Iserentant, H.; Vandekerckhove, J.; Tavernier, J. Functional Analysis of Leptin Receptor Activation Using a Janus Kinase/Signal Transducer and Activator of Transcription Complementation Assay. Mol. Endocrinol. 2004, 18, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Gao, X. Expression and function of leptin and its receptor in dairy goat mammary gland. J. Dairy Res. 2010, 77, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, F.C.; Kessel, A.G.V.; Waldner, C.; Christensen, D.A.; Laarveld, B.; Schmutz, S.M. Hot Topic: An Association Between a Leptin Single Nucleotide Polymorphism and Milk and Protein Yield. J. Dairy Sci. 2003, 86, 3164–3166. [Google Scholar] [CrossRef] [PubMed]






| Population | SNP | Genotype | Genotype Frequency (%) | Allele | Allele Frequency (%) | p-Value 1 |
|---|---|---|---|---|---|---|
| River buffalo | c.148C>T | CC | 1.0000 | C T | 1.0000 0.0000 | − |
| CT | 0.0000 | |||||
| TT | 0.0000 | |||||
| c.435G>A | AA | 0.0417 | A G | 0.1667 0.8333 | 0.53751 | |
| AG | 0.2500 | |||||
| GG | 0.7083 | |||||
| c.450G>A | AA | 0.0417 | A G | 0.2500 0.7500 | 0.65497 | |
| AG | 0.4167 | |||||
| GG | 0.5416 | |||||
| c.558G>A | AA | 0.0417 | A G | 0.1042 0.8958 | 0.06186 | |
| AG | 0.1250 | |||||
| GG | 0.8333 | |||||
| Swamp buffalo | c.148C>T | CC | 0.9756 | C T | 0.9878 0.0122 | 1.00000 |
| CT | 0.0244 | |||||
| TT | 0.0000 | |||||
| c.435G>A | AA | 0.4634 | A G | 0.5610 0.4390 | 0.00008 | |
| AG | 0.1951 | |||||
| GG | 0.3415 | |||||
| c.450G>A | AA | 0.0732 | A G | 0.1707 0.8293 | 0.03474 | |
| AG | 0.1951 | |||||
| GG | 0.7317 | |||||
| c.558G>A | AA | 0.0976 | A G | 0.2195 0.7805 | 0.05150 | |
| AG | 0.2439 | |||||
| GG | 0.6585 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Zhu, Q.; Huang, L.; Fan, X.; Teng, X.; Miao, Y. LEP Gene Promotes Milk Fat Synthesis via the JAK2-STAT3 and mTOR Signaling Pathways in Buffalo Mammary Epithelial Cells. Animals 2024, 14, 2446. https://doi.org/10.3390/ani14162446
Gao R, Zhu Q, Huang L, Fan X, Teng X, Miao Y. LEP Gene Promotes Milk Fat Synthesis via the JAK2-STAT3 and mTOR Signaling Pathways in Buffalo Mammary Epithelial Cells. Animals. 2024; 14(16):2446. https://doi.org/10.3390/ani14162446
Chicago/Turabian StyleGao, Ruixia, Qunyao Zhu, Lige Huang, Xinyang Fan, Xiaohong Teng, and Yongwang Miao. 2024. "LEP Gene Promotes Milk Fat Synthesis via the JAK2-STAT3 and mTOR Signaling Pathways in Buffalo Mammary Epithelial Cells" Animals 14, no. 16: 2446. https://doi.org/10.3390/ani14162446




