Feeding a Saccharomyces cerevisiae Fermentation Product to Mares in Late Gestation Alters the Biological Activity of Colostrum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Supplementation
2.2. Weight Determination and Diarrhea Observation
2.3. Colostrum and Blood Sample Collection
2.4. Determination Leukocyte Subpopulations
2.5. Membrane Immunofluorescence
2.6. Vaccination
2.7. Determination of Immunoglobulin G
2.8. In Vitro Determination of the Biological Activity of Colostrum
2.9. Statistical Analysis
3. Results
3.1. SCFP Supplementation Did Not Influence the Immediate Vaccination Response of Pregnant Mares
3.2. SCFP Feeding Did Not Significantly Alter Serum IgG Concentrations in Mares
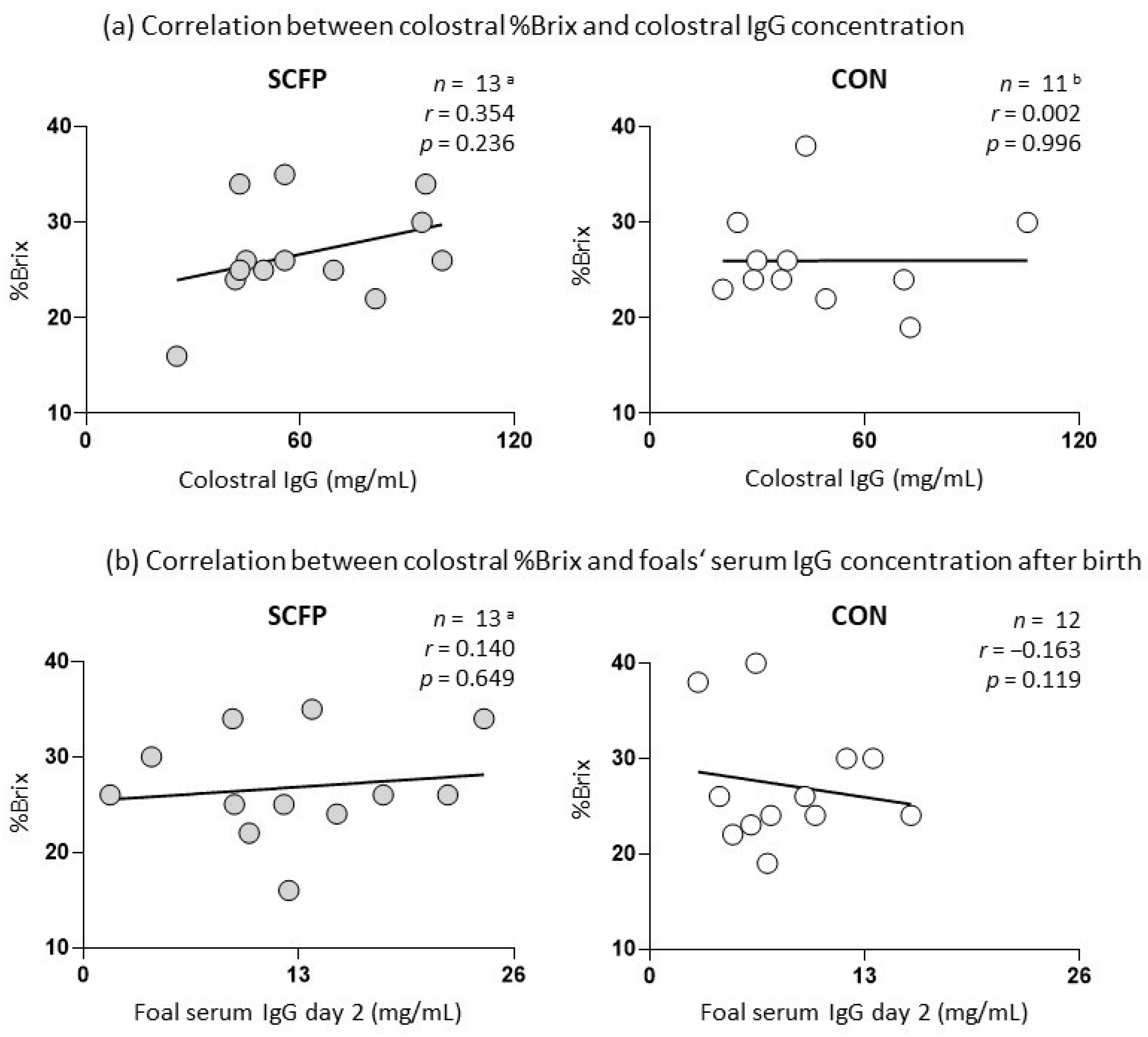
3.3. Colostrum Brix Values and Antibody Levels in Colostrum and Serum of Foals
3.4. SCFP Feeding Did Not Affect Foal Growth
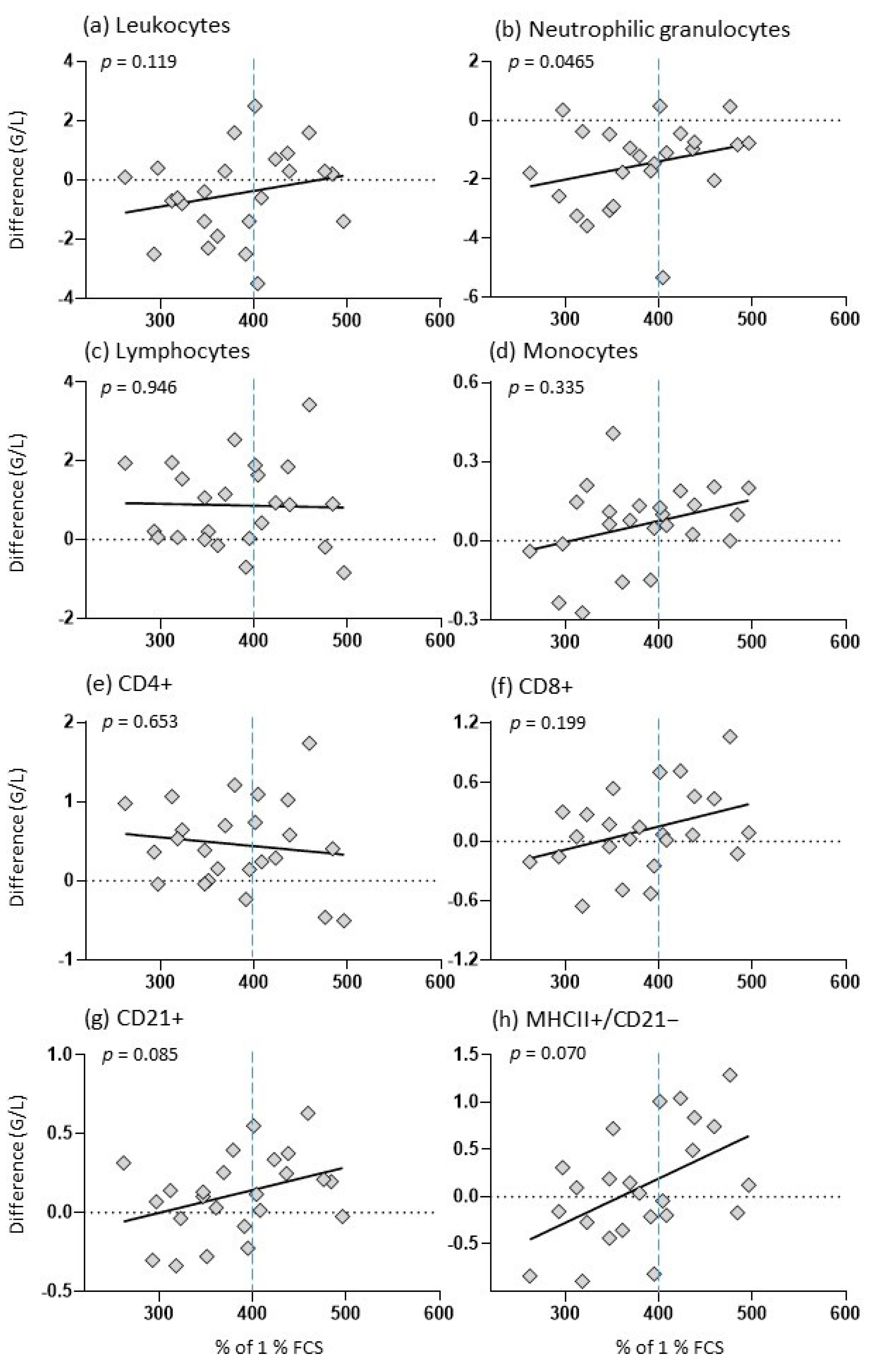
3.5. SCFP Feeding Altered the Biological Activity of Colostrum
3.6. Biological Activity of Colostrum Affects Vaccination Response of Foals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mecocci, S.; De Paolis, L.; Zoccola, R.; Fruscione, F.; De Ciucis, C.G.; Chiaradia, E.; Moccia, V.; Tognoloni, A.; Pascucci, L.; Zoppi, S.; et al. Antimicrobial and Immunomodulatory Potential of Cow Colostrum Extracellular Vesicles (ColosEVs) in an Intestinal In Vitro Model. Biomedicines 2022, 10, 3264. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Wagner, B. The development of equine immunity: Current knowledge on immunology in the young horse. Equine Vet. J. 2015, 47, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sievert, M.; Schuler, G.; Büttner, K.; Wehrend, A. Comparison of Different Methods to Determine the Absorption of Colostral IgG in Newborn Foals. J. Equine Vet. Sci. 2022, 114, 104008. [Google Scholar] [CrossRef] [PubMed]
- Jeffcott, L.B. Passive immunity and its transfer with special reference to the horse. Biol. Rev. 1972, 47, 439–464. [Google Scholar] [CrossRef]
- Sievert, M.; Krohn, J.; Wehrend, A. Immunoglobulin concentration in equine colostrum and blood of newborn foals as well as clinically relevant IgG evaluation methods—An overview. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2019, 47, 298–307. [Google Scholar] [CrossRef]
- Cash, R.S.G. Colostral quality determined by refractometry. Equine Vet. Educ. 1999, 11, 36–38. [Google Scholar] [CrossRef]
- Sobral, G.G.; Gomes Neto, O.C.; Carneiro, G.F. Effect of Supplementation with Saccharomyces cerevisiae and β-glucans to Mares during Late Gestation on Colostrum Quality and Passive Transfer of Immunity in Foals. J. Equine Vet. Sci. 2023, 121, 104168. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Timoney, J.F.; Holmes, M.A.; Karzenski, S.S.; Crisman, M.V. Immunoglobulin isotypes in sera and nasal mucosal secretions and their neonatal transfer and distribution in horses. Am. J. Vet. Res. 2000, 61, 1099–1105. [Google Scholar] [CrossRef]
- Morris, D.D.; Meirs, D.A.; Merryman, G.S. Passive transfer failure in horses: Incidence and causative factors on a breeding farm. Am. J. Vet. Res. 1985, 46, 2294–2299. [Google Scholar]
- Luft, C. Untersuchungen zur Systemischen Verfügbarkeit von Immunglobulin G Beim Neugeborenen Fohlen. Ph.D. Thesis, Ludwig-Maximilians-Universität, München, Germany, 2000. [Google Scholar]
- Warko, G.; Bostedt, H. The development of the IgG concentration in the blood serum of newborn foals. Tierarztl. Prax. 1993, 21, 528–535. [Google Scholar]
- Erhard, M.H.; Luft, C.; Remler, H.P.; Stangassinger, M. Assessment of colostral transfer and systemic availability of immunoglobulin G in new-born foals using a newly developed enzyme-linked immunosorbent assay (ELISA) system. J. Anim. Physiol. Anim. Nutr. 2001, 85, 164–173. [Google Scholar] [CrossRef]
- Markiewicz-Kęszycka, M.; Czyżak-Runowska, G.; Wójtowski, J.; Jóźwik, A.; Pankiewicz, R.; Łęska, B.; Krzyżewski, J.; Strzałkowska, N.; Marchewka, J.; Bagnicka, E. Influence of stage of lactation and year season on composition of mares’ colostrum and milk and method and time of storage on vitamin C content in mares’ milk. J. Sci. Food Agric. 2015, 95, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Venner, M.; Markus, R.; Strutzberg-Minder, K.; Nogai, K.; Beyerbach, M.; Klug, E. Evaluation of immunoglobulin G concentration in colostrum of mares by ELISA, refractometry and colostrometry. Berl. Münchener Tierärztl. Wochenschr. 2008, 121, 66–72. [Google Scholar] [CrossRef]
- Hemberg, E.; Einarsson, S.; Kútvölgyi, G.; Lundeheim, N.; Bagge, E.; Båverud, V.; Jones, B.; Morrell, J.M. Occurrence of bacteria and polymorphonuclear leukocytes in fetal compartments at parturition; relationships with foal and mare health in the peripartum period. Theriogenology 2015, 84, 163–169. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.M.; Tran, T.; Baldwin, J.L.; Pritchard, E.L. Factors that influence passive transfer of immunoglobulins in foals. J. Am. Vet. Med. Assoc. 1992, 200, 179–183. [Google Scholar] [CrossRef]
- Clabough, D.L.; Levine, J.F.; Grant, G.L.; Conboy, H.S. Factors associated with failure of passive transfer of colostral antibodies in Standardbred foals. J. Vet. Intern. Med. 1991, 5, 335–340. [Google Scholar] [CrossRef]
- Lavoie, J.P.; Spensley, M.S.; Smith, B.P.; Mihalyi, J. Colostral volume and immunoglobulin G and M determinations in mares. Am. J. Vet. Res. 1989, 50, 466–470. [Google Scholar]
- Kohn, C.W.; Knight, D.; Hueston, W.; Jacobs, R.; Reed, S.M. Colostral and serum IgG, IgA, and IgM concentrations in Standardbred mares and their foals at parturition. J. Am. Vet. Med. Assoc. 1989, 195, 64–68. [Google Scholar]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Blais, M.; Pouliot, Y.; Gauthier, S.; Boutin, Y.; Lessard, M. A gene expression programme induced by bovine colostrum whey promotes growth and wound-healing processes in intestinal epithelial cells. J. Nutr. Sci. 2014, 3, e57. [Google Scholar] [CrossRef]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune components of bovine colostrum and milk. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.J.; Matychak, M.B.; Felippe, M.J. Transfer of tumour necrosis factor-α via colostrum to foals. Vet. Rec. 2012, 170, 51. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.B.; Wagner, B.; Erb, H.N.; Ainsworth, D.M. Serum interleukin-6 (IL-6) and IL-10 concentrations in normal and septic neonatal foals. Vet. Immunol. Immunopathol. 2009, 132, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Goodman, L.B.; Wimer, C.; Freer, H.; Babasyan, S.; Wagner, B. Maternal T-lymphocytes in equine colostrum express a primarily inflammatory phenotype. Vet. Immunol. Immunopathol. 2014, 161, 141–150. [Google Scholar] [CrossRef]
- Mackenzie, C. Failure of passive transfer in foals. UK-Vet Equine 2020, 4, 62–65. [Google Scholar] [CrossRef]
- Dunière, L.; Renaud, J.B.; Steele, M.A.; Achard, C.S.; Forano, E.; Chaucheyras-Durand, F. A live yeast supplementation to gestating ewes improves bioactive molecule composition in colostrum with no impact on its bacterial composition and beneficially affects immune status of the offspring. J. Nutr. Sci. 2022, 11, e5. [Google Scholar] [CrossRef]
- Zanello, G.; Meurens, F.; Serreau, D.; Chevaleyre, C.; Melo, S.; Berri, M.; D‘Inca, R.; Auclair, E.; Salmon, H. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet. Immunol. Immunopathol. 2013, 152, 20–27. [Google Scholar] [CrossRef]
- Aoki, T.; Chiba, A.; Itoh, M.; Nambo, Y.; Yamagishi, N.; Shibano, K.I.; Cheong, S.H. Colostral and foal serum immunoglobulin G levels and associations with perinatal abnormalities in heavy draft horses in Japan. J. Equine Sci. 2020, 31, 29–34. [Google Scholar] [CrossRef]
- Blais, M.; Fortier, M.; Pouliot, Y.; Gauthier, S.F.; Boutin, Y.; Asselin, C.; Lessard, M. Colostrum whey down-regulates the expression of early and late inflammatory response genes induced by Escherichia coli and Salmonella enterica Typhimurium components in intestinal epithelial cells. Br. J. Nutr. 2015, 113, 200–211. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Pieper, R.; Kröger, S.; Martínez-Vallespín, B.; Hauser, A.E.; Niesner, R.; Vahjen, W.; Zentek, J. Porcine Colostrum Protects the IPEC-J2 Cells and Piglet Colon Epithelium against Clostridioides (syn Clostridium) difficile Toxin-Induced Effects. Microorganisms 2020, 8, 142. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Vahjen, W.; Zentek, J. Influence of high- and low-fermentable dietary fibres in sows’ diet on the colostrum potential against Clostridioides difficile toxin-induced effects in IPEC-J2 cells. J. Anim. Physiol. Anim. Nutr. 2023, 107, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, F.; Speiser, S.; Vahjen, W.; Zentek, J. Effect of different feed ingredients and additives on IPEC-J2 cells challenged with an enterotoxigenic Escherichia coli strain. Cytotechnology 2016, 68, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Cattell, M.; Marchbank, T. Marked variability in bioactivity between commercially available bovine colostrum for human use; implications for clinical trials. PLoS ONE 2020, 15, e0234719. [Google Scholar] [CrossRef]
- Langel, S.N.; Wark, W.A.; Garst, S.N.; James, R.E.; McGilliard, M.L.; Petersson-Wolfe, C.S.; Kanevsky-Mullarky, I. Effect of feeding whole compared with cell-free colostrum on calf immune status: Vaccination response. J. Dairy Sci. 2016, 99, 3979–3994. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; You, J.; Song, H.; Zhang, Y.; Lv, Y.; Qiao, H.; Tian, M.; Chen, F.; Zhang, S.; et al. The Effects of Dietary Supplementation of Saccharomyces cerevisiae Fermentation Product During Late Pregnancy and Lactation on Sow Productivity, Colostrum and Milk Composition, and Antioxidant Status of Sows in a Subtropical Climate. Front. Vet. Sci. 2020, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Macedo, R.J.; Arredondo, V.; García, F.; Aguilar, M.; Prado, O.; Rodríguez, R. Effect of supplemental yeast culture and physiological factors on colostrum and milk composition of Pelibuey ewes. Trop. Anim. Health Prod. 2012, 44, 349–354. [Google Scholar] [CrossRef]
- Shen, Y.B.; Carroll, J.A.; Yoon, I.; Mateo, R.D.; Kim, S.W. Effects of supplementing Saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets. J. Anim. Sci. 2011, 89, 2462–2471. [Google Scholar] [CrossRef]
- Scollo, A.; Borello, I.; Ghilardi, M.; Cavagnini, A. The Administration of Inactivated and Stabilized Whole-Cells of Saccharomyces cerevisiae to Gestating Sows Improves Lactation Efficiency and Post-Weaning Antimicrobial Use. Vet. Sci. 2023, 10, 576. [Google Scholar] [CrossRef]
- Le Flocʹh, N.; Achard, C.S.; Eugenio, F.A.; Apper, E.; Combes, S.; Quesnel, H. Effect of live yeast supplementation in sow diet during gestation and lactation on sow and piglet fecal microbiota, health, and performance. J. Anim. Sci. 2022, 100, skac209. [Google Scholar] [CrossRef]
- Alugongo, G.M.; Xiao, J.; Wu, Z.; Li, S.; Wang, Y.; Cao, Z. Review: Utilization of yeast of Saccharomyces cerevisiae origin in artificially raised calves. J. Anim. Sci. Biotechnol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Al-Qaisi, M.; Horst, E.A.; Mayorga, E.J.; Goetz, B.M.; Abeyta, M.A.; Yoon, I.; Timms, L.L.; Appuhamy, J.A.; Baumgard, L.H. Effects of a Saccharomyces cerevisiae fermentation product on heat-stressed dairy cows. J. Dairy Sci. 2020, 103, 9634–9645. [Google Scholar] [CrossRef]
- Kiarie, E.; Scott, M.; Krause, D.O.; Khazanehei, H.; Khafipour, E.; Nyachoti, C.M. Interactions of Saccharomyces cerevisiae fermentation product and in-feed antibiotic on gastrointestinal and immunological responses in piglets challenged with Escherichia coli K88+. J. Anim. Sci. 2012, 90 (Suppl. S4), 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Oba, P.M.; Koziol, S.A.; Applegate, C.C.; Soto-Diaz, K.; Steelman, A.J.; Panasevich, M.R.; Norton, S.A.; Swanson, K.S. Effects of a Saccharomyces cerevisiae fermentation product-supplemented diet on circulating immune cells and oxidative stress markers of dogs. J. Anim. Sci. 2022, 100, skac245. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.K.; Park, J.; Carey, J.B.; McIntyre, D.R.; Berghman, L.R. Immunomodulatory Effects of Saccharomyces cerevisiae Fermentation Product Supplementation on Immune Gene Expression and Lymphocyte Distribution in Immune Organs in Broilers. Front. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, A.; Finkler-Schade, C.; Schuberth, H.J. A Saccharomyces cerevisiae Fermentation Product (Olimond BB) Alters the Early Response after Influenza Vaccination in Racehorses. Animals 2021, 11, 2726. [Google Scholar] [CrossRef]
- Lucassen, A.; Hankel, J.; Finkler-Schade, C.; Osbelt, L.; Strowig, T.; Visscher, C.; Schuberth, H.J. Feeding a Saccharomyces cerevisiae Fermentation Product (Olimond BB) Does Not Alter the Fecal Microbiota of Thoroughbred Racehorses. Animals 2022, 12, 1496. [Google Scholar] [CrossRef]
- Terpeluk, E.R.; Schäfer, J.; Finkler-Schade, C.; Schuberth, H.J. Supplementation of Foals with a Saccharomyces cerevisiae Fermentation Product Alters the Early Response to Vaccination. Animals 2024, 14, 960. [Google Scholar] [CrossRef]
- Jeffcott, L.B. Studies on passive immunity in the foal. 1. Gamma-globulin and antibody variations associated with the maternal transfer of immunity and the onset of active immunity. J. Comp. Pathol. 1974, 84, 93–101. [Google Scholar] [CrossRef]
- Deters, E.L.; Stokes, R.S.; Genther-Schroeder, O.N.; Hansen, S.L. Effects of a Saccharomyces cerevisiae fermentation product in receiving diets of newly weaned beef steers. II. Digestibility and response to a vaccination challenge1. J. Anim. Sci. 2018, 96, 3906–3915. [Google Scholar] [CrossRef]
- Jang, Y.D.; Kang, K.W.; Piao, L.G.; Jeong, T.S.; Auclair, E.; Jonvel, S.; D‘Inca, R.; Kim, Y.Y. Effects of live yeast supplementation to gestation and lactation diets on reproductive performance, immunological parameters and milk composition in sows. Livest. Sci. 2013, 152, 167–173. [Google Scholar] [CrossRef]
- Di Costanzo, M.; De Paulis, N.; Capra, M.E.; Biasucci, G. Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy. Nutrients 2022, 14, 1766. [Google Scholar] [CrossRef]
- Brewer, M.T.; Anderson, K.L.; Yoon, I.; Scott, M.F.; Carlson, S.A. Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet. Microbiol. 2014, 172, 248–255. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar]
- Benedetto, A.; Bocca, C.; Brizio, P.; Cannito, S.; Abete, M.C.; Squadrone, S. Effects of the rare elements lanthanum and cerium on the growth of colorectal and hepatic cancer cell lines. Toxicol. In Vitro 2018, 46, 9–18. [Google Scholar] [CrossRef]
- Witkowski, J.; Polak, S.; Rogulski, Z.; Pawelec, D. In Vitro/In Vivo Translation of Synergistic Combination of MDM2 and MEK Inhibitors in Melanoma Using PBPK/PD Modelling: Part I. Int. J. Mol. Sci. 2022, 23, 12984. [Google Scholar] [CrossRef]
- Idrees, A.; Chiono, V.; Ciardelli, G.; Shah, S.; Viebahn, R.; Zhang, X.; Salber, J. Validation of in vitro assays in three-dimensional human dermal constructs. Int. J. Artif. Organs 2018, 41, 779–788. [Google Scholar] [CrossRef]
- Lowin, T.; Tingting, R.; Zurmahr, J.; Classen, T.; Schneider, M.; Pongratz, G. Cannabidiol (CBD): A killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020, 11, 714. [Google Scholar] [CrossRef]
- Weicht, R.R.; Schultz, C.R.; Geerts, D.; Uhl, K.L.; Bachmann, A.S. Polyamine Biosynthetic Pathway as a Drug Target for Osteosarcoma Therapy. Med. Sci. 2018, 6, 65. [Google Scholar] [CrossRef]
- Lowin, T.; Bleck, J.; Schneider, M.; Pongratz, G. Selective killing of proinflammatory synovial fibroblasts via activation of transient receptor potential ankyrin (TRPA1). Biochem. Pharmacol. 2018, 154, 293–302. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z.; Han, J.; Qian, L.; Wang, W.; Lei, J.; Wang, H. Endocrine, genetic, and microbiome nexus of obesity and potential role of postbiotics: A narrative review. Eat. Weight Disord. 2023, 28, 84. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Zhou, P.; Li, Z.; Zhong, W.; Zhuo, Y.; Che, L.; Xu, S.; Fang, Z.; Jiang, X.; et al. Effects of yeast culture supplementation from late gestation to weaning on performance of lactating sows and growth of nursing piglets. Animal 2022, 16, 100526. [Google Scholar] [CrossRef]
- Andersen-Nissen, E.; Fiore-Gartland, A.; Ballweber Fleming, L.; Carpp, L.N.; Naidoo, A.F.; Harper, M.S.; Voillet, V.; Grunenberg, N.; Laher, F.; Innes, C.; et al. Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk. PLoS Pathog. 2021, 17, e1009363. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems biological assessment of human immunity to BNT162b2 mRNA vaccination. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Hanson, N.Q.; Straka, R.J.; Hoke, T.R.; Ordovas, J.M.; Peacock, J.M.; Arends, V.L.; Arnett, D.K. Effect of influenza vaccine on markers of inflammation and lipid profile. J. Lab. Clin. Med. 2005, 145, 323–327. [Google Scholar] [CrossRef]
- Ivell, R.; Anand-Ivell, R. Neohormones in milk. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 419–425. [Google Scholar] [CrossRef]
- Lynn, D.J.; Pulendran, B. The potential of the microbiota to influence vaccine responses. J. Leukoc. Biol. 2018, 103, 225–231. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of immune responses to vaccination by the microbiota: Implications and potential mechanisms. Nat. Rev. Immunol. 2022, 22, 33–46. [Google Scholar] [CrossRef]

| Ingredient | SCFP a (20 g) | Mineral Bricks b (100 g) |
|---|---|---|
| Crude protein | 21.10% | |
| Crude fat | 1.90% | |
| Crude fiber | 19.20% | |
| Crude ash | 9.20% | |
| Sodium (labelled) | 0.00% | |
| Tocopherol extract | 0.090 g | 0.200 g |
| Vitamin C | 0.584 g | 0.050 g |
| Minerals (calcium carbonate) | 0.126 g | 12.00 g |
| Inactivated yeasts | 19.200 g |
| SCFP (n = 13) b | CON (n = 12) | ||||||
|---|---|---|---|---|---|---|---|
| Cell Type | Pre | Post | Difference | Pre | Post | Difference | p |
| Leukocytes | 9.12 ± 2.24 | 11.80 ± 2.69 | 2.68 ± 1.63 | 9.41 ± 2.13 | 12.56 ± 1.93 | 3.15 ± 2.91 | 0.512 |
| Granulocytes | 7.07 ± 1.87 | 10.15 ± 2.63 | 3.08 ± 1.64 | 7.46 ± 2.03 | 11.00 ± 1.97 | 3.54 ± 2.87 | 0.404 |
| Monocytes | 0.34 ± 0.16 | 0.42 ± 0.20 | 0.08 ± 0.12 | 0.36 ± 0.18 | 0.42 ± 0.15 | 0.06 ± 0.18 | 0.829 a |
| Lymphocytes | 1.71 ± 0.99 | 1.23 ± 0.56 | −0.48 ± 0.68 | 1.59 ± 1.11 | 1.14 ± 1.00 | −0.45 ± 0.66 | 0.766 |
| CD4+ T cells | 0.90 ± 0.49 | 0.63 ± 0.25 | −0.27 ± 0.39 | 0.82 ± 0.63 | 0.60 ± 0.63 | −0.23 ± 0.35 | 0.764 |
| CD8+ T cells | 0.52 ± 0.34 | 0.36 ± 0.19 | −0.16 ± 0.27 | 0.52 ± 0.37 | 0.36 ± 0.32 | −0.17 ± 0.21 | 0.966 |
| CD21+ B cells | 0.13 ± 0.10 | 0.09 ± 0.08 | −0.03 ± 0.10 | 0.11 ± 0.07 | 0.07 ± 0.06 | −0.03 ± 0.06 | 0.468 a |
| MHCII+/CD21− cells | 0.17 ± 0.16 | 0.13 ± 0.09 | −0.04 ± 0.16 | 0.21 ± 0.23 | 0.16 ± 0.16 | −0.04 ± 0.10 | 0.752 a |
| Vaccination | Parturition | |
|---|---|---|
| SCFP (n = 13) a | 13.6 ± 3.1 | 14.5 ± 2.5 |
| CON (n = 12) | 12.9 ± 2.4 | 12.3 ± 4.4 |
| p | 0.5172 | 0.1350 |
| Group 1 (SCFP) (n = 13 f) | Group 2 (CON) (n = 12) | p | ||
|---|---|---|---|---|
| Colostral %Brix a | 26 (16–35) | 25 (19–40) | 0.645 | |
| Serum protein (g/dL) a,b | 6.0 (4.6–6.6) | 6.4 (5.6–7.2) | 0.946 | |
| Serum IgG (mg/mL) c | 12.4 ± 6.4 | 8.3 ± 3.9 | 0.072 | |
| Diarrhea days d | 4 ± 6 | 3 ± 5.5 | 0.882 | |
| Daily weight gain d,e | 1.23 ± 0.3 | 1.17 ± 0.5 | 0.299 | |
| Weight (kg) d | d2 | 60.4 ± 7.8 | 56.5 ± 8.1 | 0.847 |
| d30 | 98.5 ± 15.1 | 89.9 ± 14.5 | 0.298 | |
| SCFP Group | CON Group | |||||
|---|---|---|---|---|---|---|
| n | r | p | n | r | p | |
| %Brix | 13 a | 0.0036 | 0.991 | 12 | −0.085 | 0.794 |
| Colostral IgG | 13 a | −0.222 | 0.467 | 11 b | −0.172 | 0.613 |
| Foal serum IgG day 2 | 13 a | 0.212 | 0.487 | 12 | 0.277 | 0.384 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpeluk, E.R.; Schäfer, J.; Finkler-Schade, C.; Rauch, E.; Rohn, K.; Schuberth, H.-J. Feeding a Saccharomyces cerevisiae Fermentation Product to Mares in Late Gestation Alters the Biological Activity of Colostrum. Animals 2024, 14, 2459. https://doi.org/10.3390/ani14172459
Terpeluk ER, Schäfer J, Finkler-Schade C, Rauch E, Rohn K, Schuberth H-J. Feeding a Saccharomyces cerevisiae Fermentation Product to Mares in Late Gestation Alters the Biological Activity of Colostrum. Animals. 2024; 14(17):2459. https://doi.org/10.3390/ani14172459
Chicago/Turabian StyleTerpeluk, Eva Ronja, Jana Schäfer, Christa Finkler-Schade, Elke Rauch, Karl Rohn, and Hans-Joachim Schuberth. 2024. "Feeding a Saccharomyces cerevisiae Fermentation Product to Mares in Late Gestation Alters the Biological Activity of Colostrum" Animals 14, no. 17: 2459. https://doi.org/10.3390/ani14172459
APA StyleTerpeluk, E. R., Schäfer, J., Finkler-Schade, C., Rauch, E., Rohn, K., & Schuberth, H.-J. (2024). Feeding a Saccharomyces cerevisiae Fermentation Product to Mares in Late Gestation Alters the Biological Activity of Colostrum. Animals, 14(17), 2459. https://doi.org/10.3390/ani14172459





