Genome-Wide Selection Sweep Analysis to Identify Candidate Genes with Black and Brown Color in Tibetan Sibu Yaks
Abstract
Simple Summary
Abstract
1. Introduction
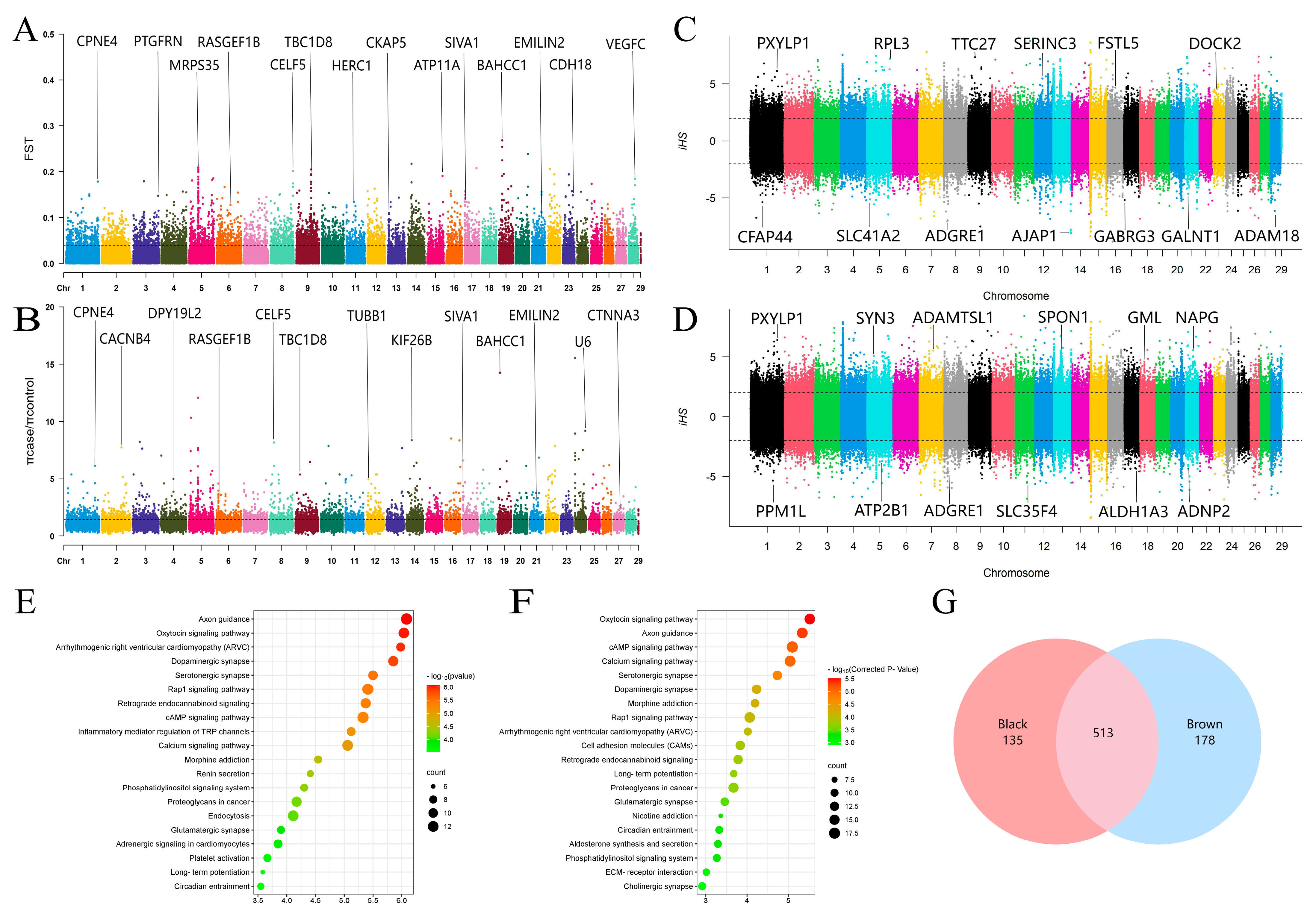
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of Functional Ingredients in Yak Milk-Derived Food on Health of Tibetan Nomads Living under High-Altitude Stress: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D.; Negrini, R.; Finlay, E.K.; Jianlin, H.; Groeneveld, E.; et al. Genetic Diversity in Farm Animals—A Review. Anim. Genet. 2010, 41 (Suppl. S1), 6–31. [Google Scholar] [CrossRef]
- Jing, X.; Ding, L.; Zhou, J.; Huang, X.; Degen, A.; Long, R. The Adaptive Strategies of Yaks to Live in the Asian Highlands. Anim. Nutr. 2022, 9, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Bano, I.; Qazi, I.H.; Matra, M.; Wanapat, M. “The Yak”—A Remarkable Animal Living in a Harsh Environment: An Overview of Its Feeding, Growth, Production Performance, and Contribution to Food Security. Front. Vet. Sci. 2023, 10, 1086985. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Peng, W.; Zhong, J.; Jiang, M. Genome-Wide Association Study of Body Weight Trait in Yaks. Animals 2022, 12, 1855. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Zheng, W.; Ren, P.; Hu, H.; Tong, X.; Zhang, S.-P.; Li, X.; Wang, H.; Jiang, J.-C.; Jin, J.; et al. Biallelic Mutations in MOS Cause Female Infertility Characterized by Human Early Embryonic Arrest and Fragmentation. EMBO Mol. Med. 2021, 13, e14887. [Google Scholar] [CrossRef]
- Centeno, P.P.; Pavet, V.; Marais, R. The Journey from Melanocytes to Melanoma. Nat. Rev. Cancer 2023, 23, 372–390. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nagata, T.; Koga, S.; Imokawa, G. Reduced Glutathione Disrupts the Intracellular Trafficking of Tyrosinase and Tyrosinase-Related Protein-1 but Not Dopachrome Tautomerase and Pmel17 to Melanosomes, Which Results in the Attenuation of Melanization. Arch. Dermatol. Res. 2014, 306, 37–49. [Google Scholar] [CrossRef]
- Amino, Y.; Takino, Y.; Kaneko, M.; Ookura, F.; Yamamoto, M.; Kashiwagi, T.; Iwasaki, K. Synthesis, Characterization, and Evaluation of Thiazolidine Derivatives of Cysteine for Suppressing Eumelanin Production. Chem. Pharm. Bull. 2016, 64, 1681–1691. [Google Scholar] [CrossRef]
- Dong, Y.; Kang, X.; Wang, C. Advances in mammalian melanin biosynthesis and its regulatory mechanisms. Life Sci. 2022, 34, 990–1000. [Google Scholar] [CrossRef]
- He, X.; Li, H.; Zhou, Z.; Zhao, Z.; Li, W. Production of Brown/Yellow Patches in the SLC7A11 Transgenic Sheep via Testicular Injection of Transgene. J. Genet. Genom. 2012, 39, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Wakamatsu, K.; Sviderskaya, E.V.; Donkin, A.J.; Montoliu, L.; Lynn Lamoreux, M.; Yu, B.; Millhauser, G.L.; Ito, S.; Barsh, G.S.; et al. Agouti Protein, Mahogunin, and Attractin in Pheomelanogenesis and Melanoblast-like Alteration of Melanocytes: A cAMP-Independent Pathway. Pigment Cell Melanoma Res. 2009, 22, 623–634. [Google Scholar] [CrossRef]
- Zdarsky, E.; Favor, J.; Jackson, I.J. The Molecular Basis of Brown, an Old Mouse Mutation, and of an Induced Revertant to Wild Type. Genetics 1990, 126, 443–449. [Google Scholar] [CrossRef]
- Cortimiglia, C.; Castiglioni, B.; Pizzi, F.; Stella, A.; Capra, E. Involvement of Tyrosinase-Related Protein 1 Gene in the Light Brown Plumage Phenotype of Falco cherrug. Anim. Genet. 2017, 48, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, V.J.; Ribani, A.; Fontanesi, L. A Premature Stop Codon in the TYRP1 Gene Is Associated with Brown Coat Colour in the European Rabbit (Oryctolagus cuniculus). Anim. Genet. 2014, 45, 600–603. [Google Scholar] [CrossRef]
- Marklund, L.; Moller, M.J.; Sandberg, K.; Andersson, L. A Missense Mutation in the Gene for Melanocyte-Stimulating Hormone Receptor (MC1R) Is Associated with the Chestnut Coat Color in Horses. Mamm. Genome 1996, 7, 895–899. [Google Scholar] [CrossRef]
- Rouzaud, F.; Martin, J.; Gallet, P.F.; Delourme, D.; Goulemot-Leger, V.; Amigues, Y.; Ménissier, F.; Levéziel, H.; Julien, R.; Oulmouden, A. A First Genotyping Assay of French Cattle Breeds Based on a New Allele of the Extension Gene Encoding the Melanocortin-1 Receptor (Mc1r). Genet. Sel. Evol. 2000, 32, 511–520. [Google Scholar] [CrossRef]
- Ollmann, M.M.; Barsh, G.S. Down-Regulation of Melanocortin Receptor Signaling Mediated by the Amino Terminus of Agouti Protein in Xenopus Melanophores. J. Biol. Chem. 1999, 274, 15837–15846. [Google Scholar] [CrossRef]
- Guo, H.; Xing, Y.; Liu, Y.; Luo, Y.; Deng, F.; Yang, T.; Yang, K.; Li, Y. Wnt/β-Catenin Signaling Pathway Activates Melanocyte Stem Cells in Vitro and in Vivo. J. Dermatol. Sci. 2016, 83, 45–51. [Google Scholar] [CrossRef]
- Dupin, E.; Le Douarin, N.M. Development of Melanocyte Precursors from the Vertebrate Neural Crest. Oncogene 2003, 22, 3016–3023. [Google Scholar] [CrossRef]
- Shibahara, S.; Takeda, K.; Yasumoto, K.; Udono, T.; Watanabe, K.; Saito, H.; Takahashi, K. Microphthalmia-Associated Transcription Factor (MITF): Multiplicity in Structure, Function, and Regulation. J. Investig. Dermatol. Symp. Proc. 2001, 6, 99–104. [Google Scholar] [CrossRef]
- Eizirik, E.; Trindade, F.J. Genetics and Evolution of Mammalian Coat Pigmentation. Annu. Rev. Anim. Biosci. 2021, 9, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Rossberg, W.; Saternus, R.; Wagenpfeil, S.; Kleber, M.; März, W.; Reichrath, S.; Vogt, T.; Reichrath, J. Human Pigmentation, Cutaneous Vitamin D Synthesis and Evolution: Variants of Genes (SNPs) Involved in Skin Pigmentation Are Associated with 25(OH)D Serum Concentration. Anticancer Res. 2016, 36, 1429–1437. [Google Scholar]
- Zhang, M.-Q.; Xu, X.; Luo, S.-J. The Genetics of Brown Coat Color and White Spotting in Domestic Yaks (Bos grunniens). Anim. Genet. 2014, 45, 652–659. [Google Scholar] [CrossRef]
- Medugorac, I.; Graf, A.; Grohs, C.; Rothammer, S.; Zagdsuren, Y.; Gladyr, E.; Zinovieva, N.; Barbieri, J.; Seichter, D.; Russ, I.; et al. Whole-Genome Analysis of Introgressive Hybridization and Characterization of the Bovine Legacy of Mongolian Yaks. Nat. Genet. 2017, 49, 470–475. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Xu, H.; Xia, X.; Luo, X.; Li, K.; Han, J.; Lei, C.; Chen, N.; Yue, X. Genomic Analysis Reveals a KIT-Related Chromosomal Translocation Associated with the White Coat Phenotype in Yak. J. Anim. Breed. Genet. 2023, 140, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, M.; Lu, Z.; Li, T.; Wang, H.; Yuan, Z.; Wei, C. Whole Genome Resequencing Reveals Selection Signals Related to Wool Color in Sheep. Animals 2023, 13, 3265. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, X.; Ma, C.; Wu, X.; ZhaXi, T.; Yin, L.; Li, W.; Li, Y.; Liang, C.; Yan, P. Whole Genome Resequencing-Based Analysis of Plateau Adaptation in Meiren Yak (Bos grunniens). Anim. Biotechnol. 2024, 35, 2298406. [Google Scholar] [CrossRef]
- Santos, W.B.; Schettini, G.P.; Maiorano, A.M.; Bussiman, F.O.; Balieiro, J.C.C.; Ferraz, G.C.; Pereira, G.L.; Baldassini, W.A.; Neto, O.R.M.; Oliveira, H.N.; et al. Genome-Wide Scans for Signatures of Selection in Mangalarga Marchador Horses Using High-Throughput SNP Genotyping. BMC Genom. 2021, 22, 737. [Google Scholar] [CrossRef]
- Zhou, J. Analysis of Genomic Data of Xinjiang Brown Cattle and Related Application Tools. Ph.D. Thesis, Ningxia University, Yinchuan, China, 2022. [Google Scholar]
- Gautier, M.; Vitalis, R. Rehh: An R Package to Detect Footprints of Selection in Genome-Wide SNP Data from Haplotype Structure. Bioinformatics 2012, 28, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Doekes, H.P.; Veerkamp, R.F.; Bijma, P.; de Jong, G.; Hiemstra, S.J.; Windig, J.J. Inbreeding Depression Due to Recent and Ancient Inbreeding in Dutch Holstein-Friesian Dairy Cattle. Genet. Sel. Evol. 2019, 51, 54. [Google Scholar] [CrossRef]
- Rodríguez-Ramilo, S.T.; Reverter, A.; Legarra, A. Islands of Runs of Homozygosity Indicate Selection Signatures in Ovis Aries 6 (OAR6) of French Dairy Sheep. JDS Commun. 2021, 2, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lu, Z.; Yuan, C.; Zhang, D.; Liu, J. Progress in the study of ROH and its application in sheep genetic breeding. China Anim. Husb. Vet. Med. 2023, 50, 3258–3266. [Google Scholar] [CrossRef]
- Kim, E.-S.; Elbeltagy, A.R.; Aboul-Naga, A.M.; Rischkowsky, B.; Sayre, B.; Mwacharo, J.M.; Rothschild, M.F. Multiple Genomic Signatures of Selection in Goats and Sheep Indigenous to a Hot Arid Environment. Heredity 2016, 116, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jara, E.; Peñagaricano, F.; Armstrong, E.; Ciappesoni, G.; Iriarte, A.; Navajas, E.A. Revealing the Genetic Basis of Eyelid Pigmentation in Hereford Cattle. J. Anim. Sci. 2022, 100, skac110. [Google Scholar] [CrossRef]
- Takeda, K.; Takahashi, N.-H.; Shibahara, S. Neuroendocrine Functions of Melanocytes: Beyond the Skin-Deep Melanin Maker. Tohoku J. Exp. Med. 2007, 211, 201–221. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Liu, B.; Zhang, Y.; Li, Y.; Li, Y.; Wang, Y.; Zhou, H. Regulatory Mechanisms of the cAMP-Responsive Element Binding Protein 3 (CREB3) Family in Cancers. Biomed. Pharmacother. 2023, 166, 115335. [Google Scholar] [CrossRef]
- Tsang, T.-F.; Chan, B.; Tai, W.C.-S.; Huang, G.; Wang, J.; Li, X.; Jiang, Z.H.; Hsiao, W.L.W. Gynostemma Pentaphyllum Saponins Induce Melanogenesis and Activate cAMP/PKA and Wnt/β-Catenin Signaling Pathways. Phytomedicine 2019, 60, 153008. [Google Scholar] [CrossRef]
- Lim, X.; Nusse, R. Wnt Signaling in Skin Development, Homeostasis, and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008029. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, K.; Deng, F.; Ye, J.; Xing, Y.; Li, Y.; Lian, X.; Yang, T. Wnt3a Promotes Melanin Synthesis of Mouse Hair Follicle Melanocytes. Biochem. Biophys. Res. Commun. 2012, 420, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Matera, I.; Watkins-Chow, D.E.; Loftus, S.K.; Hou, L.; Incao, A.; Silver, D.L.; Rivas, C.; Elliott, E.C.; Baxter, L.L.; Pavan, W.J. A Sensitized Mutagenesis Screen Identifies Gli3 as a Modifier of Sox10 Neurocristopathy. Hum. Mol. Genet. 2008, 17, 2118–2131. [Google Scholar] [CrossRef]
- Zhong, Z.; Gu, L.; Zheng, X.; Ma, N.; Wu, Z.; Duan, J.; Zhang, J.; Chen, J. Comprehensive Analysis of Spectral Distribution of a Large Cohort of Chinese Patients with Non-Syndromic Oculocutaneous Albinism Facilitates Genetic Diagnosis. Pigment Cell Melanoma Res. 2019, 32, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Loite, U.; Raam, L.; Reimann, E.; Reemann, P.; Prans, E.; Traks, T.; Vasar, E.; Silm, H.; Kingo, K.; Kõks, S. The Expression Pattern of Genes Related to Melanogenesis and Endogenous Opioids in Psoriasis. Int. J. Mol. Sci. 2021, 22, 13056. [Google Scholar] [CrossRef]
- Momtaz, S.; Niaz, K.; Maqbool, F.; Abdollahi, M.; Rastrelli, L.; Nabavi, S.M. STAT3 Targeting by Polyphenols: Novel Therapeutic Strategy for Melanoma. Biofactors 2017, 43, 347–370. [Google Scholar] [CrossRef]
- Urso, C. Spitz Tumors and Melanoma in the Genomic Age: A Retrospective Look at Ackerman’s Conundrum. Cancers 2023, 15, 5834. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Arato, K.; Lilienthal, E.; Zerweck, J.; Schutkowski, M.; Chatain, N.; Müller-Newen, G.; Becker, W.; de la Luna, S. Splice Variants of the Dual Specificity Tyrosine Phosphorylation-Regulated Kinase 4 (DYRK4) Differ in Their Subcellular Localization and Catalytic Activity. J. Biol. Chem. 2011, 286, 5494–5505. [Google Scholar] [CrossRef]
- Kim, M.; Gans, J.D.; Nogueira, C.; Wang, A.; Paik, J.-H.; Feng, B.; Brennan, C.; Hahn, W.C.; Cordon-Cardo, C.; Wagner, S.N.; et al. Comparative Oncogenomics Identifies NEDD9 as a Melanoma Metastasis Gene. Cell 2006, 125, 1269–1281. [Google Scholar] [CrossRef]
- Liu, W.; Stachura, P.; Xu, H.C.; Umesh Ganesh, N.; Cox, F.; Wang, R.; Lang, K.S.; Gopalakrishnan, J.; Häussinger, D.; Homey, B.; et al. Repurposing the Serotonin Agonist Tegaserod as an Anticancer Agent in Melanoma: Molecular Mechanisms and Clinical Implications. J. Exp. Clin. Cancer Res. 2020, 39, 38. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, H.-L.; Wen, X.-Y.; Jiang, L.; Fu, C.-H.; Hu, Y.-B.; Lei, X.-X.; Zhang, L.; Yu, X.; Yang, S.-Y.; et al. Selaginellin Inhibits Melanogenesis via the MAPK Signaling Pathway. J. Nat. Prod. 2022, 85, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Schwab, A. Cutaneous pH Landscape as a Facilitator of Melanoma Initiation and Progression. Acta Physiol. 2019, 225, e13105. [Google Scholar] [CrossRef]
- Xie, T.; Nguyen, T.; Hupe, M.; Wei, M.L. Multidrug Resistance Decreases with Mutations of Melanosomal Regulatory Genes. Cancer Res. 2009, 69, 992–999. [Google Scholar] [CrossRef]
- Boeckelmann, D.; Wolter, M.; Neubauer, K.; Sobotta, F.; Lenz, A.; Glonnegger, H.; Käsmann-Kellner, B.; Mann, J.; Ehl, S.; Zieger, B. Hermansky-Pudlak Syndrome: Identification of Novel Variants in the Genes HPS3, HPS5, and DTNBP1 (HPS-7). Front. Pharmacol. 2021, 12, 786937. [Google Scholar] [CrossRef]
- Brauer, P.M.; Tyner, A.L. RAKing in AKT: A Tumor Suppressor Function for the Intracellular Tyrosine Kinase FRK. Cell Cycle 2009, 8, 2728–2732. [Google Scholar] [CrossRef]
- Grabacka, M.M.; Wilk, A.; Antonczyk, A.; Banks, P.; Walczyk-Tytko, E.; Dean, M.; Pierzchalska, M.; Reiss, K. Fenofibrate Induces Ketone Body Production in Melanoma and Glioblastoma Cells. Front. Endocrinol. 2016, 7, 5. [Google Scholar] [CrossRef]
- Poff, A.M.; Ari, C.; Arnold, P.; Seyfried, T.N.; D’Agostino, D.P. Ketone Supplementation Decreases Tumor Cell Viability and Prolongs Survival of Mice with Metastatic Cancer. Int. J. Cancer 2014, 135, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Bahraman, A.G.; Jamshidzadeh, A.; Keshavarzi, M.; Arabnezhad, M.-R.; Mohammadi, H.; Mohammadi-Bardbori, A. α-Melanocyte-Stimulating Hormone Triggers Melanogenesis Via Activation of the Aryl Hydrocarbon Receptor Pathway in B16F10 Mouse Melanoma Cells. Int. J. Toxicol. 2021, 40, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Peripolli, E.; Metzger, J.; de Lemos, M.V.A.; Stafuzza, N.B.; Kluska, S.; Olivieri, B.F.; Feitosa, F.L.B.; Berton, M.P.; Lopes, F.B.; Munari, D.P.; et al. Autozygosity Islands and ROH Patterns in Nellore Lineages: Evidence of Selection for Functionally Important Traits. BMC Genom. 2018, 19, 680. [Google Scholar] [CrossRef]
- Gurgul, A.; Szmatoła, T.; Topolski, P.; Jasielczuk, I.; Żukowski, K.; Bugno-Poniewierska, M. The Use of Runs of Homozygosity for Estimation of Recent Inbreeding in Holstein Cattle. J. Appl. Genet. 2016, 57, 527–530. [Google Scholar] [CrossRef]
- Basang, W.-D.; Zhu, Y.-B.; Pingcuo, Z.-D.; Luosang, D.-Z.; Cidan, Y.-J.; Dawa, Y.-L.; Sun, G.-M.; Guang-Xin, E. Exploration of the Exogenous Male Yak Introduction Breeding Model and Its Effects on Tibetan Small-Sized Family Farms. Pak. Vet. J. 2021, 41, 137–141. [Google Scholar] [CrossRef]
- Leocard, S. Selective Sweep and the Size of the Hitchhiking Set. Adv. Appl. Probab. 2009, 41, 731–764. [Google Scholar] [CrossRef]
- Saif-Ur-Rehman, M.; Hassan, F.-U.; Reecy, J.; Deng, T. Whole-Genome SNP Markers Reveal Runs of Homozygosity in Indigenous Cattle Breeds of Pakistan. Anim. Biotechnol. 2023, 34, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Huang, P.; Zhang, Q.; Mimura, J.; Fujii-Kuriyama, Y.; Rannug, A.; Hökfelt, T.; Ceccatelli, S. Expression of Hypothalamic Neuropeptides after Acute TCDD Treatment and Distribution of Ah Receptor Repressor. Regul. Pept. 2004, 119, 113–124. [Google Scholar] [CrossRef]
- Liu, H.; Xi, Y.; Tang, Q.; Qi, J.; Zhou, Z.; Guo, Z.; Fan, W.; Hu, J.; Xu, Y.; Liang, S.; et al. Genetic Fine-Mapping Reveals Single Nucleotide Polymorphism Mutations in the MC1R Regulatory Region Associated with Duck Melanism. Mol. Ecol. 2023, 32, 3076–3088. [Google Scholar] [CrossRef]
- Tian, D.; Han, B.; Li, X.; Liu, D.; Zhou, B.; Zhao, C.; Zhang, N.; Wang, L.; Pei, Q.; Zhao, K. Genetic Diversity and Selection of Tibetan Sheep Breeds Revealed by Whole-Genome Resequencing. Anim. Biosci. 2023, 36, 991–1002. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, T.; Wang, Y.; Qu, H.; Shu, D.; Jia, X.; Luo, C. Genome-Wide Association Studies Provide Insight Into the Genetic Determination for Hyperpigmentation of the Visceral Peritoneum in Broilers. Front. Genet. 2022, 13, 820297. [Google Scholar] [CrossRef] [PubMed]
- Nikolovska, K.; Spillmann, D.; Haier, J.; Ladányi, A.; Stock, C.; Seidler, D.G. Melanoma Cell Adhesion and Migration Is Modulated by the Uronyl 2-O Sulfotransferase. PLoS ONE 2017, 12, e0170054. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xie, N.; Inoue, F.; Fan, S.; Saskin, J.; Zhang, C.; Zhang, F.; Hansen, M.E.B.; Nyambo, T.; Mpoloka, S.W.; et al. Integrative Functional Genomic Analyses Identify Genetic Variants Influencing Skin Pigmentation in Africans. Nat. Genet. 2024, 56, 258–272. [Google Scholar] [CrossRef]
- Adhikari, A.; Davie, J. JARID2 and the PRC2 Complex Regulate Skeletal Muscle Differentiation through Regulation of Canonical Wnt Signaling. Epigenetics Chromatin 2018, 11, 46. [Google Scholar] [CrossRef]
- Yang, Y.; Jang, G.-B.; Yang, X.; Wang, Q.; He, S.; Li, S.; Quach, C.; Zhao, S.; Li, F.; Yuan, Z.; et al. Central Role of Autophagic UVRAG in Melanogenesis and the Suntan Response. Proc. Natl. Acad. Sci. USA 2018, 115, E7728–E7737. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jang, G.-B.; Quach, C.; Liang, C. Darkening with UVRAG. Autophagy 2019, 15, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Okumoto, M.; Tsudzuki, M. Tawny: A Novel Light Coat Color Mutation Found in a Wild Population of Mus Musculus Molossinus, a New Allele at the Melanocortin 1 Receptor (Mc1r) Locus. Exp. Anim. 1999, 48, 73–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dürig, N.; Letko, A.; Lepori, V.; Hadji Rasouliha, S.; Loechel, R.; Kehl, A.; Hytönen, M.K.; Lohi, H.; Mauri, N.; Dietrich, J.; et al. Two MC1R Loss-of-Function Alleles in Cream-Coloured Australian Cattle Dogs and White Huskies. Anim. Genet. 2018, 49, 284–290. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Xu, L.; Zhang, H.; Zhu, Y.; Zhang, Q.; Zhang, C.; E, G. Genome-Wide Selection Sweep Analysis to Identify Candidate Genes with Black and Brown Color in Tibetan Sibu Yaks. Animals 2024, 14, 2458. https://doi.org/10.3390/ani14172458
Wu X, Xu L, Zhang H, Zhu Y, Zhang Q, Zhang C, E G. Genome-Wide Selection Sweep Analysis to Identify Candidate Genes with Black and Brown Color in Tibetan Sibu Yaks. Animals. 2024; 14(17):2458. https://doi.org/10.3390/ani14172458
Chicago/Turabian StyleWu, Xinming, Lu Xu, Haoyuan Zhang, Yong Zhu, Qiang Zhang, Chengfu Zhang, and Guangxin E. 2024. "Genome-Wide Selection Sweep Analysis to Identify Candidate Genes with Black and Brown Color in Tibetan Sibu Yaks" Animals 14, no. 17: 2458. https://doi.org/10.3390/ani14172458
APA StyleWu, X., Xu, L., Zhang, H., Zhu, Y., Zhang, Q., Zhang, C., & E, G. (2024). Genome-Wide Selection Sweep Analysis to Identify Candidate Genes with Black and Brown Color in Tibetan Sibu Yaks. Animals, 14(17), 2458. https://doi.org/10.3390/ani14172458



