The Regulatory Role of Myomaker in the Muscle Growth of the Chinese Perch (Siniperca chuatsi)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Design and Preparation of siRNAs
2.3. Tissue Sampling and Myomaker-siRNA Inhibition Assay
2.4. Bioinformatics Analysis of Myomaker
2.5. cDNA Synthesis and Quantitative Real-Time PCR Analysis
2.6. Histological Section and Immunofluorescence Analysis
2.7. Isolation and DAPI Staining of Single Muscle Fibers of Chinese Perch
2.8. Statistical Analysis
3. Results
3.1. Amino Acid Sequence Comparison and Homology Analysis of the Myomaker Protein
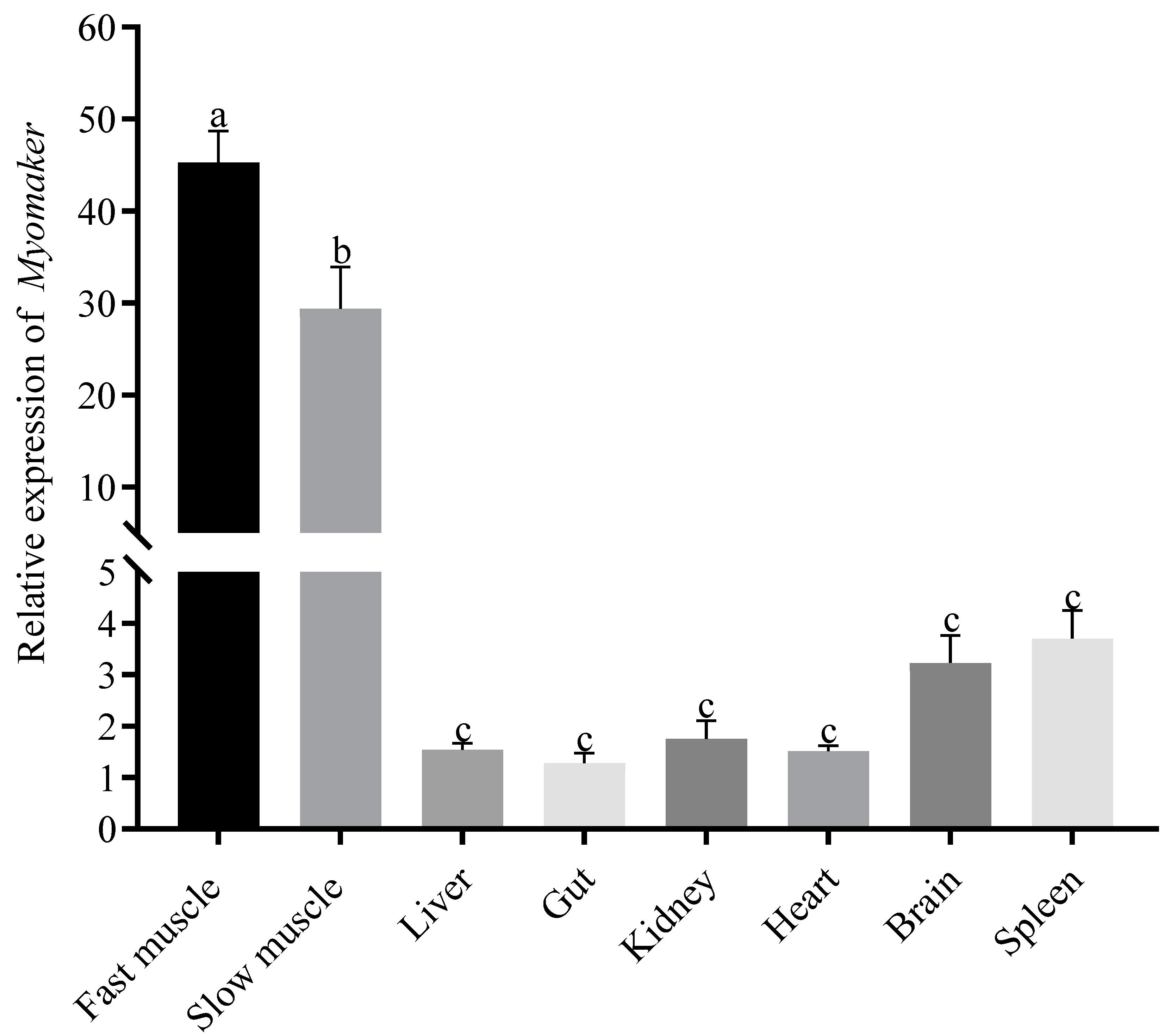
3.2. Tissue Specific Expression of Myomaker
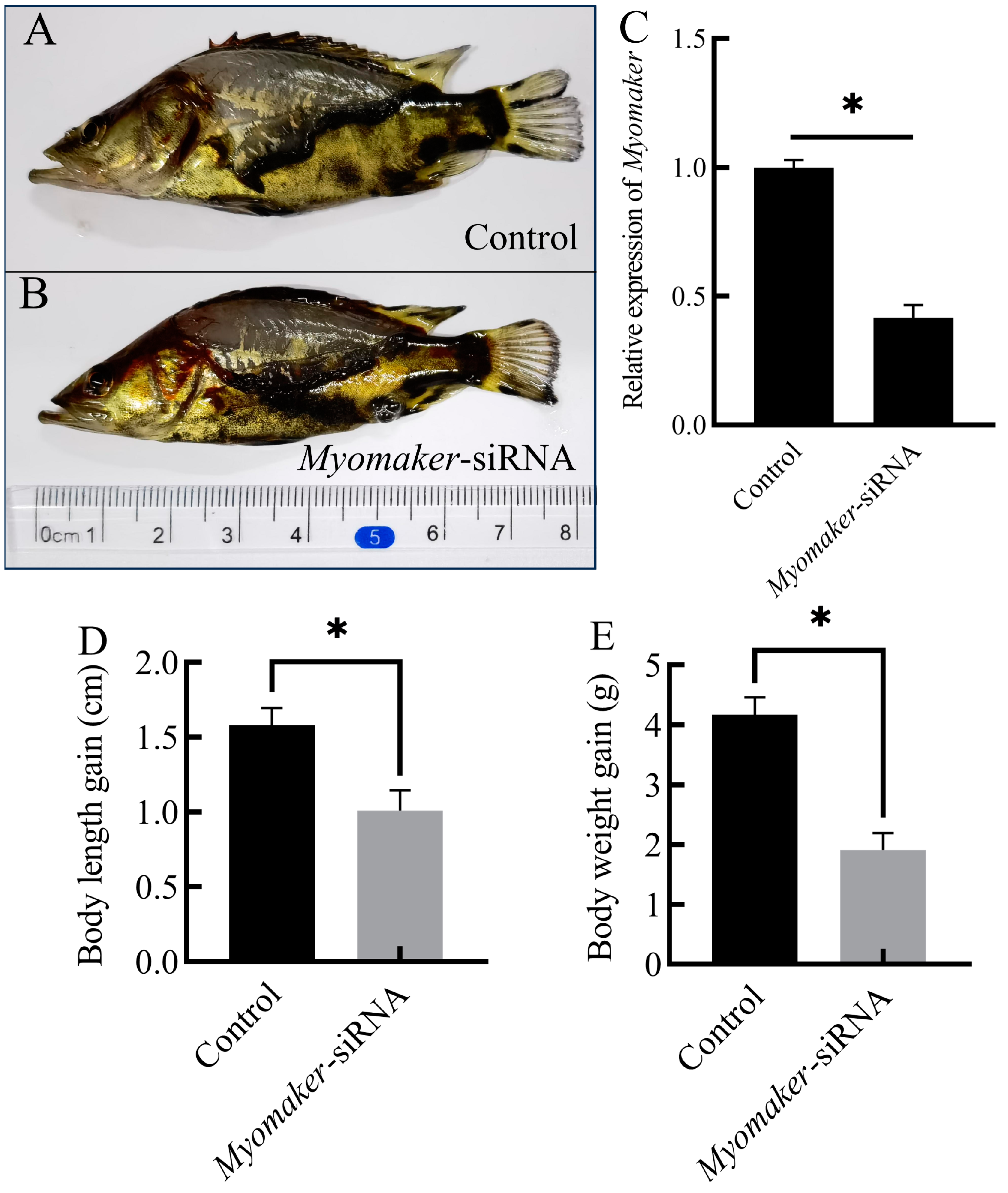
3.3. Interference with Myomaker Inhibits Growth of Chinese Perch Juvenile
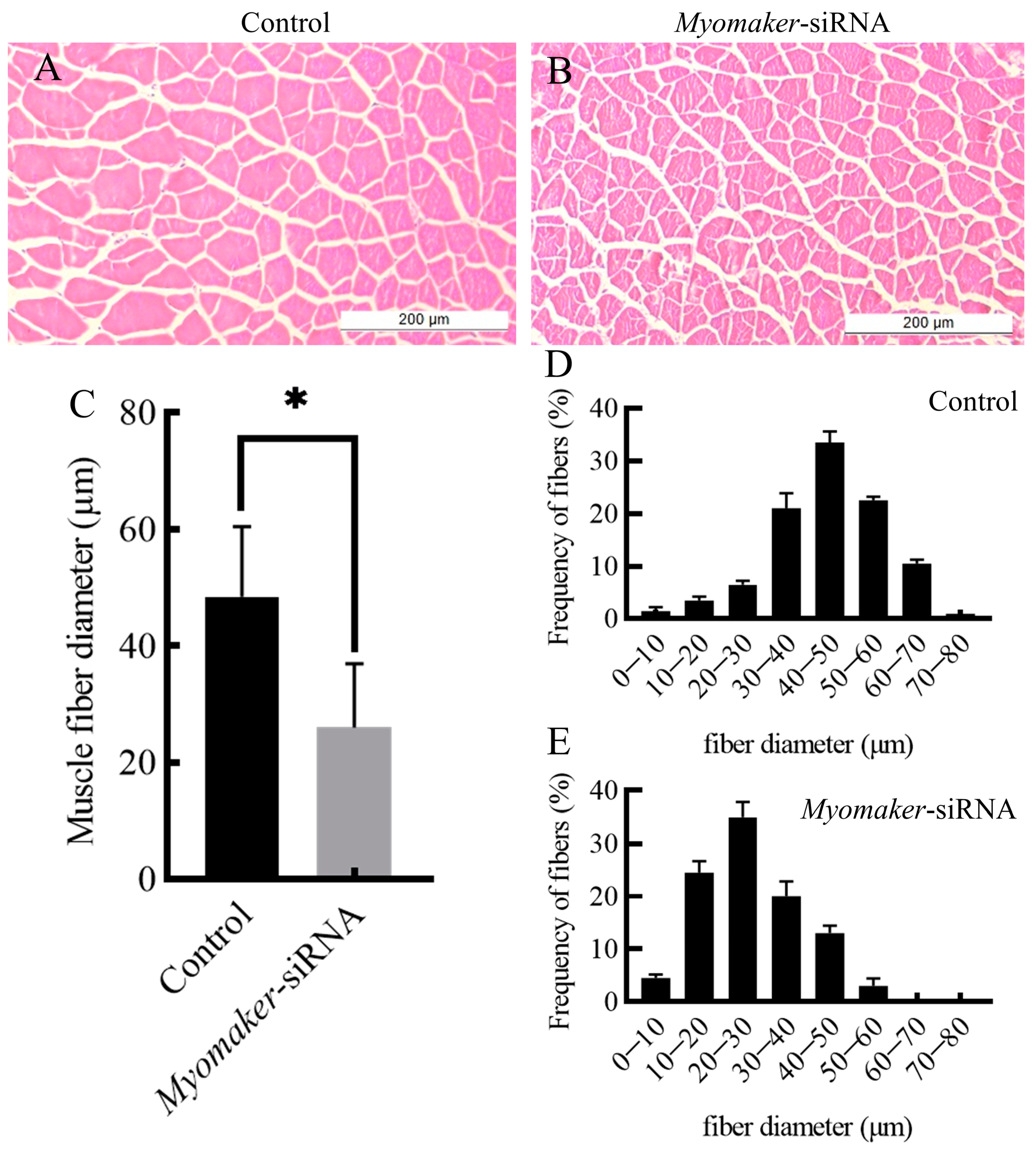
3.4. Histological Section Analysis
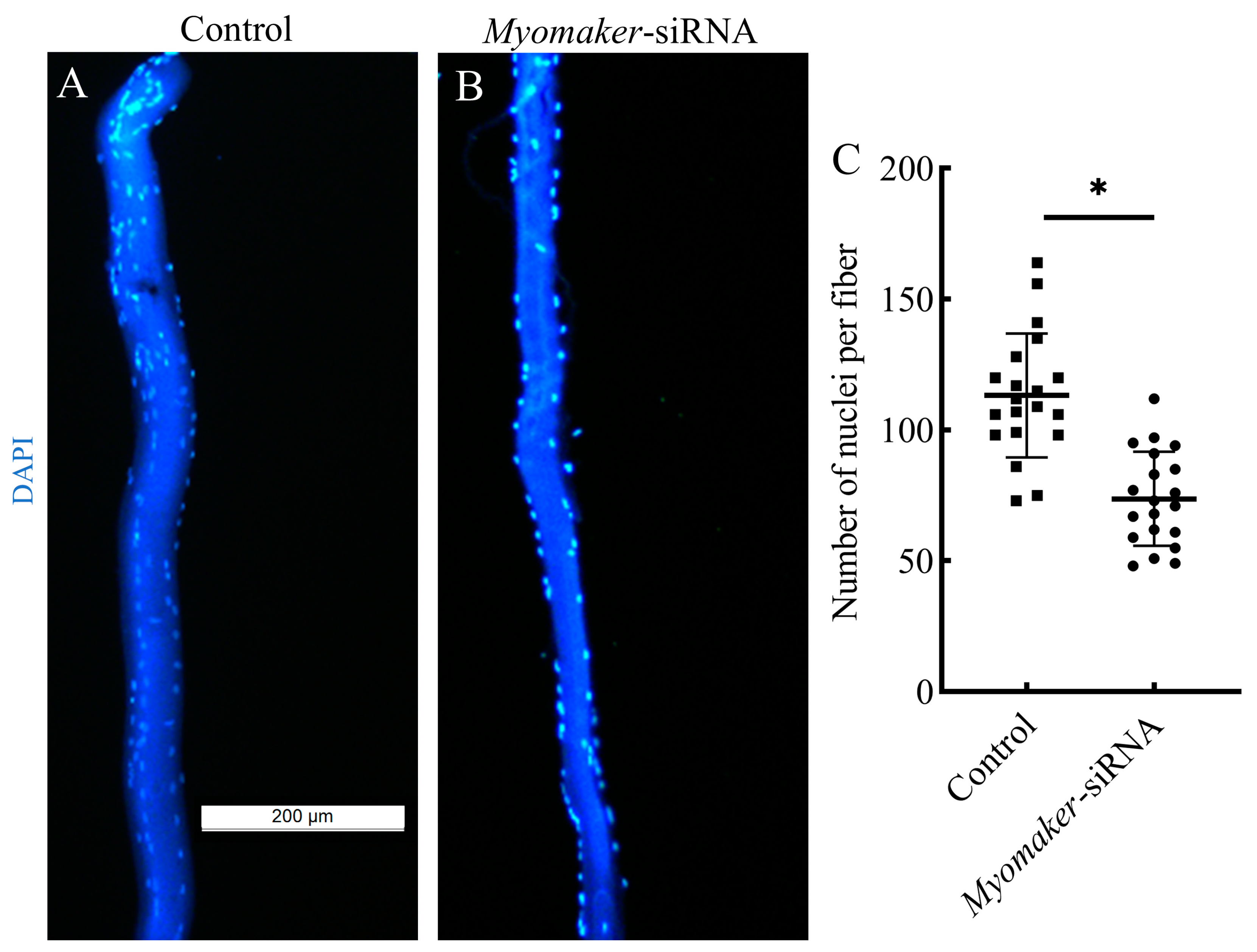
3.5. The Nuclei Number Analysis of Single Muscle Fibers
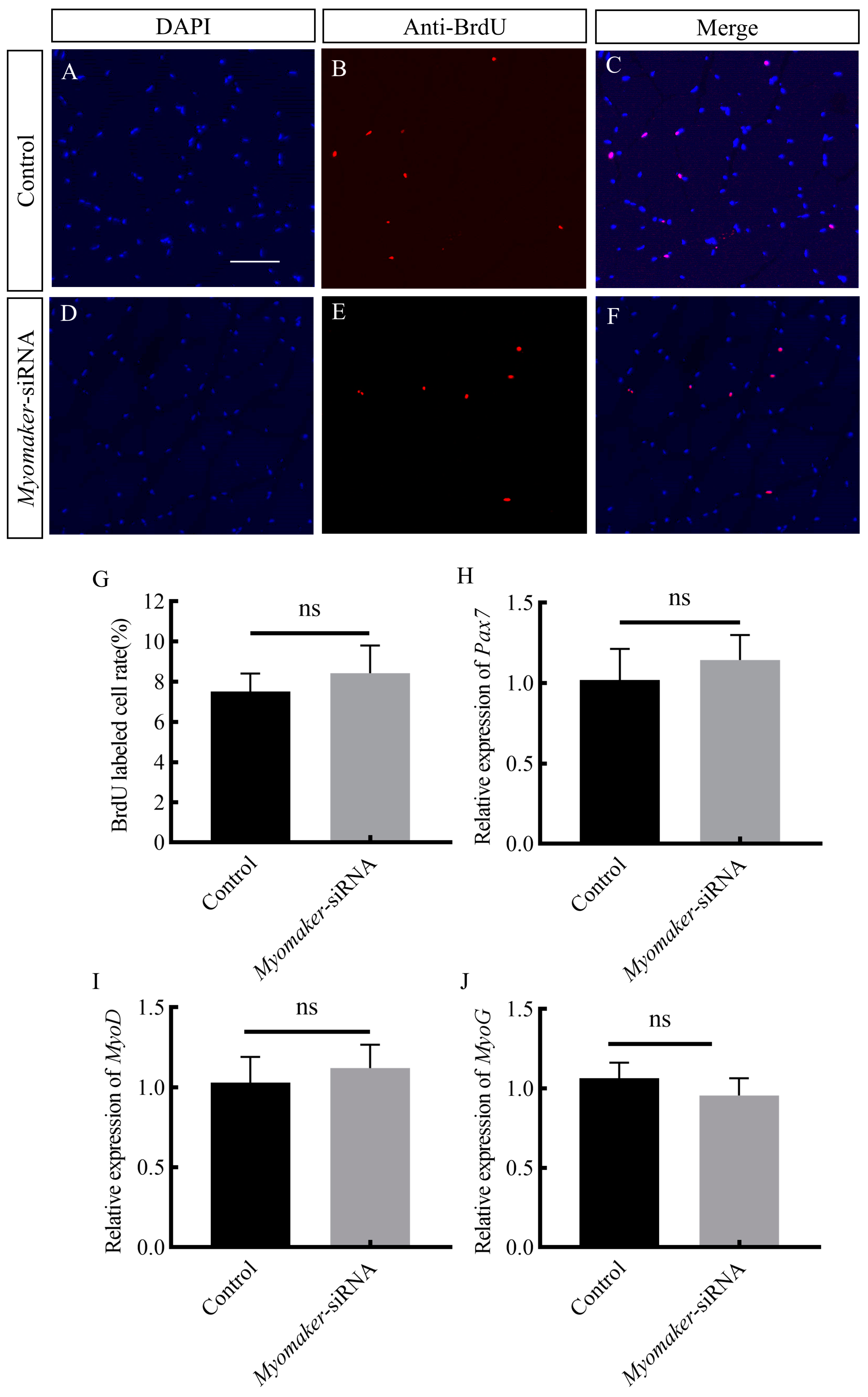
3.6. Effect of Inhibiting Myomaker on Myoblast Proliferation and Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weatherley, A.H.; Gill, H.; Casselman, J.M. The Biology of Fish Growth; Academic Press: London, UK, 1987. [Google Scholar]
- Chen, L.; Pan, Y.X.; Cheng, J.; Zhu, X.; Chu, W.Y.; Meng, Y.Y.; Bin, S.Y.; Zhang, J.S. Characterization of myosin heavy chain (MYH) genes and their differential expression in white and red muscles of Chinese perch, Siniperca chuatsi. Int. J. Biol. Macromol. 2023, 250, 125907. [Google Scholar] [CrossRef]
- Du, S.J. Molecular Regulation of Fish Muscle Development and Growth; World Scientific Publications: Singapore, 2004; pp. 339–391. [Google Scholar]
- Rossi, G.; Messina, G. Comparative myogenesis in teleosts and mammals. Cell. Mol. Life Sci. 2014, 71, 3081–3099. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, A.; Ruohonen, K.; Bjørnevik, M. Muscle fibre growth and quality in fish. Arch. Anim. Breed. 2006, 49, 137–146. [Google Scholar]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J. Neuromuscul. Dis. 2021, 8, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Perez, É.S.; Duran, B.O.S.; Zanella, B.T.T.; Dal-Pai-Silva, M. Understanding fish muscle biology in the indeterminate growth species pacu (Piaractus mesopotamicus). Comp. Biochem. Phys. A 2023, 285, 111503. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Lutfi, E.; Azizi, S.; Perelló, M.; Salmerón, C.; Riera-Codina, M.; Ibarz, A.; Fernández-Borràs, J.; Blasco, J.; Capilla, E.; et al. Understanding fish muscle growth regulation to optimize aquaculture production. Aquaculture 2017, 467, 28–40. [Google Scholar] [CrossRef]
- Johnston, I.A. Muscle development and growth: Potential implications for flesh quality in fish. Aquaculture 1999, 177, 99–115. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.W.; Qi, J.; Li, X.W.; Chen, L.; Li, M.Z.; Ma, J.D. Molecular regulation mechanism of Myomaker and Myomerger in myoblast fusion. Hereditas 2019, 41, 1110–1118. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, P.; Duan, R.; Chen, E.H. Mechanisms of myoblast fusion during muscle development. Curr. Opin. Genet. Dev. 2015, 32, 162–170. [Google Scholar] [CrossRef]
- Powell, G.T.; Wright, G.J. Jamb and Jamc Are Essential for Vertebrate Myocyte Fusion. PLoS Biol. 2011, 9, e1001216. [Google Scholar] [CrossRef]
- Si, Y.; Wen, H.; Du, S. Genetic Mutations in jamb, jamc, and myomaker Revealed Different Roles on Myoblast Fusion and Muscle Growth. Mar. Biotechnol. 2019, 21, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Vasyutina, E.; Martarelli, B.; Brakebusch, C.; Wende, H. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 8935–8940. [Google Scholar] [CrossRef]
- Millay, D.P.; O’Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Leikina, E.; Gamage, D.G.; Prasad, V.; Goykhberg, J.; Crowe, M.; Diao, J.; Kozlov, M.M.; Chernomordik, L.V.; Millay, D.P. Myomaker and Myomerger Work Independently to Control Distinct Steps of Membrane Remodeling during Myoblast Fusion. Dev. Cell 2018, 46, 767–780. [Google Scholar] [CrossRef]
- Gu, S.; Wen, C.L.; Li, J.Y.; Liu, H.H.; Huang, Q.; Zheng, J.X.; Sun, C.J.; Yang, N. Temporal Expression of Myogenic Regulatory Genes in Different Chicken Breeds during Embryonic Development. Int. J. Mol. Sci. 2022, 23, 10115. [Google Scholar] [CrossRef]
- Luo, W.; Li, E.; Nie, Q.; Zhang, X. Myomaker, Regulated by MYOD, MYOG and miR-140-3p, Promotes Chicken Myoblast Fusion. Int. J. Mol. Sci. 2015, 16, 26186–26201. [Google Scholar] [CrossRef]
- Shi, J.; Cai, M.X.; Si, Y.F.; Zhang, J.S.; Du, S.J. Knockout of myomaker results in defective myoblast fusion, reduced muscle growth and increased adipocyte infiltration in zebrafish skeletal muscle. Mol. Genet. Metab. 2018, 27, 3542–3554. [Google Scholar] [CrossRef] [PubMed]
- Biga, P.R.; Goetz, F.W. Zebrafish and giant danio as models for muscle growth: Determinate versus indeterminate growth as determined by morphometric analysis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1327–R1337. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Iturra, P.; Neira, R.; Araneda, C. Zebrafish as a model organism for nutrition and growth: Towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev. Fish Biol. Fisher. 2011, 21, 649–666. [Google Scholar] [CrossRef]
- Bi, S.; Lai, H.; Wang, G.P.; Guo, D.L.; Liu, S.; Chen, X.L.; Zhao, X.P.; Liu, X.G.; Li, G.F. Triploidy induction by hydrostatic pressure shock in mandarin fish (Siniperca chuatsi). Aquaculture 2020, 520, 734979. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Yi, H.; Su, Y.Q.; Huang, C.L.; Wei, X.C.; Chen, Q.X.; Chen, J.H.; Li, H.Y.; Bi, S.; Lai, H.; et al. Hydrostatic pressure shock induced diploid/tetraploid mosaic in mandarin fish (Siniperca chuatsi), with the observation of embryo development and change in body spots. Aquaculture 2023, 563, 738989. [Google Scholar] [CrossRef]
- Khan, T.; Weber, H.; DiMuzio, J.; Matter, A.; Dogdas, B.; Shah, T.; Thankappan, A.; Disa, J.; Jadhav, V.; Lubbers, L.; et al. Silencing Myostatin Using Cholesterol-conjugated siRNAs Induces Muscle Growth. Mol. Ther. Nucleic Acids 2016, 5, e342. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.L.; Tan, K.; Shi, L.L.; Sun, R.; Wang, W.M.; Li, Y.H. Comparison of effects of dsRNA and siRNA RNA interference on insulin-like androgenic gland gene (IAG) in red swamp crayfish Procambarus clarkii. Gene 2020, 752, 144783. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ren, L.; Liu, J.J.; Chen, L.; Cheng, J.; Chu, W.Y.; Zhang, J.S. Transcriptome analysis provides novel insights into the function of PI3K/AKT pathway in maintaining metabolic homeostasis of Chinese perch muscle. Aquacult. Rep. 2021, 21, 100838. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, E.H.; Olson, E.N. Unveiling the mechanisms of cell-cell fusion. Science 2005, 308, 369–373. [Google Scholar] [CrossRef]
- Landemaine, A.; Ramirez Martinez, A.; Monestier, O.; Sabin, N.; Rescan, P.Y.; Olson, E.N.; Gabillard, J.C. Trout myomaker contains 14 minisatellites and two sequence extensions but retains fusogenic function. J. Biol. Chem. 2019, 294, 6364–6374. [Google Scholar] [CrossRef]
- Jaberi-Lashkari, N.; Lee, B.; Calo, E. A unified view of low complexity regions (LCRs) across species. eLife 2022, 11, e77058. [Google Scholar] [CrossRef]
- Millay, D.P.; Gamage, D.G.; Quinn, M.E.; Min, Y.L.; Mitani, Y.; Bassel-Duby, R.; Olson, E.N. Structure–function analysis of myomaker domains required for myoblast fusion. Proc. Natl. Acad. Sci. USA 2016, 113, 2116–2121. [Google Scholar] [CrossRef]
- Von Heijne, G. Life and death of a signal peptide. Nature 1998, 396, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.D.; Zhu, Y.; Zhou, X.K.; Zhang, J.; Sun, J.; Li, Z.J.; Jin, L.; Long, K.; Lu, L.; Ge, L.P. miR-205 Regulates the Fusion of Porcine Myoblast by Targeting the Myomaker Gene. Cells 2023, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.C.; He, P.Y.; Liu, B.S.; Guo, H.Y.; Zhang, N.; Guo, L.; Jiang, S.G.; Zhang, D.C. Identification of Myomaker in Yellowfin Seabream (Acanthopagrus latus) (Hottuyn, 1782) and its Transcriptional Regulation by Two MyoDs. Pak. J. Zool. 2020, 53, 227. [Google Scholar] [CrossRef]
- Zhang, W.B.; Roy, S. Myomaker is required for the fusion of fast-twitch myocytes in the zebrafish embryo. Dev. Biol. 2017, 423, 24–33. [Google Scholar] [CrossRef]
- Hromowyk, K.J.; Talbot, J.C.; Martin, B.L.; Janssen, P.M.L.; Amacher, S.L. Cell fusion is differentially regulated in zebrafish post-embryonic slow and fast muscle. Dev. Biol. 2020, 462, 85–100. [Google Scholar] [CrossRef]
- Landemaine, A.; Rescan, P.Y.; Gabillard, J.C. Myomaker mediates fusion of fast myocytes in zebrafish embryos. Biochem. Bioph. Res. Commun. 2014, 451, 480–484. [Google Scholar] [CrossRef]
- Chen, B.; You, W.J.; Wang, Y.Z.; Shan, T. The regulatory role of Myomaker and Myomixer-Myomerger-Minion in muscle development and regeneration. Cell. Mol. Life Sci. 2020, 77, 1551–1569. [Google Scholar] [CrossRef]
- He, J.; Wang, F.; Zhang, P.; Li, W.J.; Wang, J.; Li, J.L.; Liu, H.J.; Chen, X.P. miR-491 inhibits skeletal muscle differentiation through targeting myomaker. Arch. Biochem. Biophys. 2017, 625, 30–38. [Google Scholar] [CrossRef]

| Genes | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Pax7 | AGCCACAACATGACTTCTCC | GTCCACCGTCTTAATGGAGG |
| MyoD | CAACGACGCCTTTGAGACCCTG | GTCCGAATCCCGCTGTAGTGT |
| MyoG | CGAGACCAACCCTTACTTCTTCCCT | GACTCCCACACAAGCCCATCAT |
| Myomaker | AGTGTTTACGGCACGGCTC | CGTGGTCAACACTCCAAACAT |
| Rpl13 | CACAAGAAGGAGAAGGCTCGGGT | TTTGGCTCTCTTGGCACGGAT |
| Items | Control (Mean ± SEM) | Myomaker-siRNA (Mean ± SEM) |
|---|---|---|
| Initial weight (g) | 2.31 ± 0.21 | 2.30 ± 0.24 |
| Final weight (g) | 6.40 ± 0.23 | 4.24 ± 0.28 |
| Initial length (cm) | 5.0 ± 0.28 | 4.9 ± 0.30 |
| Final length (cm) | 6.6 ± 0.28 | 5.9 ± 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Meng, Y.; Nie, L.; Cheng, C.; Gao, Z.; Liu, L.; Zhu, X.; Chu, W. The Regulatory Role of Myomaker in the Muscle Growth of the Chinese Perch (Siniperca chuatsi). Animals 2024, 14, 2448. https://doi.org/10.3390/ani14172448
Zeng W, Meng Y, Nie L, Cheng C, Gao Z, Liu L, Zhu X, Chu W. The Regulatory Role of Myomaker in the Muscle Growth of the Chinese Perch (Siniperca chuatsi). Animals. 2024; 14(17):2448. https://doi.org/10.3390/ani14172448
Chicago/Turabian StyleZeng, Wei, Yangyang Meng, Lingtao Nie, Congyi Cheng, Zexia Gao, Lusha Liu, Xin Zhu, and Wuying Chu. 2024. "The Regulatory Role of Myomaker in the Muscle Growth of the Chinese Perch (Siniperca chuatsi)" Animals 14, no. 17: 2448. https://doi.org/10.3390/ani14172448




