Determining the Optimal Harvesting Moment of Green Forage from Guizotia abyssinica Cultivated as a Catch Crop on Silage and Its Quality Form, Fresh or Wilted Green Material, in the Two Following Years
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishing the Field Experiment
2.2. Chemical Analysis
2.3. Statistical Analysis
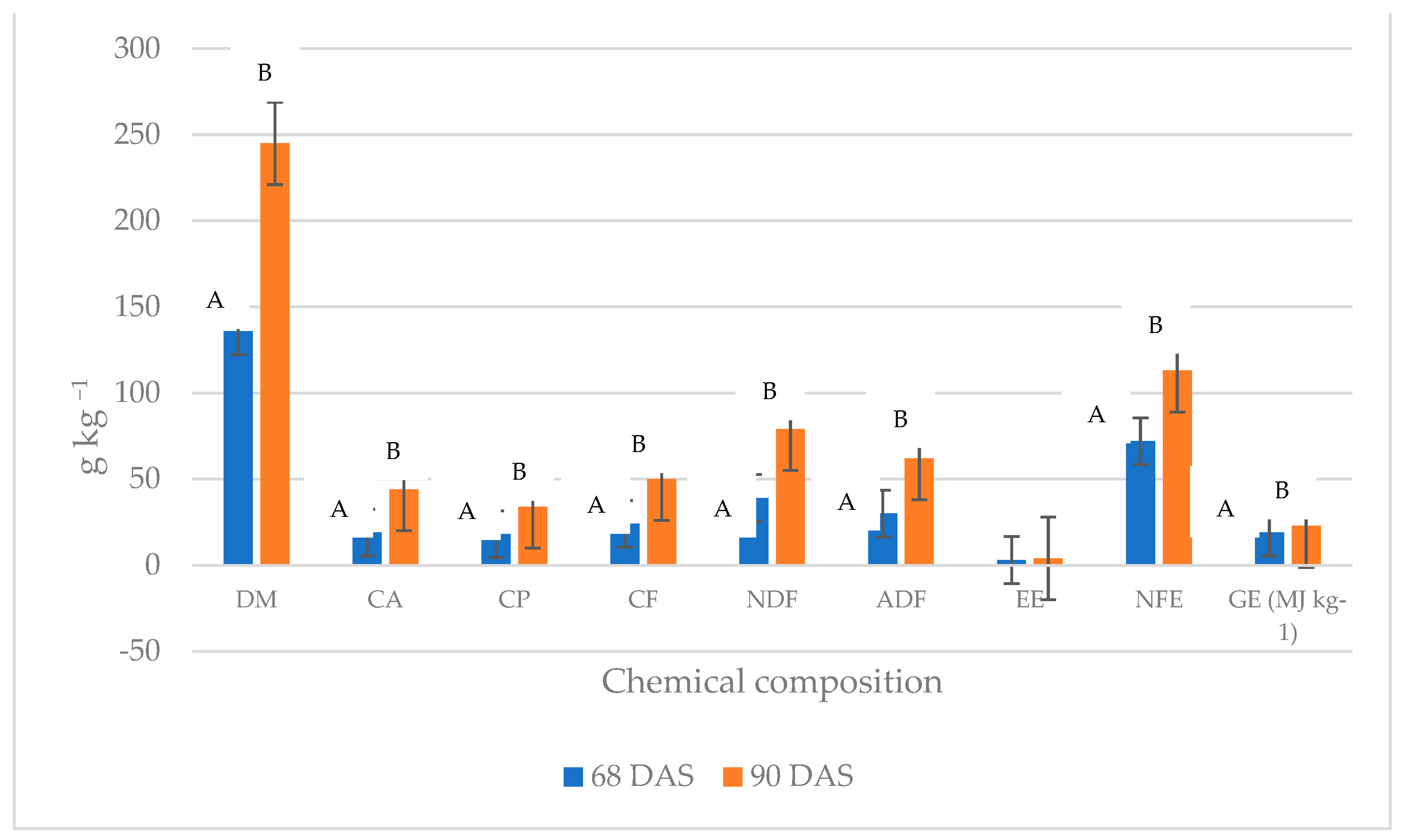
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- May, W.E.; Wood, M.D.; Del Piero, K. Niger response to nitrogen and seeding depth in the northern great plains. Agron. J. 2018, 111, 1–8. [Google Scholar] [CrossRef]
- Baagoe, J. The genus Guizotia (Compositae). A taxonomic revision. Bot. Tidsskr. 1974, 69, 1–39. [Google Scholar]
- Sandipan, P.B.; Jagtap, P.K. Honeybee—A natural pollinator in increasing the seed yield and income in the niger (Guizotia abyssinica Cass.) a traditional tribal crop of South Gujarat region. J. Plant Dev. Sci. 2015, 7, 499–502. Available online: https://jpds.co.in/wp-content/uploads/2019/07/05.-Prashant-Sandipan-766.pdf (accessed on 10 July 2024).
- Ramadan, M.F. Functional properties, nutritional value, and industrial applications of niger oilseeds (Guizotia abyssinica Cass.). Crit. Rev. Food Sci. Nutr. 2012, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K.; Saren, B.K. Productivity, water use efficiency and economics of rainfed niger (Guizotia abyssinica) as influenced by mulching and row spacing in red and lateric soil of West Bengal. India. Int. J. Bio Resour. Stress Manag. 2012, 3, 295–298. Available online: https://ojs.pphouse.org/index.php/IJBSM/article/view/275 (accessed on 10 July 2024).
- Getinet, A.; Sharma, S. Niger, promoting the conservation and use of underutilized and neglected crops. Int. Plant Genet. Resour. Inst. 1996, 51, 391. [Google Scholar]
- Weiss, E.A. Oilseed Crops. In Tropical Agriculture Series; Longman: London, UK, 1983. [Google Scholar]
- Geleta, M.; Ortiz, R. The importance of Guizotia abyssinica (niger) for sustainable food security in Ethiopia. Genet. Resour. Crop Evol. 2013, 60, 1763–1770. [Google Scholar] [CrossRef]
- Shahidi, F.; Desilva, C.; Amarowicz, R. Antioxidant activity of extracts of defatted seeds of niger (Guizotia abyssinica). J. Am. Oil Chem. Soc. 2003, 80, 443–450. [Google Scholar] [CrossRef]
- Gebeyehu, A.G.; Hammenhag, C.; Ortiz, R.; Tesfaye, K.; Geleta, M. Characterization of oilseed crop nough (Guizotia abyssinica) using agro-morphological traits. Agronomy 2021, 11, 1479. [Google Scholar] [CrossRef]
- Gogoi, B. Niger (Guizotia abyssinica L.)—A promising rabi season crop under rice-follow system in north-east India. Int. J. Agric. Sci. 2021, 12, 41–46. [Google Scholar]
- Shivarkar, H.S.; Kashid, P.S. Niger (Guizotia abyssinica): An overview. Int. J. Pharm. Chem. Biol. Sci. 2020, 10, 75–79. Available online: https://www.ijpcbs.com/articles/niger-guizotia-abyssinica-an-overview.pdf (accessed on 10 July 2024).
- Gordin, C.R.B.; de Paula Quintão Scalon, S. Temperatures and qualities of light in Niger (Guizotia abyssinica (L.f.) Cass.) seeds germination in Mato Grosso do Sul, Brazil. Acta Agron. 2017, 66, 403–407. [Google Scholar] [CrossRef]
- Stipešević, B.; Brozović, B.; Jug, D.; Jug, I.; Ranogajec, L.; Šego, D. Economic comparison of different cropping systems for niger (Guizotia abyssinica) in Croatia. In Proceedings of the TEAM 2014 6th International Scientific and Expert Conference of International TEAM Society, Kecskemét, Hungary, 10–11 November 2014. [Google Scholar]
- Sarin, R.; Sharma, M.; Khan, A.A. Studies on Guizotia abyssinica L. oil: Biodiesel synthesis and process optimization. Bioresour. Technol. 2009, 100, 4187–4192. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gai, F.; Tassone, S. Nutritional value and fatty acid profile of niger (Guizotia abyssinica) plant during its growth cycle. Livest. Res. Rural Dev. 2015, 27, 18. Available online: http://www.lrrd.org/lrrd27/1/peir27018.htm (accessed on 10 July 2024).
- Nega, A.; Melaku, S. Feed intake, digestibility and body weight change in Farta sheep fed hay supplemented with rice bran and/or noug seed (Guizotia abyssinica) meal. Trop. Anim. Health Prod. 2009, 41, 507–515. [Google Scholar] [CrossRef]
- Malik, T.A.; Mohini, M. Niger seed (Guizotia abyssinica) alters in vitro fermentation and reduces methane emission. Curr. Sci. 2021, 120, 509–513. Available online: https://www.currentscience.ac.in/show.issue.php?volume=120&issue=3 (accessed on 10 July 2024). [CrossRef]
- Sun, X.; Wang, Q.; Yang, Z.; Xie, T.; Wang, Z.; Li, S.; Wang, W. Altering methane emission, fatty acid composition, and microbial profil during in vitro ruminant fermentation by manipulating dietary fatty acid ratios. Fermentation 2022, 8, 310. [Google Scholar] [CrossRef]
- Namdari, M.; Abbasi, R.; Pirdashti, H.; Zaefarian, F. Effect of replacement ratios on plant traits and seed quality properties in intercropping of soybean (Glycine max L. Merr) and niger (Guizotia abyssinica Cass.). Iran. J. Crop Sci. 2022, 23, 373–389. Available online: https://agrobreedjournal.ir/browse.php?a_code=A-10-1296-1&sid=1&slc_lang=en (accessed on 10 July 2024).
- Szuba-Trznadel, A.; Hikawczuk, T.; Jama-Rodzeńska, A.; Król, Z.; Fuchs, B. The effect of harvest date on the chemical composition and fodder yield of Guizotia abyssinica (Guizotia abyssinica (L.f.) Cass.) under the climatic conditions of south-west Poland. Agriculture 2022, 12, 481. [Google Scholar] [CrossRef]
- Meena, M.K.; Dalei, B.B.; Predhan, K.; Senapati, N.; Phonglosa, A.; Ram, M.; Kunwar, R.; Kumari, V.; Gupta, D.; Meena, R.K. Identification and evaluation of productive mutants of niger (Guizotia abyssinica (L.f.) Cass.) for different morphological charact. Pharma. Innov. J. 2022, 11, 1388–1392. Available online: https://www.thepharmajournal.com/archives/2022/vol11issue6/PartS/11-6-170-613.pdf (accessed on 10 July 2024).
- Soil Classification: Regulation of the Council of Ministers of 12 September 2012 on the Soil Science Classification of Land: Dz.U. 2012 poz. 1246. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20120001246/O/D20121246.pdf (accessed on 6 June 2021). (In Polish)
- Saatbau Poland Company: Saatbau Poland Sp. z, o.o. Available online: https://www.saatbau.pl/asp/start,20,,1 (accessed on 10 July 2024). (In Polish).
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agric. 1996, 17, 264–268. [Google Scholar] [CrossRef]
- Deriaz, R.E. Routine analysis of carbohydrates and lignin in herbage. J. Sci. Food Agric. 1961, 12, 152–160. [Google Scholar] [CrossRef]
- Weissbach, F.; Honig, H. On the prediction and control of the fermentation process when ensiling green fodder from extensive cultivation. Landbauforsch. Völkenrode Heft 1996, 1, 10–17. (In German) [Google Scholar]
- AOAC. AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- PN-R-04013:1988; Polish Standard. Chemical Analysis of Plants—Dry Matter Determination. Polish Committee of Standardization: Warsaw, Poland, 1988. (In Polish)
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Skulmowski, J. Methods for Determining the Composition of Feeds and Their Quality; PWRiL: Warsaw, Poland, 1974. (In Polish) [Google Scholar]
- Zimmer, E. The new version of the fermentation feed key according to Flieg. Das Wirtsch. Futter 1966, 12, 299–303. (In German) [Google Scholar]
- Statistica. Data Analysis Software System, Version 13.3; Tibco Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Wang, M.; Geo, R.; Franco, M.; Hannaway, D.B.; Ke, W.; Ding, Z.; Yu, Z.; Guo, X. Effect of mixing alfalfa with whole plant corn in different proportions on fermentation characteristics and bacterial community of silage. Agriculture 2021, 11, 174. [Google Scholar] [CrossRef]
- Kaplan, M.; Kara, K.; Unlukara, A.; Kale, H.; Buyukkilic Beyiz, S.; Varol, I.S.; Kizilsimsek, M.; Kamalak, A. Water deficit and nitrogen affects yield and feed value of sorghum sudangrass silage. Agric. Water Manag. 2019, 218, 30–36. [Google Scholar] [CrossRef]
- Grant, R.J.; Ferraretto, L.F. Silage review: Silage feeding management: Silage characteristics and dairy cow feeding behaviour. J. Dairy Sci. 2018, 101, 4111–4121. [Google Scholar] [CrossRef]
- Kennedy, P.C.; Dawson, L.E.R.; Lively, F.O.; Steen, R.W.J.; Fearon, A.M.; Moss, B.W.; Kilpatrick, D.J. Effects of offering lupins/triticale, vetch/barley silages alone or in combination with grass silage on animal performance, meat quality and the fatty acid comp. J. Agric. Sci. 2018, 156, 1005–1016. [Google Scholar] [CrossRef]
- Badahir Koca, S.; Pazar, M.; Atar, B.; Yiğit, N.Ö. Investigation of the usability as raw materials an fish feed and cultivation ability of niger (Guizotia abyssinica) seed in our country. Act. Aqua. Tr. 2019, 15, 108–116. (In Turkish) [Google Scholar] [CrossRef]
- Bolsen, K.K.; Ashbell, G.; Weinber, Z.G. Silage fermentation in silage additives. Review. Asian Australian J. Animal Sci. 1996, 9, 483–489. [Google Scholar] [CrossRef]
- Ojeda, F.; Montejo, I.; Perez, G. Conservation of Mulberry as Silage. 1. Effect on Nitrogenous Compounds; FAO: Rome, Italy, 2002; Available online: https://www.fao.org/4/x9895E/x9895e0r.htm/x9895e0r.htm (accessed on 10 July 2024).
- Marsh, R. The effects of wilting on fermentation in the silo and on the nutritive value of silage. Grass Forage Sci. 1979, 34, 1–10. [Google Scholar] [CrossRef]
- Ojeda, F.; Martí, J.; Martínez, N.; Lajonchene, G. Mulberry flour: A tropical concentrate. In III International Silvopastoral Workshop “Trees and Shrubs in Livestock Farming” Memoirs; Indio Hatuey Matanzas Pasture and Forage Experimental Station: Matanzas municipality, Cuba, 1998; p. 202. (In Spanish) [Google Scholar]
- Lyimo, B.J.; Mtengeti, E.J.; Urio, N.A.; Ndemanisho, E.E. Effect of fodder grass species, wilting and ensiled amount in shopping plastic bags on silage quality. Livestock Res. Rural Dev. 2016, 28, 142. Available online: http://www.lrrd.org/lrrd28/8/lyim28142.html (accessed on 10 July 2024).
- Mtengeti, E.J.; Kavana, P.Y.; Urio, N.A.; Shem, M.N. Chemical composition and fermentative quality of fodder grasses ensiled with derinded fresh sugar cane crush. Trop. Subtrop. Agroecosystems 2006, 6, 157–165. [Google Scholar]
- Nussio, L.G. Silage production from tropical forages. In Silage Production and Utilization; Park, R.S., Stronge, M.D., Eds.; Wageningen Academic Publishers: Belfast, Northern Ireland, 2005; pp. 97–107. [Google Scholar]
- Bodarski, R.; Szyszkowska, A.; Sowiński, J. The effect of agrotechnological factors on the quality of maize-forage soybean intercrop silages. Zesz. Nauk. UP Wroc. Biol. Hod. Zwierz. 2013, 70, 9–20. Available online: http://zeszyty.bihz.up.wroc.pl/archivum/z70-2013.pdf (accessed on 10 July 2024). (In Polish).
- Ali, M.F.; Tahir, M. An overview of the factors affecting water-soluble carbohydrates concentration during ensiling of silage. J. Plant Environ. 2021, 3, 63–80. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Sloth, K.H.; Højberg, O.; Spliid, N.H.; Jensesn, C.; Thøgersen, R. Effects of microbial inoculants on corn silage fermentation, microbial contents, aerobic stability, and milk production under field conditions. J. Dairy Sci. 2010, 93, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Radkowski, A.; Radkowska, I. Evaluation of the quality and nutritive value of silages from pasture sward in some farms from South-East Poland. Wiadomości Zootech. 2014, 52, 32–37. (In Polish). Available online: https://wz.iz.edu.pl/files/WZ_2014_1_art05.pdf (accessed on 10 July 2024). (In Polish).
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I.; Bocianowski, J.; Cyplik, A.; Wolski, K.; Bujak, H. Effect of amino acids and effective microorganisms on meadow silage chemical composition. Agronomy 2021, 11, 1198. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Rossi, L.G.; Andrade, M.E.B.; Rabelo, C.H.S.; Siqueira, G.R.; Vicente, E.F.; Silva, W.L.; Silva, M.M.; Reis, R.A. Flint corn silage management: Influence of maturity stage, inoculation with Lentilactobacillus buchneri, and storage time on fermentation pattern, aerobic stability, and nutritional characteristics. Front. Microbiol. 2023, 14, 1223717. [Google Scholar] [CrossRef] [PubMed]
- Morrison, I.M. Changes in the cell wall components of laboratory silages and the effect of various additives on these changes. J. Agric. Sci. 1979, 93, 581–586. [Google Scholar] [CrossRef]
- Rezende, A.V.; Rabelo, C.H.; Veiga, R.M.; Andrade, L.P.; Härter, C.J.; Rabelo, F.H.; Basso, F.C.; Reis, R.A. Rehydration of corn grain with acid whey improves the silage quality. Anim. Feed Sci. Technol. 2014, 197, 213–221. [Google Scholar] [CrossRef]
- Khandaker, Z.H.; Uddin, M.J. Cost-effective preservation technique of green fodder and its impact on quality of silage. Livest. Res. Rural. Develop. 2013, 25, 233–243. Available online: http://www.lrrd.org/lrrd25/4/khan25070.htm (accessed on 10 July 2024).
- Gerlach, K.; Daniel, J.L.P.; Jobim, C.C.; Nussio, L.G. A data analysis of the effect of acidic acid on dry matter intake in dairy cattle. Anim. Feed Sci. Technol. 2021, 272, 114782. [Google Scholar] [CrossRef]
- Han, K.J.; Collins, M.; Newman, M.C.; Dougherty, C.T. Effects of forage length and bale chamber pressure on pearl millet silage. Crop Sci. 2006, 46, 337–344. [Google Scholar] [CrossRef]
- Weiss, K.; Kroschewski, B.; Auerbach, H.U. The influence of delayed sealing and repeated air ingress during the storage of maize silage on fermentation patterns, yeast development and aerobic stability. Fermentation 2022, 8, 48. [Google Scholar] [CrossRef]
- Wang, M.; Xu, S.; Wang, T.; Jia, T.; Xu, Z.; Wang, X.; Yu, Z. Effect of inoculants and storage temperature on the microbial, chemical and mycotoxin composition of corn silage. Asian Australian J. Animal Sci. 2018, 31, 1903–1912. [Google Scholar] [CrossRef]
- Selwet, M. Effect of organic acids and bacterial-enzymatic preparations on the number of fungal populations and silage aerobic stability. Bull. Vet. Inst. Pulawy 2006, 50, 215–220. [Google Scholar]
- Keles, G.; Kiely, P.O.; Lenehan, J.J.; Forristal, P.D. Conservation characteristics of baled grass silages differing in duration of wilting, bale density and number of layers of plastic stretch-film. Int. J. Account. Financ. Report. 2009, 48, 21–34. Available online: https://www.jstor.org/stable/25594961 (accessed on 10 July 2024).
- Whiter, A.G.; Kung, L. The effect of a dry or liquid application of Lactobacillus plantarum MTD1 on the fermentation of lucerne silage. J. Dairy Sci. 2001, 84, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Rizk, C.; Mustafa, A.F.; Phillip, L.E. Effect of inoculation of high dry matter lucerne silage on ensiling characteristics, ruminal nutrient degradability and dairy cow performance. J. Sci. Food Agric. 2005, 85, 743–750. [Google Scholar] [CrossRef]
- Greenhill, W.L. Plant juices in relation to silage fermentation. III. Effect of water activity of juice. Grass Forage Sci. 2006, 19, 336–339. [Google Scholar] [CrossRef]
- Woolford, M.K. The Silage Fermentation. In Microbiological Series, 14th ed.; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1984. [Google Scholar]
- Meeske, R. The Effect of Inoculants on Silage Fermentation Properties and on Animal Production; University of Stellenbosch: Stellenbosch, South Africa, 2000; Available online: http://hdl.handle.net/10019.1/14770 (accessed on 10 July 2024).
- Flynn, A. Factors affecting the feeding value of silage. In. Recent Developments in Ruminant Nutrition, 2nd ed.; Butterworths: London, UK, 1988; pp. 265–273. [Google Scholar]



| Year (Previous Crop) | Material for Silage | |
|---|---|---|
| Fresh | Wilted | |
| 2018 (winter rape) | n = 9 (1–9) | n = 9 (10–18) |
| 2019 (spring rape) | n = 9 (19–27) | n = 9 (28–36) |
| Specification | DAS | p-Value | ||
|---|---|---|---|---|
| 58 | 68 | 90 | ||
| 2018 | ||||
| WSC (g kg−1 DM) | 120 C ± 7.3 | 214 B ± 11.4 | 281 A ± 6.2 | 0.000 |
| BC (g lactic acid kg−1 DM) | 60 A ± 2.7 | 40 A ± 3.11 | 30 B ± 2.7 | 0.000 |
| FC (DM (%)+ 8 WSC/BC) | 25.70 C ± 5.00 | 56.10 B ± 7.26 | 96.03 A ± 4.45 | 0.000 |
| 2019 | ||||
| WSC (g kg−1 DM) | 161.2 Aa ± 6.1 | 225.2 b ± 5.7 | 292.0 B ± 5.1 | 0.000 |
| BC (g lactic acid kg−1 DM) | 47.95 ± 4.5 | 41.14 A ± 2.3 | 26.14 B ± 2.5 | 0.000 |
| FC (DM (%)+ 8 WSC/BC) | 37.36 A ± 5.3 | 57.99 B ± 4.00 | 111.06 C ± 3.8 | 0.000 |
| Treatment | DM | CP | CF | EE | CA | NFE | NDF | ADF | GE |
|---|---|---|---|---|---|---|---|---|---|
| % | g·kg−1 DM | MJ·kg−1 DM | |||||||
| Silage | |||||||||
| Fresh 2018 | 12.71 Bb | 149.13 | 167.74 Bb | 25.26 Aa | 144.91 | 512.95 ab | 271.93 Bc | 211.25 Cb | 18.77 Aa |
| Wilted 2018 | 17.07 Aa | 160.27 | 224.35 ab | 17.90 Bbc | 158.15 | 439.32 b | 361.98 ab | 309.16 ABa | 19.77 Bb |
| Fresh 2019 | 14.42 Bb | 114.77 | 182.82 Bb | 19.49 b | 141.24 | 541.67 a | 300.29 bc | 229.14 BCb | 18.15 Bc |
| Wilted 2019 | 19.33 Aa | 123.84 | 244.04 Aa | 13.91 Bc | 153.98 | 464.23 ab | 398.12 Aa | 334.04 Aa | 20.06 Aa |
| p-value | 0.000 | 0.064 | 0.001 | 0.000 | 0.141 | 0.026 | 0.003 | 0.001 | 0.000 |
| SEM | 0.794 | 7.311 | 10.062 | 1.300 | 2.964 | 14.816 | 16.607 | 16.947 | 0.238 |
| Year | |||||||||
| 2018 | 14.89 B | 154.70 A | 196.05 | 21.58 A | 151.53 | 476.14 | 316.95 | 260.20 | 19.27 |
| 2019 | 16.87 A | 119.30 B | 213.43 | 16.70 B | 147.61 | 502.95 | 349.20 | 281.59 | 19.11 |
| Silage material | |||||||||
| Fresh | 13.56 B | 131.95 | 175.28 B | 22.38 A | 143.08 b | 527.31 A | 286.11 B | 220.20 B | 18.46 B |
| Wilted | 18.20 A | 142.06 | 234.20 A | 15.90 B | 156.06 a | 451.78 B | 380.05 A | 321.60 A | 19.91 A |
| p-value | |||||||||
| Year | 0.006 | 0.013 | 0.095 | 0.001 | 0.458 | 0.219 | 0.091 | 0.201 | 0.259 |
| Silage material | 0.000 | 0.391 | 0.000 | 0.000 | 0.033 | 0.006 | 0.001 | 0.000 | 0.000 |
| Y*S | 0.619 | 0.928 | 0.808 | 0.397 | 0.962 | 0.927 | 0.823 | 0.826 | 0.009 |
| Treatment | DM | CP | CF | EE | CA | NFE | NDF | ADF | GE |
|---|---|---|---|---|---|---|---|---|---|
| % | g·kg −1 DM | MJ·kg−1 DM | |||||||
| Silage | |||||||||
| Fresh 2018 | 25.73 AB | 112.33 Bc | 163.39 Bc | 25.84 A | 155.08 Aa | 477.20 | 252.51 Cd | 184.19 B | 22.85 b |
| Wilted 2018 | 28.03 A | 149.41 Aa | 210.25 ab | 24.92 A | 221.24 bc | 460.35 | 305.82 Bc | 254.51 C | 24.18 a |
| Fresh 2019 | 23.33 B | 117.27 Bbc | 201.08 b | 19.43 B | 138.04 Bc | 458.59 | 339.04 Aa | 250.58 B | 22.79 b |
| Wilted 2019 | 25.55 AB | 122.38 Bb | 239.30 Aa | 22.15 B | 208.52 ab | 493.24 | 382.54 Bb | 319.40 A | 24.11 a |
| p-value | 0.003 | 0.000 | 0.000 | 0.000 | 0.005 | 0.572 | 0.000 | 0.000 | 0.003 |
| SEM | 0.555 | 4.397 | 8.788 | 2.252 | 11.882 | 9.252 | 14.566 | 14.856 | 0.224 |
| Year | |||||||||
| 2018 | 26.88 A | 130.87 A | 186.82 B | 25.38 A | 188.16 | 468.77 | 279.16 B | 219.35 B | 23.51 |
| 2019 | 24.44 B | 119.83 B | 220.19 A | 20.79 B | 173.28 | 465.91 | 360.79 A | 284.99 A | 23.45 |
| Silage material | |||||||||
| Fresh | 24.53 B | 114.80 B | 182.24 B | 22.63 | 146.56 B | 465.45 | 295.77 B | 217.39 B | 22.82 B |
| Wilted | 26.79 A | 135.89 A | 224.77 A | 23.54 | 214.88 A | 469.24 | 344.18 A | 286.96 A | 24.15 A |
| p-value | |||||||||
| Year | 0.002 | 0.000 | 0.002 | 0.000 | 0.280 | 0.885 | 0.000 | 0.000 | 0.799 |
| Silage material | 0.004 | 0.000 | 0.000 | 0.327 | 0.001 | 0.848 | 0.000 | 0.000 | 0.000 |
| Y*S | 0.947 | 0.000 | 0.584 | 0.069 | 0.870 | 0.314 | 0.450 | 0.931 | 0.993 |
| Treatment | Share of Acids (%) | N-NH3 %Ntotal | pH | Silage Quality according to Flieg–Zimmer Scale | |||
|---|---|---|---|---|---|---|---|
| Acetic acid | Butyric acid | Lactic acid | Score (Points) * | Quality ** | |||
| Silage | |||||||
| Fresh 2018 | 19.23 B | 0.00 | 80.77 A | 0.61 A | 4.18 a | 89.5 B | very good |
| Wilted 2018 | 28.47 A | 0.00 | 71.53 B | 0.29 B | 3.91 Bb | 97.3 A | very good |
| Fresh 2019 | 17.50 B | 0.00 | 82.50 A | 0.63 A | 4.39 Aa | 98.0 A | very good |
| Wilted 2019 | 18.55 B | 0.00 | 81.45 A | 0.26 B | 3.91 Bb | 98.0 A | very good |
| p-value | 0.001 | - | 0.001 | 0.000 | 0.000 | 0.000 | - |
| SEM | 1.417 | - | 1.417 | 0.055 | 0.064 | 1.109 | - |
| Year | |||||||
| 2018 | 23.9 A | 0.0 | 76.21 B | 0.45 | 4.05 | 93.4 B | very good |
| 2019 | 18.0 B | 0.0 | 82.02 A | 0.44 | 4.15 | 98.0 A | very good |
| Silage material | |||||||
| Fresh | 18.37 B | 0.00 | 81.63 A | 0.62 A | 4.29 A | 93.8 B | very good |
| Wilted | 23.51 A | 0.00 | 76.49 B | 0.27 B | 3.91 B | 97.7 A | very good |
| p-value | |||||||
| Year | 0.001 | - | 0.001 | 0.859 | 0.074 | 0.000 | - |
| Silage material | 0.003 | - | 0.003 | 0.000 | 0.000 | 0.000 | - |
| Year*Silage material | 0.009 | - | 0.009 | 0.520 | 0.064 | 0.000 | - |
| Treatment | Share of Acids (%) | N-NH3 %Ntotal | pH | Silage Quality according to Flieg–Zimmer Scale | |||
|---|---|---|---|---|---|---|---|
| Acetic Acid | Butyric Acid | Lactic Acid | Score (Points) * | Quality ** | |||
| Silage | |||||||
| Fresh 2018 | 16.2 a | 0.00 | 83.9 | 0.42 b | 4.12 b | 98.00 | very good |
| Wilted 2018 | 18.8 ab | 0.00 | 81.8 | 0.61 Aa | 4.18 ab | 97.33 | very good |
| Fresh 2019 | 18.0 ab | 0.00 | 82.5 | 0.63 Aa | 4.39 a | 98.00 | very good |
| Wilted 2019 | 19.3 b | 0.00 | 80.7 | 0.19 Bc | 4.13 b | 98.00 | very good |
| p-value | 0.027 | - | 0.129 | 0.000 | 0.019 | 0.441 | - |
| SEM | 0.443 | - | 0.494 | 0.056 | 0.040 | 0.167 | - |
| Year | |||||||
| 2018 | 17.52 | 0.00 | 82.81 | 0.52 a | 4.15 | 97.7 | very good |
| 2019 | 18.71 | 0.00 | 81.62 | 0.41 b | 4.26 | 98.0 | very good |
| Silage material | |||||||
| Fresh | 17.73 | 0.00 | 82.28 | 0.62 A | 4.29 a | 97.7 | very good |
| Wilted | 18.40 | 0.00 | 82.13 | 0.31 B | 4.13 b | 98.0 | very good |
| Year | 0.087 | 0.183 | 0.028 | 0.066 | 0.347 | - | |
| Silage material | 0.295 | 0.868 | 0.000 | 0.015 | 0.347 | - | |
| Year*Silage material | 0.011 | 0.047 | 0.013 | 0.089 | 0.347 | - | |
| Treatment | Lactic Acid | Acetic Acid | Propionic Acid | Ethanol | Butyric Acid | Valeric Acid | N-NH3 %Ntotal |
|---|---|---|---|---|---|---|---|
| g kg−1 | |||||||
| Silage | |||||||
| Fresh 2018 | 20.353 | 0.847 B | 0.027 ab | 1.602 ab | 0.227 | 0.006 B | 0.051 Aa |
| Wilted 2018 | 20.695 | 0.775 B | 0.030 Aa | 2.329 a | 0.004 | 0.001 B | 0.027 BCb |
| Fresh 2019 | 14.942 | 0.713 B | 0.013 Bc | 0.604 a | 0.016 | 0.037 A | 0.046 ABa |
| Wilted 2019 | 14.379 | 1.547 A | 0.017 bc | 1.391 ab | 0.009 | 0.010 B | 0.019Cb |
| p-value | 0.147 | 0.000 | 0.006 | 0.016 | 0.300 | 0.000 | 0.000 |
| SEM | 1.294 | 0.107 | 0.002 | 0.220 | 0.048 | 0.004 | 0.004 |
| Year | |||||||
| 2018 | 20.524 a | 0.811 B | 0.028 A | 1.965 A | 0.115 | 0.004 B | 0.039 |
| 2019 | 14.660 b | 1.130 A | 0.015 B | 0.998 B | 0.013 | 0.024 A | 0.032 |
| Silage material | |||||||
| Fresh | 17.537 | 1.161 A | 0.023 | 1.860 a | 0.007 | 0.006 B | 0.023 B |
| Wilted | 17.647 | 0.780 B | 0.020 | 1.103 b | 0.121 | 0.022 A | 0.049 A |
| p-value | |||||||
| Year | 0.029 | 0.005 | 0.000 | 0.008 | 0.288 | 0.000 | 0.117 |
| Silage material | 0.961 | 0.002 | 0.291 | 0.026 | 0.239 | 0.000 | 0.000 |
| Year*Silage material | 0.843 | 0.001 | 0.745 | 0.916 | 0.267 | 0.001 | 0.661 |
| Treatment | Lactic Acid | Acetic Acid | Propionic Acid | Ethanol | Butyric Acid | Valeric Acid | N-NH3 %Ntotal |
|---|---|---|---|---|---|---|---|
| g kg−1 | |||||||
| Silage | |||||||
| Fresh 2018 | 17.64 | 0.75 a | 0.09 A | 0.11 b | 0.01 | 0.02 ab | 0.04 a |
| Wilted 2018 | 18.69 | 0.81 a | 0.03 B | 1.60 a | 0.05 | 0.01 b | 0.05 Aa |
| Fresh 2019 | 13.58 | 0.43 b | 0.02 B | 0.00 b | 0.03 | 0.05 a | 0.02 Bb |
| Wilted 2019 | 15.61 | 0.75 a | 0.01 B | 0.60 ab | 0.02 | 0.04 a | 0.05 Aa |
| p-value | 0.237 | 0.010 | 0.000 | 0.011 | 0.168 | 0.012 | 0.001 |
| SEM | 0.941 | 0.053 | 0.009 | 0.223 | 0.008 | 0.006 | 0.004 |
| Year | |||||||
| 2018 | 18.164 | 0.783 a | 0.057 A | 0.858 | 0.028 | 0.013 B | 0.043 a |
| 2019 | 14.594 | 0.586 b | 0.018 B | 0.302 | 0.022 | 0.043 A | 0.032 b |
| Silage material | |||||||
| Fresh | 15.611 | 0.589 b | 0.055 A | 0.057 B | 0.017 | 0.035 | 0.026 B |
| Wilted | 17.147 | 0.780 a | 0.020 B | 1.103 A | 0.034 | 0.022 | 0.049 A |
| p-value | |||||||
| Year | 0.071 | 0.015 | 0.000 | 0.074 | 0.657 | 0.003 | 0.019 |
| Silage material | 0.397 | 0.017 | 0.000 | 0.005 | 0.248 | 0.099 | 0.000 |
| Year*Silage material | 0.782 | 0.075 | 0.003 | 0.141 | 0.061 | 0.918 | 0.135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szuba-Trznadel, A.; Hikawczuk, T.; Jama-Rodzeńska, A.; Kamińska, J.; Svecnjak, Z.; Król, Z.; Fuchs, B. Determining the Optimal Harvesting Moment of Green Forage from Guizotia abyssinica Cultivated as a Catch Crop on Silage and Its Quality Form, Fresh or Wilted Green Material, in the Two Following Years. Animals 2024, 14, 2455. https://doi.org/10.3390/ani14172455
Szuba-Trznadel A, Hikawczuk T, Jama-Rodzeńska A, Kamińska J, Svecnjak Z, Król Z, Fuchs B. Determining the Optimal Harvesting Moment of Green Forage from Guizotia abyssinica Cultivated as a Catch Crop on Silage and Its Quality Form, Fresh or Wilted Green Material, in the Two Following Years. Animals. 2024; 14(17):2455. https://doi.org/10.3390/ani14172455
Chicago/Turabian StyleSzuba-Trznadel, Anna, Tomasz Hikawczuk, Anna Jama-Rodzeńska, Joanna Kamińska, Zlatko Svecnjak, Zygmunt Król, and Bogusław Fuchs. 2024. "Determining the Optimal Harvesting Moment of Green Forage from Guizotia abyssinica Cultivated as a Catch Crop on Silage and Its Quality Form, Fresh or Wilted Green Material, in the Two Following Years" Animals 14, no. 17: 2455. https://doi.org/10.3390/ani14172455
APA StyleSzuba-Trznadel, A., Hikawczuk, T., Jama-Rodzeńska, A., Kamińska, J., Svecnjak, Z., Król, Z., & Fuchs, B. (2024). Determining the Optimal Harvesting Moment of Green Forage from Guizotia abyssinica Cultivated as a Catch Crop on Silage and Its Quality Form, Fresh or Wilted Green Material, in the Two Following Years. Animals, 14(17), 2455. https://doi.org/10.3390/ani14172455







