Effects of Feeding Rates on Growth Performance and Liver Glucose Metabolism in Juvenile Largemouth Bronze Gudgeon (Coreius guichenoti)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Breeding Management
2.2. Sample Collection and Growth Index Calculation
2.3. Liver Physiological Parameters Detection
2.4. Liver Tissue Section Examination
2.5. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR Analysis
2.6. Data Statistics and Analysis
3. Results
3.1. Effects of Feeding Rates on the Growth Performance
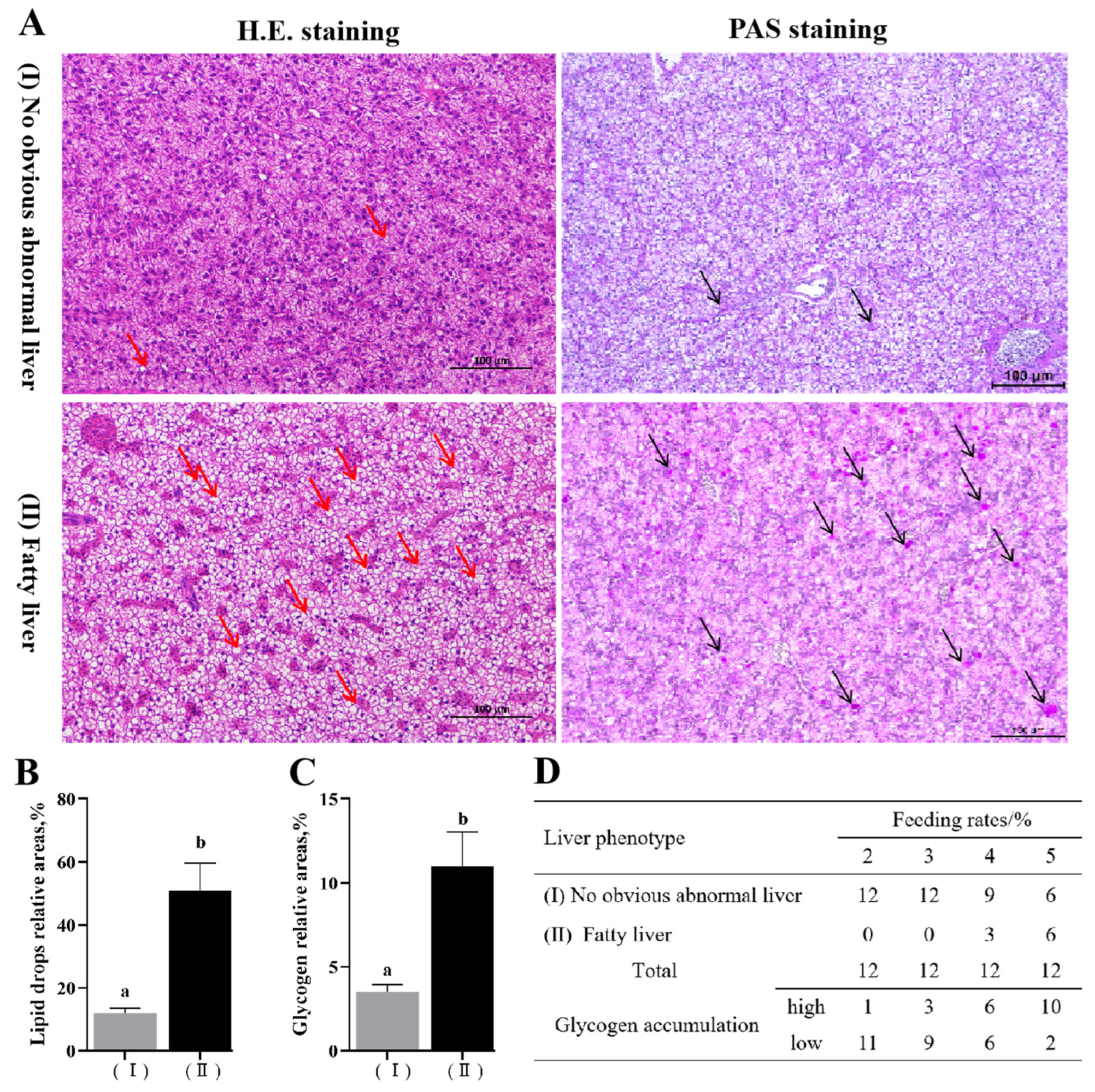
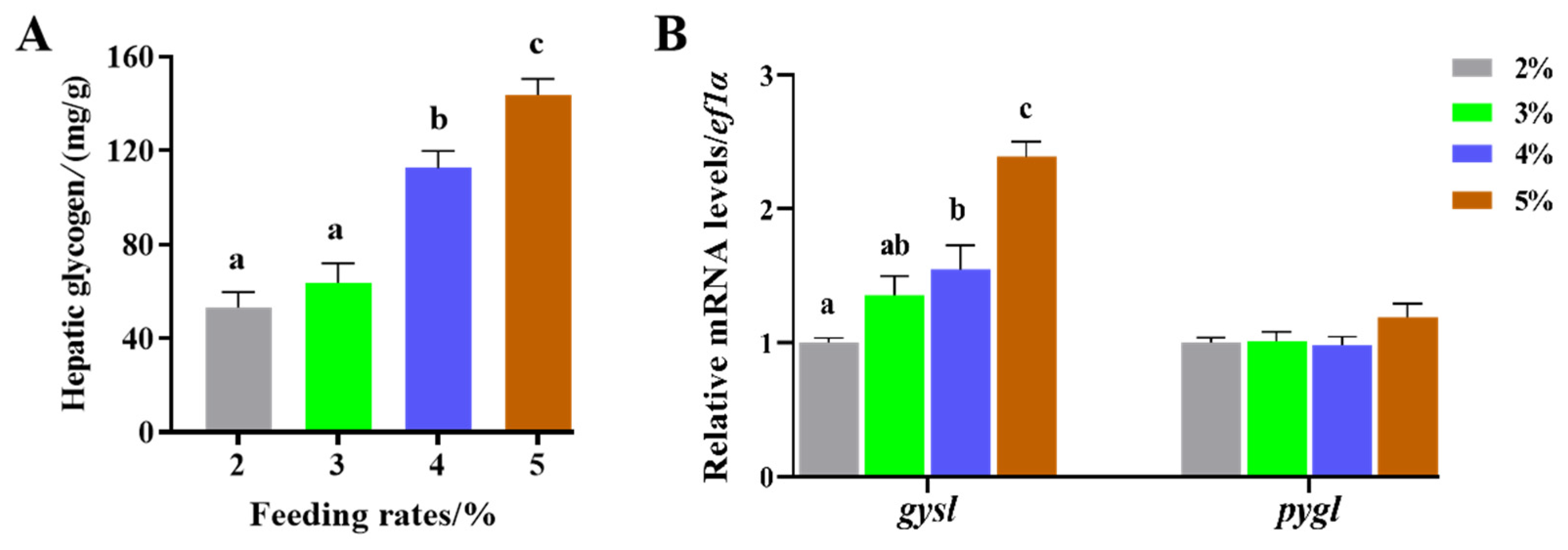
3.2. Effects of Feeding Rates on Liver Tissue Sections and Glycogen Synthesis
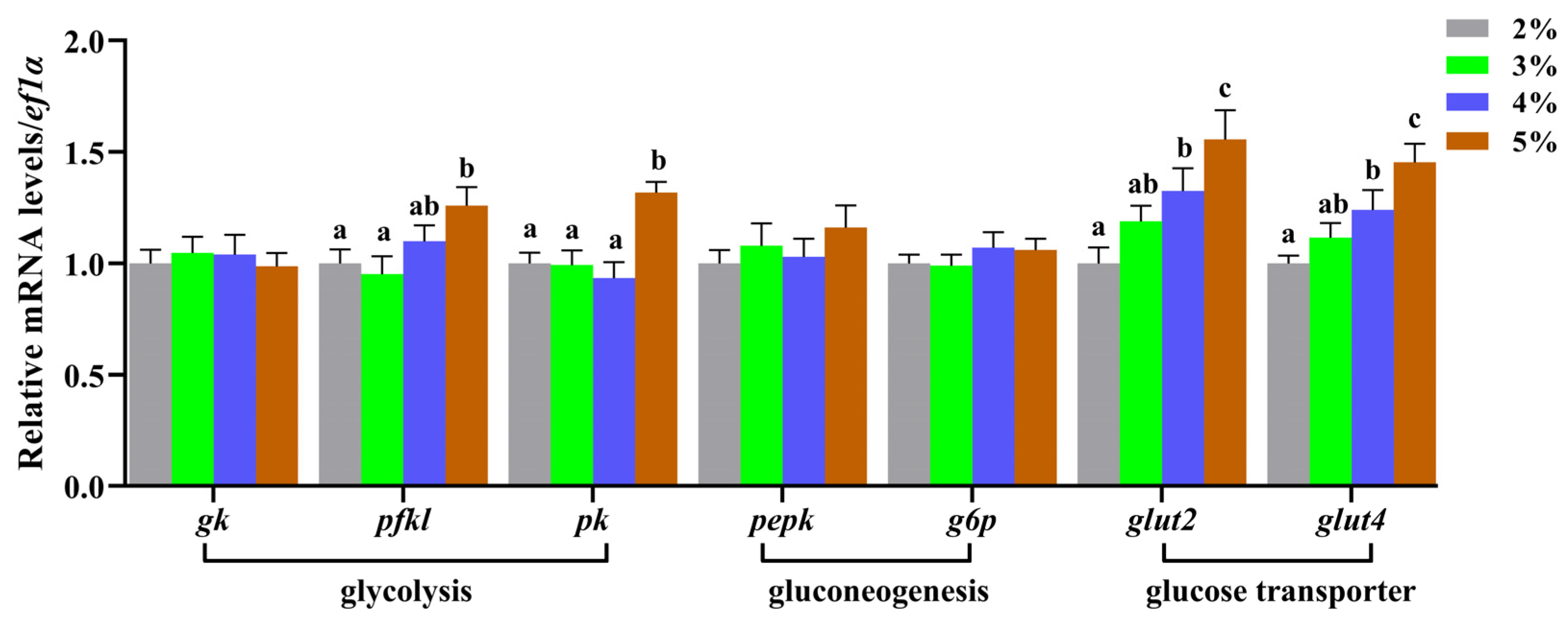
3.3. Effects of Feeding Rate on Plasma Glucose, Liver Glycolysis, and Gluconeogenesis Metabolism-Related Enzyme Activities and Gene Expression
4. Discussion
4.1. Effects of Feeding Rate on the Growth Performance
4.2. Effects of Feeding Rate on Glucose Metabolism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, Q.X.; Wang, Z.J.; Zhang, H.X. Study on the hepatopancreases of Coreius heterodon (Bleeker) and Coreius guichenoti (Sauvage et Dabry) in Yangtze River. J. Quanzhou Norm. Univ. 2001, 19, 69–74. (In Chinese) [Google Scholar]
- Jiang, W.; Liu, H.Z.; Duan, Z.H.; Cao, W.X. Seasonal variation in drifting eggs and larvae in the upper Yangtze, China. Zool. Sci. 2010, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fujiwara, M.; Zhang, W.W.; Lin, P.C.; Liu, H.Z. The impact of dams on the population viability of a migratory fish in the Yangtze River, China. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1509–1519. [Google Scholar] [CrossRef]
- Xiong, M.H.; Shao, K.; Li, W.T.; Zhu, B. Research progress on resources variation and protection of Coreius guichenoti. Yangtze River 2023, 54, 63–71. (In Chinese) [Google Scholar]
- Liao, X.; Yu, X.; Chang, J.B.; Tong, J. Polymorphic microsatellites in largemouth bronze gudgeon (Coreius guichenoti) developed from repeat-enriched libraries and cross-species amplifications. Mol. Ecol. Notes 2007, 7, 1104–1107. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhu, Y.J.; He, Y.F.; Chen, J.W.; Feng, Y.B.; Li, X.; Xiong, B.X.; Yang, D.G. Effects of Temperature Reduction and MS-222 on Water Quality and Blood Biochemistry in Simulated Transport Experiment of Largemouth Bronze Gudgeon, Coreius guichenoti. J. World Aquac. Soc. 2014, 45, 493–507. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Yang, J.; Li, Y.; Chen, P.; Qu, H.T. Feeding frequency affects liver health in largemouth bronze gudgeon Coreius guichenoti: Implications for lipid metabolism, oxidative stress, and inflammation response. Aquac. Rep. 2024, 35, 101941. [Google Scholar] [CrossRef]
- Bu, X.; Lian, X.; Zhang, Y.; Yang, C.; Cui, C.; Che, J.; Tang, B.; Su, B.; Zhou, Q.; Yang, Y. Effects of feeding rates on growth, feed utilization, and body composition of juvenile Pseudobagrus ussuriensis. Aquac. Int. 2017, 25, 1821–1831. [Google Scholar] [CrossRef]
- Liu, W.; Wen, H.; Jiang, M.; Wu, J.J.; Wu, F. Effects of feeding rate and feeding frequency on growth performance and liver health for juvenile generically improved farmed tiplapid, Oreochromis Niloticus. Freshw. Fish. 2019, 49, 84–93. (In Chinese) [Google Scholar]
- Liu, K.; He, J.Z.; Feng, P.F.; Ma, H.W.; Luo, X. Effects of feeding frequency and level on the digestive enzymes and fat metabolism enzyme in the hepatopancreas of juvenile Leiocassis longirostris. J. Yunnan Agric. Univ. (Nat. Sci.) 2019, 34, 779–784. (In Chinese) [Google Scholar]
- He, B.; Zhou, B.; Xie, H.; Hu, Z.T.; Wang, B.; Zhang, J.L.; Li, Q.; Zhao, F.; Liu, X.; Li, Q. Effect of feeding level on growth, digestive and metabolic enzymes and antioxidant capacity in juvenile Yangtze sturgeon (Acipenser dabryanus). Aquaculture 2023, 567, 739265. [Google Scholar] [CrossRef]
- Escriva, F.; Gavete, M.L.; Fermin, Y.; Pérez, C.; Carrascosa, J.M. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: Role of adiposity. J. Endocrinol. 2007, 194, 131–141. [Google Scholar] [CrossRef]
- Desai, A.S.; Singh, R.K. The effects of water temperature and ration size on growth and body composition of fry of common carp, Cyprinus carpio. J. Therm. Biol. 2009, 34, 276–280. [Google Scholar] [CrossRef]
- Greene, D.H.; Selivonchick, D.P. Lipid metabolism in fish. Prog. Lipid Res. 1987, 26, 53–85. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2006, 143, 89–96. [Google Scholar] [CrossRef]
- Wu, B.; Luo, Y.P.; Xie, X.J. Chemical composition and energy density in juvenile Coreius guichenoti. J. Southwest Univ. (Nat. Sci.) 2008, 30, 62–67. (In Chinese) [Google Scholar]
- Dong, C.; Luo, A.H.; Chen, X.J.; Wang, C.Y.; Tan, H.Y.; Yang, Z. Nutrient composition analysis of Coreius guichenoti at different ages and gonad development stages. J. Hydroecology 2022, 43, 108–115. (In Chinese) [Google Scholar]
- Liu, F.; Dang, S.G.; Wang, W.J.; Cao, W.X. Feeding habits of Coreius guichenoti (Sauvage et Dabry) in the upper Yangtze River. Acta Hydrobiol. Sin. 2012, 36, 1081–1086. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.T.; Chen, P.; Lu, X.B.; Guo, B.F.; Wen, Z.H. Effects of feeding strategy on growth, digestive enzymes, and liver structure in juvenile Coreius guichienoti. Prog. Fish. Sci. 2024, 45, 95–104. (In Chinese) [Google Scholar]
- Chen, P.; Zhao, Y.; Yang, Y.J.; Yang, J.; Qu, H.T. Effects of high feeding rate on hepatic antioxidant, immune function and lipid metabolism in juvenile largemouth bronze gudgeon (Coreius guichenoti). Chin. J. Anim. Nutr. 2023, 35, 5870–5885. (In Chinese) [Google Scholar]
- Chen, P.; Zhu, Y.P.; Wu, X.F.; Gu, X.; Xue, M.; Liang, X.F. Metabolic adaptation to high-starch diet in largemouth bass (Micropterus salmoides) was associated with the restoration of metabolic functions via inflammation, bile acid synthesis and energy metabolism. Br. J. Nutr. 2023, 129, 381–394. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, L.J.; Li, K.; Bureau, D.P. Effects of feeding frequency and ration level on growth, feed utilization and nitrogen waste output of cuneate drum (Nibea miichthioides) reared in net pens. Aquaculture 2007, 271, 350–356. [Google Scholar] [CrossRef]
- Lupatsch, I.; Santos, G.A.; Schrama, J.W.; Verreth, J.A.J. Effect of stocking density and feeding level on energy expenditure and stress responsiveness in European sea bass Dicentrarchus labrax. Aquaculture 2010, 298, 245–250. [Google Scholar] [CrossRef]
- Li, X.M.; Wu, X.B.; Gong, J.L.; Zhu, Y.J.; Yang, D.G. Comparative analysis of liver transcriptome of parent and offspring Coreius guichenoti. Acta Hydrobiol. Sin. 2020, 44, 774–780. (In Chinese) [Google Scholar]
- Chen, P.; Yang, J.; Zhao, Y.; Qu, H.T. Analysis of fatty liver phenotype in juvenile largemouth bronze gudgeon on based on transcriptome sequencing. China Feed 2024, 11, 121–128. (In Chinese) [Google Scholar]
- Liu, Y.; Wang, Y.J.; Tian, X.E.; Huang, X. Effect of ration levels on growth, feed utilization efficiency and body biochemical composition of Misgurnus anguillicaudatus. Feed Ind. 2011, 32, 26–28. (In Chinese) [Google Scholar]
- Wei, Y.L.; Wang, J.X.; Xu, H.G.; Liang, M.Q. Study on optimum feeding frequency and feeding level of juvenile Takifugu rubripes. Chin. J. Anim. Nutr. 2021, 33, 1755–1765. (In Chinese) [Google Scholar]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Wu, S.S.; Deng, Y.; Huo, H.H.; Peng, M. Research progress on glucose transporter 4 in glucose metabolism in fish: A review. Chin. J. Fish. 2023, 5, 138–147. (In Chinese) [Google Scholar]
- Zhao, N.N.; Cui, Y.T.; Wang, Z.K.; Wang, C.; Zhang, Z.H.; Deng, Z.T.; Zhao, R.Y.; Sun, J.F.; Wang, R.J.; Li, Y.Q. Effects of feeding frequency on expression of genes related to metabolism and PI3K signaling pathway in Litopenaeus vannamei. Oceanol. Et Limnol. Sin. 2022, 5, 1189–1196. (In Chinese) [Google Scholar]
- Yang, L.P.; Zhi, S.Y.; Yang, G.K.; Qin, C.B.; Yan, X.; Niu, M.M.; Zhang, W.L.; Liu, M.Y.; Zhao, M.J.; Nie, G.X. Molecular identification of glucose transporter 4: The responsiveness to starvation, glucose, insulin and glucagon on glucose transporter 4 in common carp (Cyprinus carpio L.). J. Fish Biol. 2021, 99, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Mou, M.M.; Pu, D.C.; Lin, S.M.; Chen, Y.J.; Luo, L. Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass, Micropterus Salmoides. Aquac. 2019, 498, 482–487. [Google Scholar] [CrossRef]
- Panserat, S.; Plagnes-Juan, E.; Kaushik, S. Nutritional regulation and tissue specificity of gene expression for proteins involved in hepatic glucose metabolism in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2001, 204, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Products (bp) |
|---|---|---|---|
| ef1α | TGGGTGTTGGACAAACTGAA | CAACACCACCAGCAACAATC | 190 |
| gk | GTCCCCATATCAGGGTGTCTT | CAACCGTTGTCAGAAGTCCAT | 163 |
| pk | ACTGGACACCAAAGGACCAG | GCTGGGATAATCCAACCAGA | 157 |
| pfkl | AGACTGCAGAAAGGGCAAAA | TTCTCTGCGGAAGGTCTTGT | 154 |
| pepck | ACCTGCACCTGGAATCAAAC | ACACACCATGACGCCAGTTA | 236 |
| g6p | TTCTCGTCTCTGAACCGTGAT | GAACAGTGGGAAGAGGGAAAC | 163 |
| glut2 | CAGTTGCAACACCCAGCTAA | GGGCAGACGAACTCTCACTC | 243 |
| glut4 | CCATGCCAATGATGAAGTTG | TGACAGGAGACTGTGCCATC | 193 |
| gysl | TTGCATAAATGGCCCTCTTC | CCTGCCAAAACCAACAACTT | 199 |
| pygl | TCTTTGACCAGCGTGAAGTG | CTCGGTGTAACCGGTGATCT | 153 |
| Feeding Rates (%) | ANOVA p Value | ||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | ||
| initial body weight (g) | 4.95 ± 0.12 | 5.01 ± 0.11 | 4.93 ± 0.08 | 4.98 ± 0.10 | 0.898 |
| final body weight (g) | 8.61 ± 0.26 a | 10.68 ± 0.37 b | 12.59 ± 0.43 c | 14.17 ± 0.35 d | <0.001 |
| WGR (%) | 73.94 ± 4.23 a | 113.17 ± 4.54 b | 155.38 ± 5.32 c | 184.54 ± 5.65 d | <0.001 |
| SGR (%/d) | 0.99 ± 0.08 a | 1.35 ± 0.07 b | 1.67 ± 0.08 c | 1.86 ± 0.06 d | <0.001 |
| FCR | 0.46 ± 0.02 a | 0.53 ± 0.01 b | 0.55 ± 0.02 b | 0.54 ± 0.01 b | 0.012 |
| CF (g/cm3) | 1.42 ± 0.10 | 1.49 ± 0.08 | 1.48 ± 0.08 | 1.45 ± 0.06 | 0.905 |
| SR (%) | 97.33 ± 0.67 | 98.67 ± 0.67 | 98.67 ± 1.33 | 96.67 ± 0.67 | 0.344 |
| Feeding Rates (%) | ANOVA p Value | ||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | ||
| Hematological parameters | |||||
| Glucose (mmol/L) | 4.82 ± 0.25 a | 5.54 ± 0.45 a | 6.82 ± 0.56 b | 7.21 ± 0.56 b | 0.018 |
| Hepatic parameters | |||||
| TP (g/L) | 5.01 ± 0.31 | 4.15 ± 0.24 | 4.81 ± 0.45 | 4.61 ± 0.49 | 0.449 |
| Glucose (mmol/g prot) | 3.92 ± 0.14 ab | 4.68 ± 0.35 b | 3.37 ± 0.65 ab | 3.25 ± 0.43 a | 0.101 |
| GK (U/g prot) | 0.90 ± 0.19 | 1.03 ± 0.15 | 1.35 ± 0.12 | 1.33 ± 0.19 | 0.197 |
| PFKL (U/g prot) | 5.54 ± 0.47 a | 6.68 ± 0.33 ab | 5.55 ± 0.57 a | 7.64 ± 0.96 b | 0.072 |
| PK (U/g prot) | 129.50 ± 15.22 a | 141.05 ± 5.05 a | 147.44 ± 21.32 a | 210.60 ± 26.49 b | 0.019 |
| PC (U/g prot) | 3.92 ± 0.24 | 4.43 ± 0.37 | 3.74 ± 0.35 | 4.93 ± 0.54 | 0.160 |
| PEPCK (U/g prot) | 4.83 ± 1.04 | 5.14 ± 0.35 | 5.87 ± 0.41 | 5.29 ± 0.44 | 0.666 |
| G6P (U/mg prot) | 159.45 ± 19.02 | 180.16 ± 19.21 | 199.36 ± 14.43 | 179.87 ± 21.13 | 0.543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Qu, H.; Yang, J.; Zhao, Y.; Cheng, X.; Jiang, W. Effects of Feeding Rates on Growth Performance and Liver Glucose Metabolism in Juvenile Largemouth Bronze Gudgeon (Coreius guichenoti). Animals 2024, 14, 2466. https://doi.org/10.3390/ani14172466
Chen P, Qu H, Yang J, Zhao Y, Cheng X, Jiang W. Effects of Feeding Rates on Growth Performance and Liver Glucose Metabolism in Juvenile Largemouth Bronze Gudgeon (Coreius guichenoti). Animals. 2024; 14(17):2466. https://doi.org/10.3390/ani14172466
Chicago/Turabian StyleChen, Pei, Huantao Qu, Jing Yang, Yu Zhao, Xu Cheng, and Wei Jiang. 2024. "Effects of Feeding Rates on Growth Performance and Liver Glucose Metabolism in Juvenile Largemouth Bronze Gudgeon (Coreius guichenoti)" Animals 14, no. 17: 2466. https://doi.org/10.3390/ani14172466





