The Distribution, Diversity, and Control of Dirofilariosis in Brazil: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
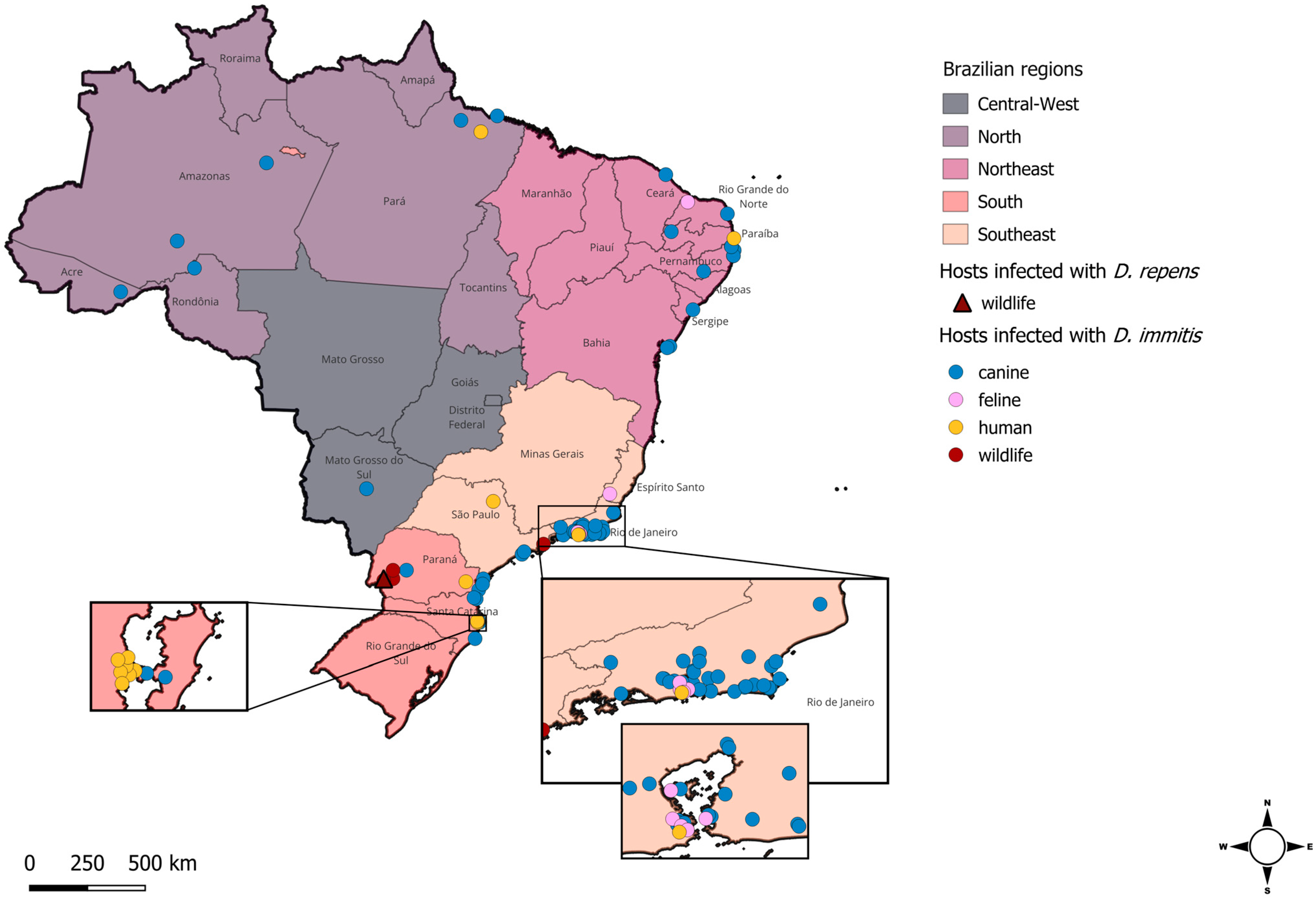
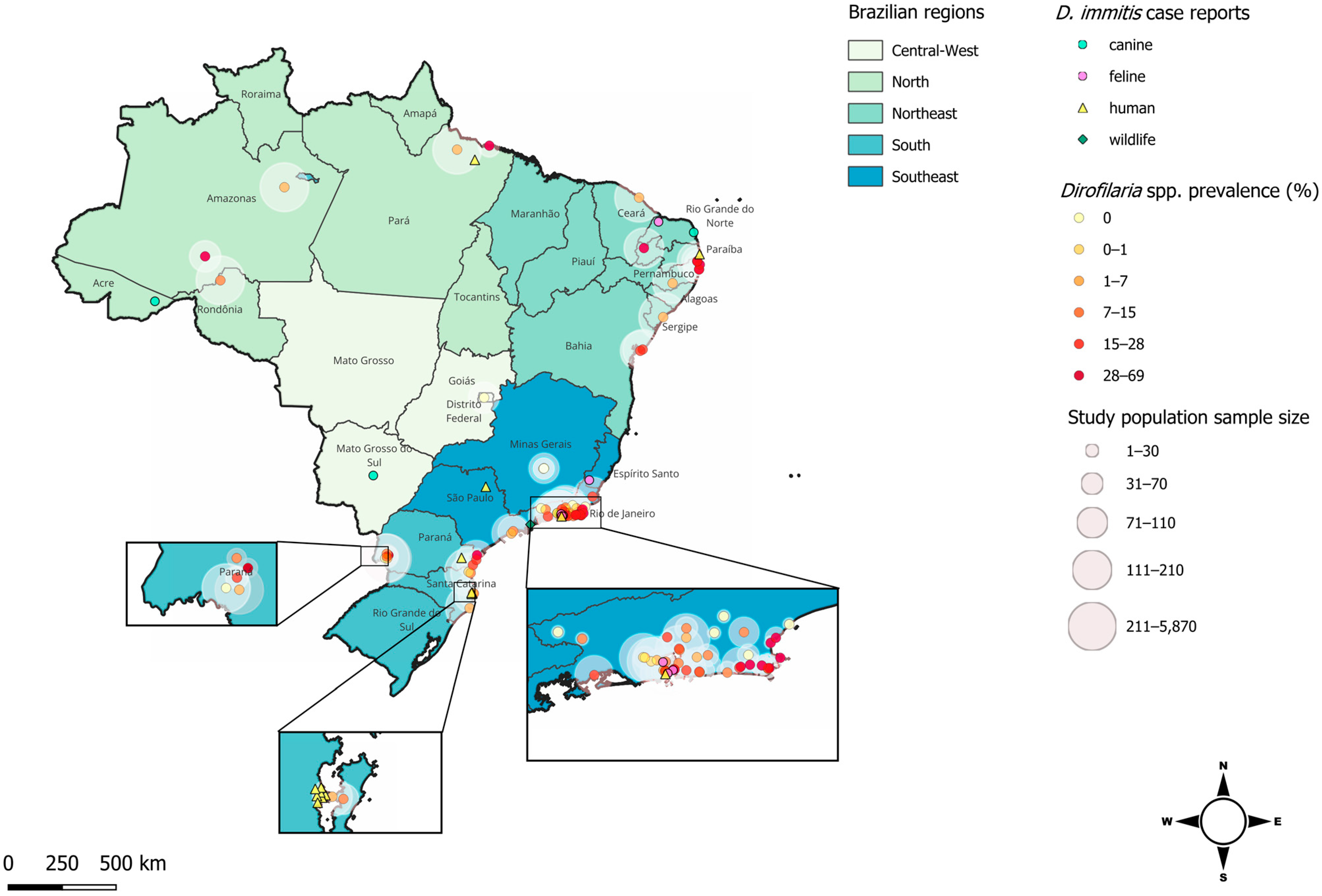
2. Distribution of Dirofilaria spp. in Brazil
2.1. Canine Hosts
| Region | State | City | Filarial Species | Prevalence | Positives/Total | Diagnostic Method | Authors |
|---|---|---|---|---|---|---|---|
| North | AM | Lábrea | D. immitis | 44.4% | 44/99 | PCR (cox1) | [25] |
| AM | Manaus | D. immitis | 3.7% | 28/766 | Thick blood smear | [27] | |
| PA | Ilha do Algodoal | D. immitis | 35.8% | 24/67 | Knott’s method and PCR (12S) | [26] | |
| PA | Ilha de Marajó | D. immitis | 2.1% | 9/418 | PCR (5.8S-ITS2-28S) | [34] | |
| RO | Porto Velho | D. immitis | 12.8% | 93/727 | Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea) | [35] | |
| AC | Rio Branco | D. immitis | Case Report | 1/1 | Clinical examination, Microscopy, SNAP 4Dx Plus Test (IDEXX Laboratories), Echocardiogram | [36] | |
| Northeast | BA | Lauro de Freitas | D. immitis | 20.3% | 30/148 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] |
| BA | Salvador | D. immitis | 20.0% | 24/120 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] | |
| CE | Fortaleza | D. immitis | 1.1% | 26/2400 | Blood smear | [28] | |
| PB | Sousa | D. immitis | 33.6% | 48/140 | Knott’s method, Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Republic of Korea), and PCR (COI) | [37] | |
| PE | Recife | D. immitis | 36.7% | 22/60 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] | |
| PE | Itamaracá | D. immitis | 49.5% | 54/109 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] | |
| PE | Recife | D. immitis | 11.5% | 12/104 | Knott’s method | [38] | |
| PE | Goiana | D. immitis | 36.3% | 74/204 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [39] | |
| PE | São Joaquim de Bicas | D. immitis | 0.0% | 0/103 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [39] | |
| PE | Garanhuns | D. immitis | 5.5% | 11/201 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [40] | |
| PE | Goiana | D. immitis | 32.0% | 32/100 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [41] | |
| SE | Aracaju | D. immitis | 3.7% | 14/378 | Knott’s method | [29] | |
| RN | Natal | D. immitis | Case report | 1/1 | Skin cytology, histopathology, Knott’s method, Thick smear, PCR | [42] | |
| Southeast | RJ | Cabo Frio | D. immitis | 30.1% | 31/103 | Microscopy, SNAP 4Dx Plus Test (IDEXX Laboratories), and PCR (COI) | [22] |
| RJ | Mangaratiba | D. immitis | 16.3% | 23/141 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] | |
| RJ | Niterói | D. immitis | 58.6% | 92/157 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] | |
| RJ | Cabo Frio | D. immitis | 27.5% | 11/40 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] | |
| RJ | Armação de Búzios | D. immitis | 62.2% | 23/37 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [30] | |
| RJ | Rio de Janeiro | D. immitis | 7.0% | 30/428 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [23] | |
| RJ | Rio de Janeiro | D. immitis | 21.6% | 44/204 | Microfilariae and antigen detection | [43] | |
| RJ | Rio de Janeiro | D. immitis | 5.8% | 6/103 | Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea) | [44] | |
| RJ | Araruama | D. immitis | 25.0% | 1/4 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Barra de São João | D. immitis | 27.8% | 5/18 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Cabo Frio | D. immitis | 14.6% | 12/82 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Campos | D. immitis | 17.5% | 29/166 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Iguaba Grande | D. immitis | 30.0% | 3/10 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Macaé | D. immitis | 0.0% | 0/18 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Maricá | D. immitis | 24.8% | 61/246 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Rio das Ostras | D. immitis | 31.5% | 17/54 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [42] | |
| RJ | Saquarema | D. immitis | 9.4% | 3/32 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Cachoeiras de Macacu | D. immitis | 0.0% | 0/17 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Metropolitan Region | D. immitis | 15.2% | 14/92 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Itaboraí | D. immitis | 7.1% | 2/28 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Magé | D. immitis | 27.3% | 3/11 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Niterói | D. immitis | 21.1% | 22/104 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Nova Iguaçu | D. immitis | 5.4% | 2/37 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Rio de Janeiro | D. immitis | 20.0% | 2/10 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | São Gonçalo | D. immitis | 15.2% | 14/92 | Direct examination and SNAP 4Dx Plus Test (IDEXX Laboratories) | [45] | |
| RJ | Maricá | D. immitis | 7.5% | 6/80 | Knott’s method | [46] | |
| RJ | São Pedro da Aldeia | D. immitis | 43.4% | 23/53 | Knott’s method | [46] | |
| RJ | Maricá/Niterói | D. immitis | 24.1% | 33/137 | Knott’s method | [47] | |
| RJ | Guapimirim | D. immitis | 1.5% | 20/1372 | Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea), ELISA, and Knott’s method | [48] | |
| RJ | São João de Meriti | D. immitis | 0.2% | 1/667 | Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea), ELISA, and Knott’s method | [48] | |
| RJ | Nova Iguaçu | D. immitis | 0.9% | 2/226 | Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea), ELISA, and Knott’s method | [48] | |
| RJ | Magé | D. immitis | 8.2% | 156/1896 | Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea), ELISA, and Knott’s method | [48] | |
| RJ | Duque de Caxias | D. immitis | 1.8% | 107/5870 | Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea), ELISA, and Knott’s method | [48] | |
| RJ | Ilha do Governador | D. immitis | 30.2% | 19/63 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [49] | |
| RJ | Araruama | D. immitis | 68.9% | 115/167 | Knott’s method and SNAP 4Dx Plus Test (IDEXX Laboratories) | [32] | |
| RJ | Aldeia Velha | D. immitis | 7.5% | 8/106 | Knott’s method and SNAP 4Dx Plus Test (IDEXX Laboratories) | [32] | |
| RJ | São Vicente | D. immitis | 0.0% | 0/60 | Knott’s method and SNAP 4Dx Plus Test (IDEXX Laboratories) | [32] | |
| MG | Igarapé | D. immitis | 0.0% | 0/50 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [42] | |
| MG | São Joaquim de Bicas | D. immitis | 0.0% | 0/50 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [42] | |
| MG | Uberlândia | D. immitis | Case Report | 1/1 | Urinalysis, Thick blood smear | [50] | |
| SP | Guarujá | D. immitis | 2.8% | 4/142 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [31] | |
| SP | Bertioga | D. immitis | 7.6% | 7/92 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [31] | |
| South | PR | Parque Nacional Iguaçu | D. immitis | 22% | 11/50 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [5] |
| PR | Parque Nacional Iguaçu | D. immitis | 0.0% | 0/225 | Knott’s method, Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea), and PCR (myoHC and hsp70) | [51] | |
| PR | Guaratuba | D. immitis | 24.5% | 12/49 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [31] | |
| PR | Guaraqueçaba | D. immitis | 31.8% | 7/22 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [31] | |
| PR | Pontal do Paraná | D. immitis | 26.3% | 31/118 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [31] | |
| SC | Florianópolis | D. immitis | 2.1% | 3/146 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [31] | |
| SC | Araquari | D. immitis | 7.3% | 11/150 | Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [31] | |
| SC | Laguna | D. immitis | 4.6% | 11/238 | Knott’s method, Immunochromatography (CHW Ag 2.0 Test Kit, Alere Bionote Inc., Gyeonggi-do, Republic of Korea), and PCR (surface antigen gene) | [52] | |
| SC | Joinville | D. immitis | 0.7% | 3/429 | Knott’s method and SNAP 4Dx Plus Test (IDEXX Laboratories) | [53] | |
| Central-West | MT | Pantanal—Barão de Melgaço | D. immitis | 7.1% | 6/84 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [33] |
| DF | Brasilia | D. immitis | 0.0% | 0/100 | SNAP 4Dx Plus Test (IDEXX Laboratories) | [42] | |
| MS | Campo Grande | D. immitis | Case Report | 1/1 | Knott’s method, SNAP 4Dx Plus Test (IDEXX Laboratories) and PCR (12S) | [34] |
2.2. Feline Hosts
2.3. Wildlife Hosts
2.4. Human Hosts
3. Genomic Diversity of Dirofilaria in South America
4. Treatment and Prophylaxis Available in Brazil
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capelli, G.; Genchi, C.; Baneth, G.; Bourdeau, P.; Brianti, E.; Cardoso, L.; Danesi, P.; Fuehrer, H.P.; Giannelli, A.; Ionică, A.M.; et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasites Vectors 2018, 11, 663. [Google Scholar] [CrossRef]
- Ranjbar-Bahadori, S.; Veshgini, A.; Shirani, D.; Eslami, A.; Mohieddin, H.; Shemshadi, B.; Masooleh, R. Epidemiological aspects of canine dirofilariasis in the north of Iran. Iran. J. Parasitol. 2011, 6, 73–80. [Google Scholar] [PubMed] [PubMed Central]
- Tarish, J.H.; Al-Saqur, I.M.; Al-Abbassy, S.N.; Kadhim, F.S. The prevalence of parasitic helminths in stray dogs in the Baghdad area, Iraq. Ann. Trop. Med. Parasitol. 1986, 80, 329–331. [Google Scholar] [CrossRef]
- Genchi, C.; Kramer, L.H. The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.F.D.; da Silva, M.X.; Magalhães-Matos, P.C.; de Albuquerque, A.C.A.; Tebaldi, J.H.; Mathias, L.A.; Lux Hoppe, E.G. Filarial nematodes with zoonotic potential in ring-tailed coatis (Nasua nasua Linnaeus, 1766, Carnivora: Procyonidae) and domestic dogs from Iguaçu National Park, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- López, J.; Valiente-Echeverría, F.; Carrasco, M.; Mercado, R.; Abarca, K. Identificación morfológica y molecular de filarías caninas en una comuna semi-rural de la Región Metropolitana, Chile. Rev. Chil. Infectol. 2012, 29, 248–289. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.; Castañeda, S.; Muñoz, M.; Flórez, A.; Pinilla, J.C.; Ramírez, J.D. The first report of Dirofilaria repens infection in dogs from Colombia. Parasitol. Res. 2023, 122, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariosis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Overview on Dirofilaria immitis in the Americas, with notes on other filarial worms infecting dogs. Vet. Parasitol. 2020, 282, 109113. [Google Scholar] [CrossRef]
- Alsarraf, M.; Carretón, E.; Ciuca, L.; Diakou, A.; Dwużnik-Szarek, D.; Fuehrer, H.-P.; Genchi, M.; Ionică, A.M.; Kloch, A.; Kramer, L.H.; et al. Diversity and geographic distribution of haplotypes of Dirofilaria immitis across European endemic countries. Parasites Vectors 2023, 16, 325. [Google Scholar] [CrossRef]
- Vicente, J.J.; de Oliveira Rodrigues, H.; Gomes, D.C.; Pinto, R.M. Nematóides do Brasil. Parte V: Nematóides de mamíferos. Rev. Bras. Zool. 1997, 14 (Suppl. 1), 1–452. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Dirofilariosis in the Americas: A more virulent Dirofilaria immitis? Parasites Vectors 2013, 6, 288. [Google Scholar] [CrossRef]
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Chapter 4: Heartworm disease in animals and humans. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 66, pp. 193–285. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Dominguez, J.M.L. The Coastal Zone of Brazil: An Overview. J. Coast. 2006, 16–20. Available online: http://www.jstor.org/stable/25741527 (accessed on 14 August 2024).
- Labarthe, N.; Serrão, M.L.; Fontenele Melo, Y.; de Oliveira, S.J.; Lourenço-de-Oliveira, R. Mosquito frequency and feeding habits in an enzootic canine dirofilariosis area in Niterói, state of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 1998, 93, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bendas, A.J.R.; Branco, A.S.; da Silva, B.R.S.A.; Paiva, J.P.; de Miranda, M.G.N.; Mendes-de-Almeida, F.; Labarthe, N.V. Mosquito abundance in a Dirofilaria immitis hotspot in the eastern state of Rio de Janeiro, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100320. [Google Scholar] [CrossRef]
- Silva-Araújo, A. Filaria immitis e a Filaria sanguinolenta no Brasil. Gaz. Med. Bahia 1878, 3, 295–312. [Google Scholar]
- Magalhães, O.S. Descrição de uma espécie de filaria encontrada no coração humano. Rev. Cursos Práticos Theor. Fac. Med. Rio Jan. 1887, 3, 129–215. [Google Scholar]
- Travassos, L. Notas Helmintolojicas. Rev. Sci. 1920, 4, 152. [Google Scholar]
- Lent, H.; de Freitas, J.F.T. Dirofilariose sub-cutanea dos cães no Brasil. Mem. Inst. Oswaldo Cruz 1937, 32, 443–448. [Google Scholar] [CrossRef][Green Version]
- Trancoso, T.A.L.; da Conceição Lima, N.; Barbosa, A.S.; Leles, D.; Fonseca, A.B.M.; Labarthe, N.V.; Bastos, O.M.P.; Uchôa, C.M.A. Detection of Dirofilaria immitis using microscopic, serological and molecular techniques among dogs in Cabo Frio, RJ, Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e017219. [Google Scholar] [CrossRef] [PubMed]
- Mendes-de-Almeida, F.; Alves, L.C.; do Amaral Fernandes, P.; de Menezes Leivas, R.; Labarthe, N. Infection with Dirofilaria immitis and Other Infections in Cats and Dogs from Rio de Janeiro, Brazil: The Need for Prophylactic Enforcement. Acta Parasitol. 2021, 66, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Milanez de Campos, J.R.; Barbas, C.S.V.; Filomeno, L.T.B.; Fernandez, A.; Minamoto, H.; Filho, J.V.B.; Jatene, F.B. Human pulmonary dirofilariosis. Chest 1997, 112, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Soares, H.S.; Camargo, L.M.A.; Gennari, S.M.; Labruna, M.B. Survey of canine tick-borne diseases in Lábrea, Brazilian Amazon: ‘accidental’ findings of Dirofilaria immitis infection. Rev. Bras. Parasitol. Vet. 2014, 23, 473–480. [Google Scholar] [CrossRef]
- Moreira, H.R.; Madeira, E.A.O.; Cunha, D.N.L.; Scofield, A.; Góes-Cavalcante, G.; Abel, I.; Guimarães, R.J.P.S.; Fernandes, J.I. Dirofilaria immitis infection in dogs in Algodoal Island, Brazilian Amazon. Pesq. Vet. Bras. 2019, 39, 510–515. [Google Scholar] [CrossRef]
- Barbosa, U.C.; Nava, A.F.D.; Ferreira Neto, J.V.; Dias, C.A.; da Silva, V.C.; de Mesquita, H.G.; de Moreira Sampaio, R.T.; Barros, W.G.; de Sousa Farias, E.; da Silva, T.R.R.; et al. Dirofilaria immitis is endemic in rural areas of the Brazilian Amazonas state capital, Manaus. Rev. Bras. Parasitol. Vet. 2023, 32, e000223. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Lima, G.R.F.; de Araújo, V.M.J.; Teixeira, G.G.; Coelho, J.M.A.; de Azevedo Farzat, F.; da Silva Oliveira, E.; Pinheiro, V.C.; da Silva Mendes, A.L.; Ramires, P.; et al. Perfil epidemiológico, hematológico e bioquímico em cães com Dirofilaria sp. no Ceará. Res. Soc. Dev. 2021, 10, e23010817252. [Google Scholar] [CrossRef]
- Lee, D.; Lopes da Silva, P.; Bezerra, T.L.; Lima, V.F.S.; Meira-Santos, P.O. Identification of zoonotic microfilariae in canine blood samples from the city of Aracaju (Sergipe, northeastern Brazil). SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Labarthe, N.V.; Pereira Paiva, J.; Reifur, L.; Mendes-de-Almeida, F.; Merlo, A.; Carvalho Pinto, C.J.; Juliani, P.S.; Ornelas de Almeida, M.A.; Câmara Alves, L. Updated canine infection rates for Dirofilaria immitis in areas of Brazil previously identified as having a high incidence of heartworm-infected dogs. Parasites Vectors 2014, 7, 493. [Google Scholar] [CrossRef]
- Willi, L.M.V.; Mendes-de-Almeida, F.; da Silva Freitas de Souza, C.; Laeta, T.; Paiva, J.P.; de Miranda, M.G.N.; Knackfuss, F.B.; Labarthe, N. Serological evidence of canine exposure to arthropod-borne pathogens in different landscapes in Rio de Janeiro, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 40–44. [Google Scholar] [CrossRef]
- Alberigi, B.; Labarthe, N.; Cardoso, F.; Cunha, C.; Almeida, C.; Souza, C.; Mendes-de-Almeida, F. Serological evidence of canine arthropod-borne infections in an ecotone area of a natural reserve at the Pantanal, Brazil. Braz. J. Vet. Med. 2019, 41, e103719. [Google Scholar] [CrossRef]
- Soares, R.L.; Franco, P.A.; da Silva Orti, K.F.M.; da Silva, A.O.; Coelho, M.L.; do Nascimento Ramos, C.A. First canine dirofilariosis report (Dirofilaria immitis) in Campo Grande, Mato Grosso do Sul, Brazil. Acta Vet. Bras. 2020, 14, 152–155. [Google Scholar] [CrossRef]
- de Argôlo, E.G.G.; Reis, T.; Fontes, D.A.T.; Gonçalves, E.C.; Giese, E.G.; de Vasconcelos Melo, F.T.; dos Santos, J.N.; Furtado, A.P. Canine filariasis in the Amazon: Species diversity and epidemiology of these emergent and neglected zoonoses. PLoS ONE 2018, 13, e0200419. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, G.M.; da Cruz, E.N.; Cunha, P.N.A.; Camargo, L.M.A. Canine heartworm disease in Porto Velho: First record, distribution map and occurrence of positive mosquitoes. Rev. Bras. Parasitol. Vet. 2013, 22, 559–564. [Google Scholar] [CrossRef]
- Gomes Zanfagnini, L.; Carvalho Bento, G.K.; Fernandes Nunes da Silva Malavazi, P.; Figueiredo Souza, S.; Duarte Pacheco, A. Primeira descrição de dirofilariose canina alóctone em Rio Branco, Acre: Relato de caso. Rev. Med. Vet. 2024, 48, 1–9. [Google Scholar] [CrossRef]
- Soares, L.A.; Matias, I.C.; Silva, S.S.; Ramos, M.E.O.; Silva, A.P.; Barretto, M.L.M.; Brasil, A.W.L.; Silva, M.L.C.R.; Galiza, G.J.N.; Maia, L.A. Parasitological, serological and molecular diagnosis of Dirofilaria immitis in dogs in Northeastern Brazil. Exp. Parasitol. 2022, 236–237, 108233. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.A.N.; de Oliveira do Rêgo, A.G.; de Farias Firmino, E.D.; do Nascimento Ramos, C.A.; de Carvalho, G.A.; Dantas-Torres, F.; Otranto, D.; Alves, L.C. Filarioids infecting dogs in northeastern Brazil. Vet. Parasitol. 2016, 226, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Figueredo, L.A.; da Silva Sales, K.G.; de Oliveira Miranda, D.E.; de Almeida Alexandre, J.L.; da Silva, Y.Y.; da Silva, L.G.; Valle, G.R.; Ribeiro, V.M.; Otranto, D.; et al. Prevalence and incidence of vector-borne pathogens in unprotected dogs in two Brazilian regions. Parasites Vectors 2020, 13, 195. [Google Scholar] [CrossRef]
- de Macedo, L.O.; Bezerra-Santos, M.A.; Filho, C.R.C.U.; da Silva Sales, K.G.; de Sousa-Paula, L.C.; da Silva, L.G.; Dantas-Torres, F.; do Nascimento Ramos, R.A.; Otranto, D. Vector-borne pathogens of zoonotic concern in dogs from a Quilombola community in northeastern Brazil. Parasitol. Res. 2022, 121, 3305–3311. [Google Scholar] [CrossRef]
- Figueredo, L.A.; da Silva Sales, K.G.; Deuster, K.; Pollmeier, M.; Otranto, D.; Dantas-Torres, F. Exposure to vector-borne pathogens in privately owned dogs living in different socioeconomic settings in Brazil. Vet. Parasitol. 2017, 243, 18–23. [Google Scholar] [CrossRef]
- da Silva, W.I.; Gomes, A.R.D.; de Francisco, M.C.; da Silva, J.M.; de Oliveira Filho, H.S.; Feitosa, T.F.; Vilela, V.L.R. Subcutaneous dirofilariosis due to Dirofilaria immitis in a dog in Brazil: First report. Rev. Bras. Parasitol. Vet. 2023, 32, e001423. [Google Scholar] [CrossRef] [PubMed]
- Moraes-da-Silva, M.d.F.C.V.; Mendes-de-Almeida, F.; Abdalla, L.; Merlo, A.; Paiva, J.P.; Labarthe, N.V. Selamectin for the prevention of canine Dirofilaria immitis infection: Field efficacy in client-owned dogs in a high-risk area. Parasites Vectors 2016, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.L.G.; Almeida, M.B.; Santos, A.C.M.; Mattos, A.V.; Oliveira, A.C.; Barros, R.S.; Campos, V.D.D.; Souza, W.N.; Balthazar, A.; Lautenschlager, M. Serological Investigation of Heartworm (Dirofilaria immitis) Infection in Military Dogs from Rio de Janeiro, Brazil. J. Anim. Vet. Adv. 2016, 6, 1332. [Google Scholar] [CrossRef]
- Gonçalves, G.P.; Xavier, S.G.; da Conceição Lima, N.; Bendas, A.J.R. Frequency of Dirofilaria immitis infection in blood donor dogs of the Rio de Janeiro state. Braz. J. Vet. Med. 2023, 45, e002223. [Google Scholar] [CrossRef]
- Alberigi, B.; Carvalho, E.; Mendes-de-Almeida, F.; Labarthe, N.; Scott, F.B. Dogs infected by Dirofilaria immitis: A threat to the health of human and non-human animals in Rio de Janeiro, Brazil. Braz. J. Vet. Med. 2023, 45, e001723. [Google Scholar] [CrossRef]
- Silva, M.S.G.; Leles, D.; Sudré, A.P.; Millar, P.R.; Uchôa, F.; Brener, B. Prevalence and Molecular Characterization of Dirofilaria immitis (Filarioidea: Onchocercidae) in Dogs from Endemic Areas of Rio De Janeiro State, Brazil. J. Parasitol. 2019, 105, 387. [Google Scholar] [CrossRef]
- de Andrade Vieira, V.M.; Martiniano, N.O.M.; da Silva, P.P.; Paulino, É.T.; do Amaral Fernandes, P.; Labarthe, N.; Gazêta, G.S.; de Moraes Neto, A.H.A. Molecular characterization of canine filarioids in a previously non-endemic area of Rio de Janeiro State, Brazil. Parasitol. Res. 2022, 121, 925–932. [Google Scholar] [CrossRef]
- de Almeida, G.L.G.; de Almeida, M.B.; Santos, A.C.M.; Ballot, S.; Vargas, Â.; de Campos, V.D.D.; de Oliveira Lemos, N.M.; de Oliveira, T.R. Serological evidence of canine vector-borne diseases caused by Anaplasma spp., Borrelia burgdorferi, Ehrlichia canis and Dirofilaria immitis in dogs from Governador Island, Rio de Janeiro, Brazil. Tradit. Mod. Vet. Med. 2023, 8, 52–58. [Google Scholar]
- de Faria Naves, J.H.; de Carvalho, P.R.; Fonseca, T.F.; Guiotoku, M.R.M. Microfilaruria por Dirofilaria immitis em um cão na cidade de Uberlândia—Minas Gerais. Pubvet 2021, 15, 1–4. [Google Scholar] [CrossRef]
- Figuerêdo Duarte Moraes, M.; de Souza Pollo, A.; Lux Hoppe, E.G. Filarids (Spirurida: Onchocercidae) in wild carnivores and domestic dogs from the Brazilian Atlantic Forest. PLoS Negl. Trop. Dis. 2022, 16, e0010213. [Google Scholar] [CrossRef]
- Sebolt, A.P.R.; Snak, A.; de Lima, F.R.; Pilati, G.V.T.; de Quadros, R.M.; Miletti, L.C.; Chryssafidis, A.L.; de Moura, A.B. Prevalence and risk factors for Dirofilaria immitis in dogs from Laguna, Santa Catarina, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2022, 29, 100697. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, A.K.; Frondana, L.; Martins, I.H.R.; Longhi, C.E.; Fialkowski, M.M.; Milczewski, V. Occurrence of filarid parasites in household and sheltered dogs in the city of Joinville—Santa Catarina, Brazil. Ciênc. Anim. Bras. 2019, 20, e-53529. [Google Scholar] [CrossRef]
- Alberigi, B.; Campos, D.R.; Branco, A.S.; Bendas, A.; Brum, R.P.; Calixto, R.; Alves, L.C.; Pinheiro Júnior, J.W.; Knackfuss, F.B.; Labarthe, N.; et al. Feline heartworm in clinical settings in a high canine prevalence area. Front. Vet. Sci. 2022, 9, 819082. [Google Scholar] [CrossRef]
- Pereira, B.B.; Bastos, B.F.; Keidel, L.; Leles, D.; Brener, B. Feline heartworm (Dirofilaria immitis) infection: First case report of serological diagnosis in Brazil, confirmed by molecular assay. An. Acad. Bras. Ciênc. 2018, 90 (Suppl. 1), 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Alberigi, B.; de Oliveira, A.C.; Vieira, G.S.R.; do Amaral Fernandes, P.; Labarthe, N.; Mendes-de-Almeida, F. Unusual feline Dirofilaria immitis infection: A case report. Rev. Bras. Parasitol. Vet. 2020, 29, e008420. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.S.; Mendes-de-Almeida, F.; Faria, M.C.F.; de Souza-Dantas, L.M.; Labarthe, N.V. Dirofilaria immitis (Leidy, 1856) no entorno de um caso felino: Um estudo sobre sua transmissão. Rev. Bras. Parasitol Vet. 2009, 18, 14–18. [Google Scholar] [CrossRef]
- Bitencourt Vidal, M.L.; Silveira, D.S.; Martins, I.V.F.; Boeloni, J.N.; de Carvalho Nunes, L. Rare case of Dioctophyme renale (Nematoda: Enoplida) and Dirofilaria sp. (Nematoda: Spirurida) in the subcutaneous tissue of a cat in Espírito Santo, Brazil. Heliyon 2021, 7, e06092. [Google Scholar] [CrossRef]
- Moraes, M.F.D.; de Souza Pollo, A.; Marques, K.C.; de Souza Góis, R.C.; Ferreira, M.B.; da Silva, A.M.; de Lucena, R.B.M.; Batista, J.S.; Filgueira, K.; Sellera, F.P.; et al. Report of Dirofilaria immitis infection with acute cardiopulmonary complications in a cat from Northeastern Brazil. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e186835. [Google Scholar] [CrossRef]
- Vezzani, D.; Carbajo, A.E. Spatial and temporal transmission risk of Dirofilaria immitis in Argentina. Int. J. Parasitol. 2006, 36, 1463–1472. [Google Scholar] [CrossRef]
- Filoni, C.; de Jesus Pena, H.F.; Gennari, S.M.; Cristo, D.S.; Torres, L.N.; Catão-Dias, J.L. Heartworm (Dirofilaria immitis) disease in a Brazilian oncilla (Leopardus tigrinus). Pesq. Vet. Bras. 2009, 29, 474–478. [Google Scholar] [CrossRef]
- Araújo, R.B.; Estradioto, L.; de Souza Coelho, M.; de Araújo, V.B.; Kusano, L.D.C.; da Silva, L.L.G. Dirofilariose pulmonar—Um atípico diagnóstico de um nódulo pulmonar. Rev. Col. Bras. Cir. 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Dogs, cats, parasites, and humans in Brazil: Opening the black box. Parasites Vectors 2014, 7, 22. [Google Scholar] [CrossRef]
- Ferrari, P.A.; Grisolia, A.; Reale, S.; Liotta, R.; Mularoni, A.; Bertani, A. A rare case of human pulmonary dirofilariosis with nodules mimicking malignancy: Approach to diagnosis and treatment. J. Cardiothorac. 2018, 13, 65. [Google Scholar] [CrossRef]
- Silva, M.J.; Costa, A.R.; Calvinho, P. Human pulmonary dirofilariosis: A pitfall in solitary pulmonary nodule. Pulmonology 2022, 28, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Sá, G.; Barradas, P.; Amorim, I.; Cardoso, L.; Mesquita, J. Correspondence: “The One Health concept applied to dirofilariosis—A zoonotic disease”. Pulmonology 2023, 29, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Cavallazzi, R.S.; Cavallazzi, A.C.; Souza, I.V.; de Deus Cardoso, J.J. Dirofilariose pulmonar humana: Relato de sete casos. J. Bras. Pneumol. 2002, 28, 100–102. [Google Scholar] [CrossRef][Green Version]
- Otranto, D.; Diniz, D.G.; Dantas-Torres, F.; Casiraghi, M.; de Almeida, I.N.; de Almeida, L.N.; dos Santos, J.N.; Furtado, A.P.; de Almeida Sobrinho, E.F.; Bain, O. Human intraocular filariasis caused by Dirofilaria sp. nematode, Brazil. Emerg. Infect. Dis. 2011, 17, 863–866. [Google Scholar] [CrossRef]
- Pereira, L.L.; Coletta, R.D.; Monteiro, L.C.; Ferreira, V.Y.N.; Leon, J.E.; Bonan, P.R.F. Dirofilariosis involving the oral cavity: Report of the first case from South America. Rev. Soc. Bras. Med. Trop. 2015, 48, 361–363. [Google Scholar] [CrossRef]
- Rodrigues-Silva, R.; de Alcantara Guerra, R.J.; de Almeida, F.B.; Machado-Silva, J.R.; de Paiva, D.D. Dirofilaríase pulmonar humana no Estado do Rio de Janeiro, Brasil: Relato de um caso. Rev. Soc. Bras. Med. Trop. 2004, 37, 56–59. [Google Scholar] [CrossRef][Green Version]
- Doltrário, A.B.; Valim, N.C.; Dellaspora, E.A.P.B.; Gaspar, G.G.; Puga, F.G.; Fabro, A.T.; Brunaldi, M.O.; Martinez, R. Human pulmonary dirofilariosis with secondary myocarditis. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180461. [Google Scholar] [CrossRef]
- Furtado, A.P.; Do Carmo, E.S.; Giese, E.G.; Vallinoto, A.C.R.; Lanfredi, R.M.; Santos, J.N. Detection of dog filariasis in Marajo Island, Brazil by classical and molecular methods. Parasitol. Res. 2009, 105, 1509–1515. [Google Scholar] [CrossRef]
- To, K.K.W.; Wong, S.S.Y.; Poon, R.W.S.; Trendell-Smith, N.J.; Ngan, A.H.Y.; Lam, J.W.K.; Tang, T.H.C.; AhChong, A.-K.; Kan, J.C.-H.; Chan, K.-H.; et al. A novel Dirofilaria species causing human and canine infections in Hong Kong. J. Clin. Microbiol. 2012, 50, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Atapattu, U.; Koehler, A.V.; Huggins, L.G.; Wiethoelter, A.; Traub, R.J.; Colella, V. Dogs are reservoir hosts of the zoonotic Dirofilaria sp. ‘hongkongensis’ and potentially of Brugia sp. Sri Lanka genotype in Sri Lanka. One Health 2023, 17, 100625. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. On the validity of “Candidatus Dirofilaria hongkongensis” and on the use of the provisional status Candidatus in zoological nomenclature. Parasites Vectors 2020, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, D.; Moré, G.; Eiras, D. Evidencias sobre una nueva especie del género Dirofilaria en perros de Neuquén, Argentina. Analecta Vet. 2017, 37, 010. [Google Scholar] [CrossRef][Green Version]
- Huggins, L.G.; Atapattu, U.; Young, N.D.; Traub, R.J.; Colella, V. Development and validation of a long-read metabarcoding platform for the detection of filarial worm pathogens of animals and humans. BMC Microbiol. 2024, 24, 28. [Google Scholar] [CrossRef]
- Jacobson, L.S. DiGangi, B.A. An accessible alternative to melarsomine: “Moxi-Doxy” for treatment of adult heartworm infection in dogs. Front. Vet. Sci. 2021, 8, 702018. [Google Scholar] [CrossRef]
- McCall, J.W. The safety-net story about macrocyclic lactone heartworm preventives: A review, an update, and recommendations. Vet. Parasitol. 2005, 133, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bendas, A.J.R. Avaliação da Associação de Moxidectina 2.5% e Imidacloprida 10% Tópica com Doxiciclina no Tratamento de cães (Canis familiaris Linnaeus, 1758) Naturalmente Infectados por Dirofilaria immitis (Leidy, 1856). Master’s Thesis, Universidade Federal Fluminense, Niterói, Brazil, 2018. [Google Scholar]
- Alberigi, B.; da Silva Freitas de Souza, C.; Fernandes, J.I.; Merlo, A.; Labarthe, N. Use of Slow-Release Injectable Moxidectin for Treatment of Dirofilaria immitis Infection During Pregnancy. Front. Vet. Sci. 2020, 6, 440. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Tasker, S.; Hartmann, K.; Belák, S.; Addie, D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Hofmann-Lehmann, R.; Hosie, M.; et al. Dirofilarioses in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2020, 22, 442–451. [Google Scholar] [CrossRef]
- de Carvalho, B.M.; Perez, L.P.; de Oliveira, B.F.A.; da Silva Viana Jacobson, L.; Horta, M.A.; Sobral, A.; de Souza Hacon, S. Vector-borne diseases in Brazil: Climate change and future warming scenarios. Sustain. Debate 2020, 11, 361–404. [Google Scholar] [CrossRef]
- Paul, M.; King, L.; Carlin, E.P. Zoonoses of people and their pets: A US perspective on significant pet-associated parasitic diseases. Trends Parasitol. 2010, 26, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Best practices for preventing vector-borne diseases in dogs and humans. Trends Parasitol. 2016, 32, 43–55. [Google Scholar] [CrossRef]
- IBGE. Rio de Janeiro. 2022. Available online: https://www.ibge.gov.br/cidades-e-estados/rj (accessed on 23 April 2024).
- Lins-de-Barros, F.M. Integrated coastal vulnerability assessment: A methodology for coastal cities management integrating socioeconomic, physical and environmental dimensions—Case study of Região dos Lagos, Rio de Janeiro, Brazil. Ocean Coast. Manag. 2017, 149, 1–11. [Google Scholar] [CrossRef]
- Perles, L.; Dantas-Torres, F.; Krücken, J.; Morchón, R.; Walochnik, J.; Otranto, D. Zoonotic dirofilariases: One, no one, or more than one parasite. Trends Parasitol. 2024, 40, 257–270. [Google Scholar] [CrossRef]
- Ferreira, M.A.M.; Leite, Y.L.R.; Junior, C.C.; Vicente, C.R. Impact of climate change on public health in Brazil. Public Health Chall. 2023, 2, e62. [Google Scholar] [CrossRef]
- Nava, A.; Shimabukuro, J.S.; Chmura, A.A.; Luz, S.L.B. The impact of global environmental changes on infectious disease emergence with a focus on risks for Brazil. ILAR J. 2017, 58, 393–400. [Google Scholar] [CrossRef]
- Nelson, C.T.; McCall, J.W.; Jones, S.; Moorhead, A. Current Canine Guidelines for the Prevention, Diagnosis, and Management of Heartworm (Dirofilaria immitis) Infection in Dogs; AHS: Wilmington, DE, USA, 2020; Available online: https://d3ft8sckhnqim2.cloudfront.net/images/pdf/AHS_Canine_Guidelines_11_13_20.pdf?1605556516 (accessed on 9 July 2024).
- Kaplan, B.; Kahn, L.H.; Monath, T.P.; Woodall, J. “ONE HEALTH” and parasitology. Parasites Vectors 2009, 2, 36. [Google Scholar] [CrossRef]
| Region | State | City | Filarial Species | Prevalence | Positives/Total | Diagnostic Method | Authors |
|---|---|---|---|---|---|---|---|
| Southeast | RJ | Rio de Janeiro | D. immitis | 0.9% | 5/556 | SNAP Feline Triple Test (IDEXX Laboratories, Westbrook, ME, USA) | [24] |
| RJ | Rio de Janeiro | D. immitis | 1.2% | 7/586 | SNAP Feline Triple Test (IDEXX Laboratories, Westbrook, ME, USA) | [54] | |
| RJ | Niterói | D. immitis | Case report | 1/1 | PCR (12S and cox1) | [55] | |
| RJ | Ilha do Governador | D. immitis | Case report | 1/1 | Blood smear, Knott’s method, SNAP Feline Triple Test (IDEXX Laboratories, Westbrook, ME, USA) and echocardiogram | [56] | |
| RJ | Rio de Janeiro | D. immitis | Case report | 1/1 | Clinical signs, radiography, and necropsy | [57] | |
| ES | Alegre | D. immitis | Case report | 1/1 | Morphological | [58] | |
| Northeast | RN | Mossoró | D. immitis | Case report | 1/1 | PCR (18s, MyoHC and hsp70) | [59] |
| Region | State | City | Animal | Filarial Species | Prevalence | Positives/Total | Diagnostic Method | Authors |
|---|---|---|---|---|---|---|---|---|
| South | PR | Parque Nacional Iguaçu | Ring-tailed coatis (Nasua nasua) | D. immitis | 10.7% | 8/75 | Knott’s method, Histochemical, Serology (Witness Dirofilaria, Zoetis®, Parsippany, NJ, USA) | [5] |
| PR | Parque Nacional Iguaçu | Ring-tailed coatis (Nasua nasua) | D. repens | 33.3% | 25/75 | Knott’s method, Histochemical | [5] | |
| PR | Parque Nacional Iguaçu | Ring-tailed coatis (Nasua nasua) | D. immitis | 1.48% | 2/135 | Knott’s method, Immunochromatography and PCR (myoHC and hsp70) | [51] | |
| Southeast | SP | Ubatuba | Brazilian little spotted cat (Leopardus tigrinus) | D. immitis | Case report | 1/1 | Clinical signs, radiography, and necropsy | [61] |
| Region | State | City | Patient | Symptoms | Location | Authors |
|---|---|---|---|---|---|---|
| North | PA | Belém | M, 16 yo | Yes | Eye | [68] |
| Northeast | PB | João Pessoa | F, 65 yo | Yes | Oral cavity | [69] |
| Southeast | RJ | Rio de Janeiro | F, 45 yo | Yes | Lung | [70] |
| SP | Ribeirão Preto * | M, 67 yo | Yes | Lung | [71] | |
| South | PR | Curitiba | F, 50 yo | Yes | Lung | [62] |
| SC | Florianópolis | M, 75 yo | No | Lung | [67] | |
| SC | Florianópolis | M, 42 yo | No | Lung | [67] | |
| SC | Florianópolis | M, 70 yo | Yes | Lung | [67] | |
| SC | Florianópolis | M, 55 yo | Yes | Lung | [67] | |
| SC | Florianópolis | M, 44 yo | Yes | Lung | [67] | |
| SC | Florianópolis | F, 69 yo | Yes | Lung | [67] | |
| SC | Florianópolis | F, 63 yo | Yes | Lung | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chocobar, M.L.E.; Schmidt, E.M.d.S.; Weir, W.; Panarese, R. The Distribution, Diversity, and Control of Dirofilariosis in Brazil: A Comprehensive Review. Animals 2024, 14, 2462. https://doi.org/10.3390/ani14172462
Chocobar MLE, Schmidt EMdS, Weir W, Panarese R. The Distribution, Diversity, and Control of Dirofilariosis in Brazil: A Comprehensive Review. Animals. 2024; 14(17):2462. https://doi.org/10.3390/ani14172462
Chicago/Turabian StyleChocobar, Marianna Laura Elis, Elizabeth Moreira dos Santos Schmidt, William Weir, and Rossella Panarese. 2024. "The Distribution, Diversity, and Control of Dirofilariosis in Brazil: A Comprehensive Review" Animals 14, no. 17: 2462. https://doi.org/10.3390/ani14172462
APA StyleChocobar, M. L. E., Schmidt, E. M. d. S., Weir, W., & Panarese, R. (2024). The Distribution, Diversity, and Control of Dirofilariosis in Brazil: A Comprehensive Review. Animals, 14(17), 2462. https://doi.org/10.3390/ani14172462










