Effects of Intermittent and Chronic Hypoxia on Fish Size and Nutrient Metabolism in Tiger Puffer (Takifugu rubripes)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Culture and Hypoxia Conditions Management
2.2. Fish Sampling and Indicators Calculating
2.3. Proximate Analysis of Diet and Whole Fish Compositions
2.4. Biochemical Indexes Assays
2.5. Extraction of RNA and qPCR
2.6. Fatty Acid Compositions Analysis
2.7. Statistical Analysis
3. Results
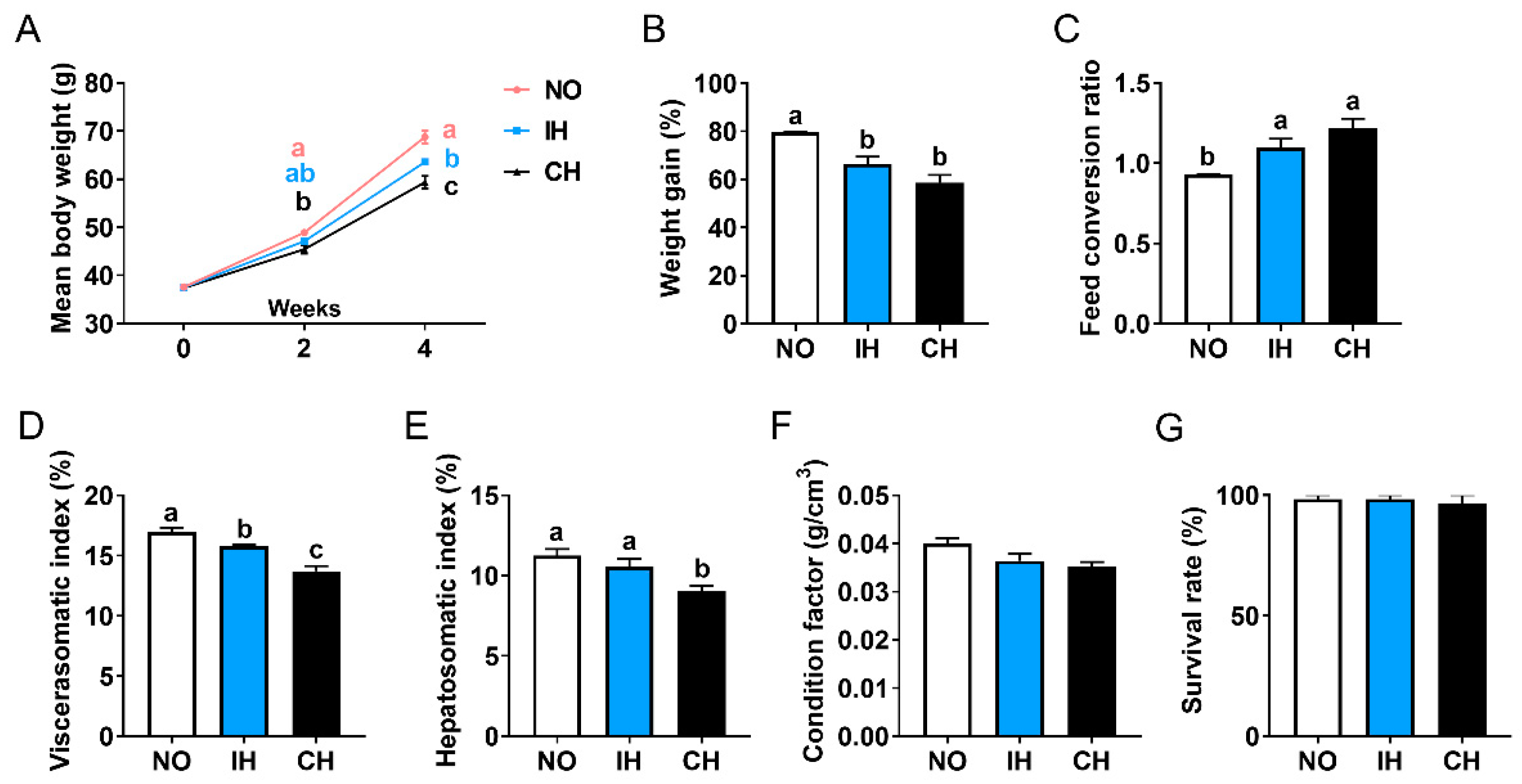
3.1. Effect of Intermittent and Chronic Hypoxia on Fish Size and Organ Weight
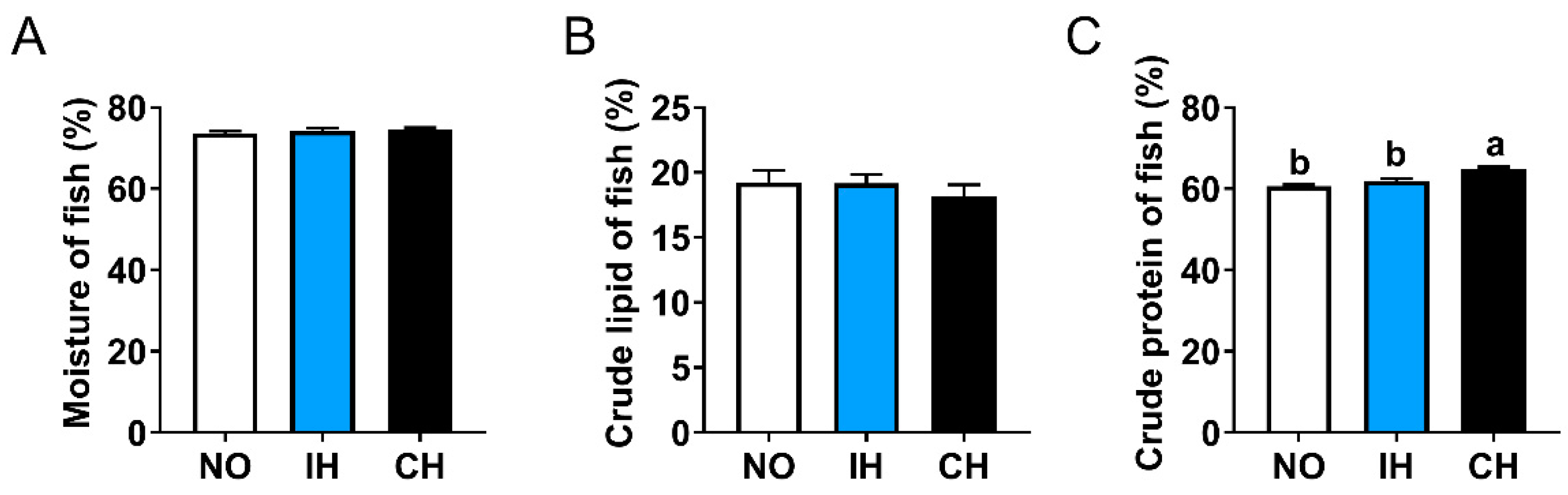
3.2. Effects of Intermittent and Chronic Hypoxia on Whole Fish Compositions
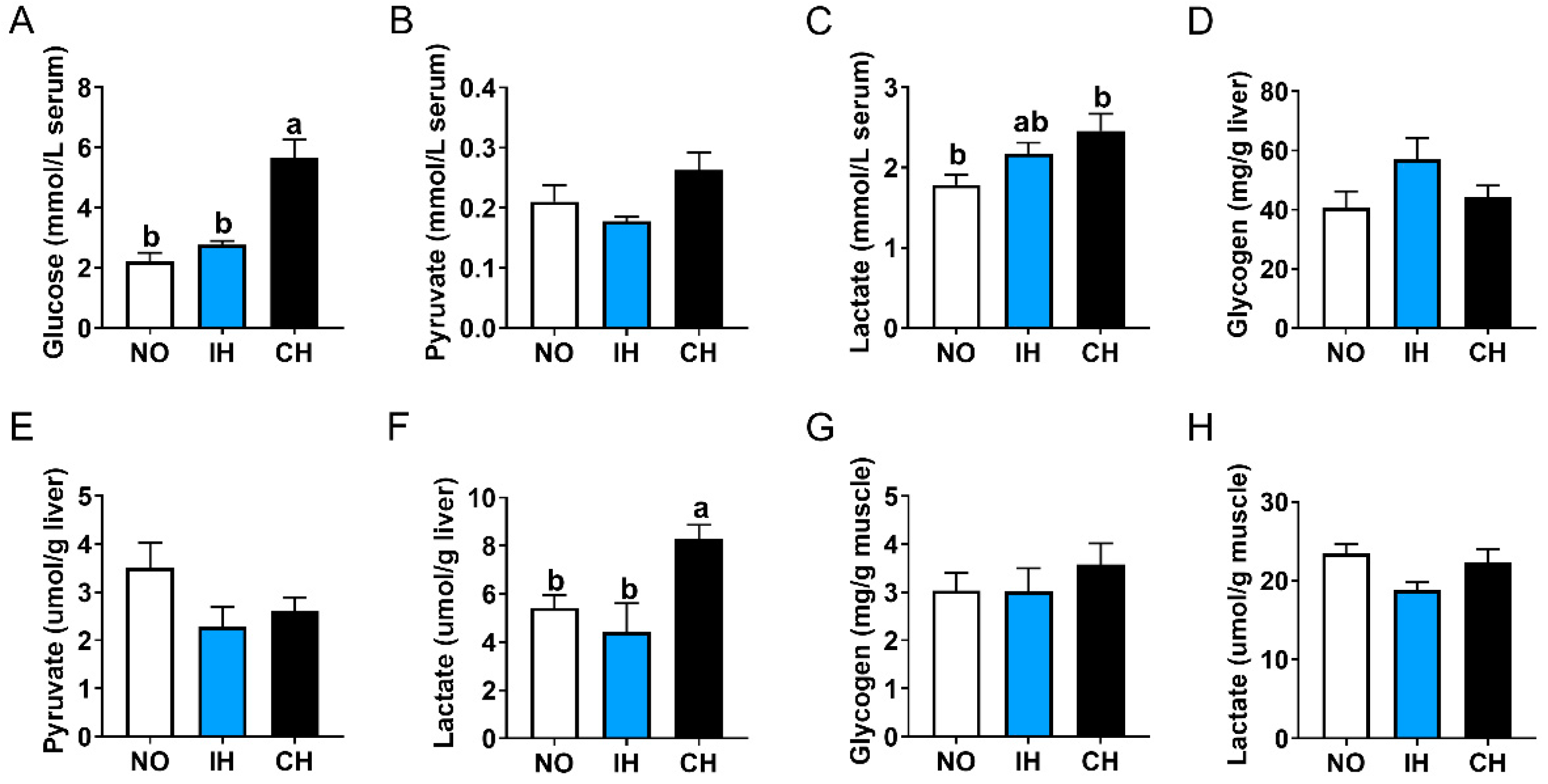
3.3. Effects of Intermittent and Chronic Hypoxia on Glucose Metabolism
3.4. Effects of Intermittent and Chronic Hypoxia on Hifα and Glycolysis Pathway
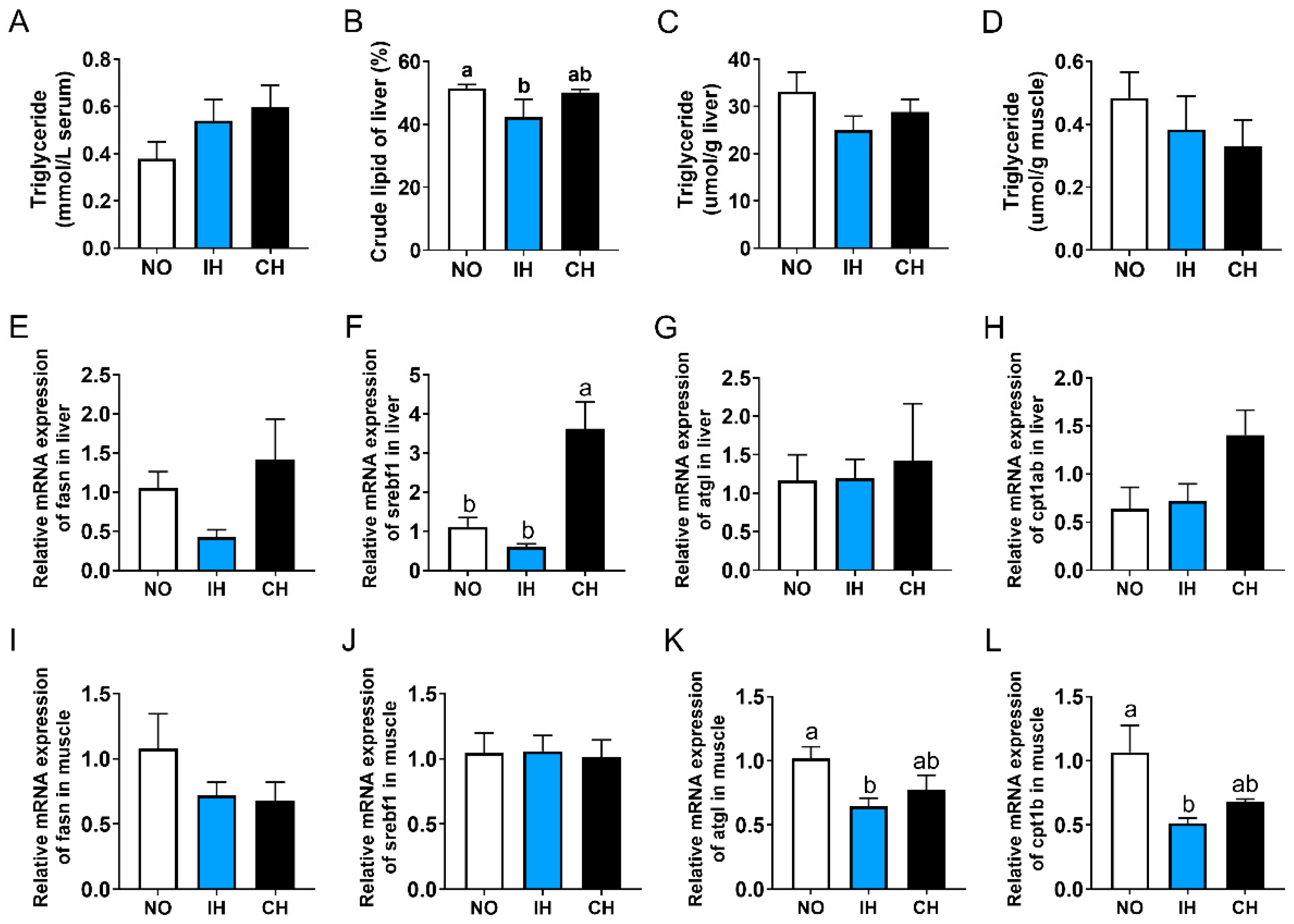
3.5. Effects of Intermittent and Chronic Hypoxia on Lipid Metabolism
3.6. Effects of Intermittent and Chronic Hypoxia on Fatty Acids Compositions
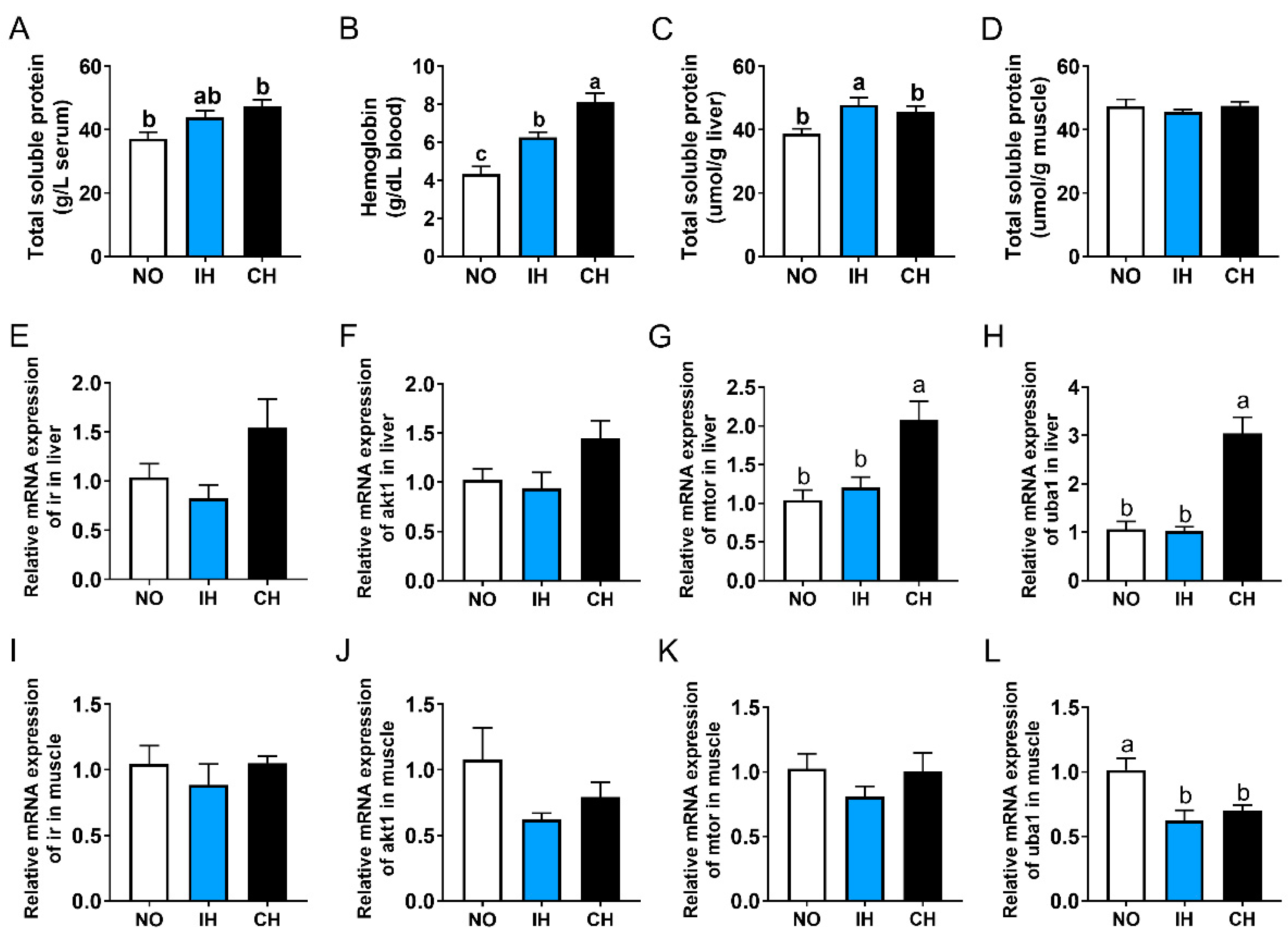
3.7. Effects of Intermittent and Chronic Hypoxia on Protein Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekau, W.; Auel, H.; Portner, H.O.; Gilbert, D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 2010, 7, 1669–1699. [Google Scholar] [CrossRef]
- Karim, M.R.; Sekine, M.; Ukita, M. Simulation of eutrophication and associated occurrence of hypoxic and anoxic condition in a coastal bay in Japan. Mar. Pollut. Bull. 2002, 45, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N.; Turner, R.E.; Wiseman, W.J. Gulf of Mexico hypoxia, A.K.A. “The dead zone”. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, C.T.; Yue, J.; Luo, Y.; Qiao, F.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. High-carbohydrate diet promotes the adaptation to acute hypoxia in zebrafish. Fish Physiol. Biochem. 2020, 46, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.; De Lázaro, O.; Cortés, P.; Oyarzún-Salazar, R.; Paschke, K.; Vargas-Chacoff, L. Hypoxia modulates the transcriptional immunological response in Oncorhynchus kisutch. Fish Shellfish Immun. 2020, 106, 1042–1051. [Google Scholar] [CrossRef]
- Phan-Van, M.; Rousseau, D.; De Pauw, N. Effects of fish bioturbation on the vertical distribution of water temperature and dissolved oxygen in a fish culture-integrated waste stabilization pond system in Vietnam. Aquaculture 2008, 281, 28–33. [Google Scholar] [CrossRef]
- Chen, X.; Wei, G.; Xie, L.; Deng, W.; Sun, Y.; Wang, Z.; Ke, T. Biological controls on diurnal variations in seawater trace element concentrations and carbonate chemistry on a coral reef. Mar. Chem. 2015, 176, 1–8. [Google Scholar] [CrossRef]
- Howarth, R.W.; Hayn, M.; Marino, R.M.; Ganju, N.; Foreman, K.; McGlathery, K.; Giblin, A.E.; Berg, P.; Walker, J.D. Metabolism of a nitrogen-enriched coastal marine lagoon during the summertime. Biogeochemistry 2013, 118, 1–20. [Google Scholar] [CrossRef]
- Gattuso, A.; Garofalo, F.; Cerra, M.C.; Imbrogno, S. Hypoxia Tolerance in Teleosts: Implications of Cardiac Nitrosative Signals. Front. Physiol. 2018, 9, 366. [Google Scholar] [CrossRef]
- Breitburg, D. Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuar. Coast. 2002, 25, 767–781. [Google Scholar] [CrossRef]
- Keeling, R.F.; Kortzinger, A.; Gruber, N. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M. Oxidative phosphorylation at the fin de siecle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Goda, N.; Kanai, M. Hypoxia-inducible factors and their roles in energy metabolism. Int. J. Hematol. 2012, 95, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Gaspar, J.M.; Velloso, L.A. Hypoxia inducible factor as a central regulator of metabolism—Implications for the development of obesity. Front. Neurosci. 2018, 12, 813. [Google Scholar] [CrossRef]
- Schwab, L.P.; Peacock, D.L.; Majumdar, D.; Ingels, J.F.; Jensen, L.C.; Smith, K.D.; Cushing, R.C.; Seagroves, T.N. Hypoxia-inducible factor 1 alpha promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res. 2012, 14, R6. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Simopoulos, C.; Turley, H.; Talks, K.; Gatter, K.C.; Harris, A.L.; Tumour Angiogenesis Res, G. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int. J. Radiat. Oncol. 2002, 53, 1192–1202. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Skarlatos, J.; Corti, L.; Blandamura, S.; Piazza, M.; Gatter, K.C.; Harris, A.L. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001, 61, 1830–1832. [Google Scholar]
- Makino, Y.; Cao, R.; Svensson, K.; Bertilsson, G.; Asman, M.; Tanaka, H.; Cao, Y.; Berkenstam, A.; Poellinger, L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 2001, 414, 550–554. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, L.; Yao, Q.; Li, Y.; Zhou, J.; Liu, Y.; Duan, C. Molecular, functional, and gene expression analysis of zebrafish hypoxia-inducible factor-3α. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2012, 303, R1165–R1174. [Google Scholar] [CrossRef]
- Zhang, P.; Yao, Q.; Lu, L.; Li, Y.; Chen, P.J.; Duan, C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014, 6, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.B.; Souza-Castro, N.; Almeida-Val, V.M.F. Acute hypoxia up-regulates HIF-1α and VEGF mRNA levels in Amazon hypoxia-tolerant Oscar (Astronotus ocellatus). Fish Physiol. Biochem. 2016, 42, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Qi, C.; Li, E.; Du, Z.; Qin, J.G.; Chen, L. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 2018, 495, 187–195. [Google Scholar] [CrossRef]
- Bergstedt, J.H.; Pfalzgraff, T.; Skov, P.V. Hypoxia tolerance and metabolic coping strategies in Oreochromis niloticus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 257, 110956. [Google Scholar] [CrossRef]
- Yang, S.; Yan, T.; Wu, H.; Xiao, Q.; Fu, H.M.; Luo, J.; Zhou, J.; Zhao, L.L.; Wang, Y.; Yang, S.Y.; et al. Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides). Fish Shellfish Immun. 2017, 67, 449–458. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; Wu, H.; Liu, Q.; Liao, L.; Luo, J.; Lian, W.Q.; Cui, C.; Jin, L.; Ma, J.D.; et al. Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides). Sci. Total Environ. 2020, 713, 135157. [Google Scholar] [CrossRef]
- Stump, E.; Ralph, G.M.; Comeros-Raynal, M.T.; Matsuura, K.; Carpenter, K.E. Global conservation status of marine pufferfishes (Tetraodontiformes: Tetraodontidae). Glob. Ecol. Conserv. 2018, 14, 10. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Phys. Part D Genom. Proteom. 2006, 1, 145–152. [Google Scholar] [CrossRef]
- Japan embraces CRISPR-edited fish. Nat. Biotechnol. 2022, 40, 10. [CrossRef]
- Guo, B.; Zou, M.; Gan, X.; He, S. Genome size evolution in pufferfish: An insight from BAC clone-based Diodon holocanthus genome sequencing. BMC Genom. 2010, 11, 396. [Google Scholar] [CrossRef]
- Ogawa, K.; Matsushita, Y.; Sawada, S.; Amimoto, T. Spatiotemporal distribution of the harmful red tide of Karenia mikimotoi and damage to cultured tiger puffer fisheries in southern Harima-nada, eastern Seto Inland Sea, Japan in the summer of 2022. Nippon Suisan Gakk. 2023, 89, 424–437. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, Y.; Ma, Z.; Wang, X.; Su, P.; Wang, H.; Liu, Y. Life cycle assessment of tiger puffer (Takifugu rubripes) farming: A case study in Dalian, China. Sci. Total Environ. 2022, 823, 153522. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, X.; Li, L.Y.; Qiao, F.; Zhang, M.L.; Du, Z.Y. High protein intake promotes the adaptation to chronic hypoxia in zebrafish (Danio rerio). Aquaculture 2021, 535, 736356. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Gregoire, M.; Chavez, F.P.; Conley, D.J.; Garcon, V.; Gilbert, D.; Gutierrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, 46. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, B.G.; McClelland, G.B.; Rees, B.B.; Scott, G.R. Distinct metabolic adjustments arise from acclimation to constant hypoxia and intermittent hypoxia in estuarine killifish (Fundulus heteroclitus). J. Exp. Biol. 2018, 221, jeb190900. [Google Scholar] [CrossRef]
- He, Y.; Yu, H.; Zhang, Z.; Zhang, J.; Kang, S.; Zhang, X. Effects of chronic hypoxia on growth performance, antioxidant capacity and protein turnover of largemouth bass (Micropterus salmoides). Aquaculture 2022, 561, 738673. [Google Scholar] [CrossRef]
- Vanderplancke, G.; Claireaux, G.; Quazuguel, P.; Huelvan, C.; Corporeau, C.; Mazurais, D.; Zambonino-Infante, J.L. Exposure to chronic moderate hypoxia impacts physiological and developmental traits of European sea bass (Dicentrarchus labrax) larvae. Fish Physiol. Biochem. 2015, 41, 233–242. [Google Scholar] [CrossRef]
- Sun, S.; Yang, M.; Fu, H.; Ge, X.; Zou, J. Altered intestinal microbiota induced by chronic hypoxia drives the effects on lipid metabolism and the immune response of oriental river prawn Macrobrachium nipponense. Aquaculture 2020, 526, 735431. [Google Scholar] [CrossRef]
- Obirikorang, K.A.; Acheampong, J.N.; Duodu, C.P.; Skov, P.V. Growth, metabolism and respiration in Nile tilapia (Oreochromis niloticus) exposed to chronic or periodic hypoxia. Comp. Biochem. Phys. Part A Mol. Integr. Physiol. 2020, 248, 110768. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.M.; Yan, G.J.; Zhang, A.J.; Cao, Z.D.; Fu, S.J. Effects of stable and diel-cycling hypoxia on hypoxia tolerance, postprandial metabolic response, and growth performance in juvenile qingbo (Spinibarbus sinensis). Aquaculture 2014, 428, 21–28. [Google Scholar] [CrossRef]
- Burt, K.; Hamoutene, D.; Perez-Casanova, J.; Gamperl, A.K.; Volkoff, H. The effect of intermittent hypoxia on growth, appetite and some aspects of the immune response of Atlantic salmon (Salmo salar). Aquac. Res. 2013, 45, 124–137. [Google Scholar] [CrossRef]
- Cai, X.; Zhou, Z.; Zhu, J.; Liao, Q.; Zhang, D.; Liu, X.; Wang, J.; Ouyang, G.; Xiao, W. Zebrafish Hif3α modulates erythropoiesis via regulation of gata1 to facilitate hypoxia tolerance. Development 2020, 147, dev185116. [Google Scholar] [CrossRef]
- Ma, Q.; Luo, Y.; Zhong, J.; Limbu, S.M.; Li, L.Y.; Chen, L.Q.; Qiao, F.; Zhang, M.L.; Lin, Q.; Du, Z.Y. Hypoxia tolerance in fish depends on catabolic preference between lipids and carbohydrates. Zool. Res. 2023, 44, 954. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Jiang, X.; Zou, S. Transcription of blunt snout bream (Megalobrama amblycephala) HIF3α and its localization in the nucleus under both normoxic and hypoxic conditions. Biochem. Biophys. Res. Commun. 2018, 500, 443–449. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, H.; Wei, Y.; Liang, M. Effects of acute hypoxia on nutrient metabolism and physiological function in turbot, Scophthalmus maximus. Fish Physiol. Biochem. 2023, 50, 367–383. [Google Scholar] [CrossRef]
- Townley, I.K.; Karchner, S.I.; Skripnikova, E.; Wiese, T.E.; Hahn, M.E.; Rees, B.B. Sequence and functional characterization of hypoxia-inducible factors, HIF1α, HIF2α a, and HIF3α, from the estuarine fish, Fundulus heteroclitus. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2017, 312, R412–R425. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, Z.; Chen, X.; Wang, Z.; Zhang, D.; Liang, L.; Mu, W. Molecular characterization and expression analysis of hypoxia-inducible factor-1 alpha, factor-2 alpha, and factor-3 alpha and physiological response to hypoxia exposure in Amur minnow (Phoxinus lagowskii). Aquacult. Int. 2022, 30, 607–632. [Google Scholar] [CrossRef]
- Garcia-Marquez, J.; Alvarez-Torres, D.; Cerezo, I.M.; Dominguez-Maqueda, M.; Figueroa, F.L.; Alarcon, F.J.; Acien, G.; Martinez-Manzanares, E.; Abdala-Diaz, R.T.; Bejar, J.; et al. Combined dietary administration of Chlorella fusca and ethanol-inactivated Vibrio proteolyticus modulates intestinal microbiota and gene expression in Chelon labrosus. Animals 2023, 13, 3325. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Rahman, M.S.; Horiguchi, T.; Thomas, P. Upregulation of hypoxia-inducible factor (HIF)-1α and HIF-2α mRNA levels in dragonet Callionymus valenciennei exposed to environmental hypoxia in Tokyo Bay. Mar. Pollut. Bull. 2012, 64, 1339–1347. [Google Scholar] [CrossRef]
- Mu, W.; Wen, H.; Li, J.; He, F. HIFs genes expression and hematology indices responses to different oxygen treatments in an ovoviviparous teleost species Sebastes schlegelii. Mar. Environ. Res. 2015, 110, 142–151. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, X.; Li, S.; Zhou, H.; Chen, W.; Ren, L.; Zhou, X.; Zheng, H.; Shi, R. Hypoxia Induces Dysregulation of Lipid Metabolism in HepG2 Cells via Activation of HIF-2α. Cell. Physiol. Biochem. 2014, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Pichavant, K.; Person-Le-Ruyet, J.; Le Bayon, N.; Severe, A.; Le Roux, A.; Boeuf, G. Comparative effects of long-term hypoxia on growth, feeding and oxygen consumption in juvenile turbot and European sea bass. J. Fish Biol. 2001, 59, 875–883. [Google Scholar] [CrossRef][Green Version]
- Borowiec, B.G.; Darcy, K.L.; Gillette, D.M.; Scott, G.R. Distinct physiological strategies are used to cope with constant hypoxia and intermittent hypoxia in killifish (Fundulus heteroclitus). J. Exp. Biol. 2015, 218, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.; Ning, Z.; Chen, Y.; Zhou, H.; Mu, W. The effects of sustained and diel-cycling hypoxia on high-latitude fish Phoxinus lagowskii. Comp. Biochem. Phys. Part D Genom. Proteom. 2023, 45, 101059. [Google Scholar] [CrossRef]
- Yao, T.; Wang, S.; Xu, K.; Zhang, X.; Zhang, T.; Wang, J.; Wang, Z.; Mu, W. Biochemical, histological, and gene expression analysis in brain and heart in Phoxinus lagowskii under sustained and diel-cycling hypoxia. J. World Aquacult. Soc. 2022, 53, 860–878. [Google Scholar] [CrossRef]

| Dietary Ingredient (% of Dry Matter) | |
|---|---|
| Fishmeal | 41 |
| Soybean protein concentrate | 25 |
| Wheat meal | 21 |
| Fish oil | 6 |
| Soybean lecithin | 1.5 |
| Vitamin premix a | 0.4 |
| Mineral premix b | 0.8 |
| Choline chloride | 0.5 |
| Butylated hydroxytoluene | 0.02 |
| Dimethyl-beta-propiothetin | 0.1 |
| Ca(H2PO4)2 | 1.5 |
| Vitamin C | 0.5 |
| Carboxymethyl cellulose | 1.68 |
| Total | 100 |
| Proximate compositions: | |
| Moisture (%) | 9.31 |
| Crude fat (%) | 10.62 |
| Crude protein (%) | 49.46 |
| Ash (%) | 8.65 |
| Gene Name | Sequences (5′ to 3′) Forward and Reverse | Product Length | GenBank NO. |
|---|---|---|---|
| hif3α/hif1αl | AAGCATCAGCATCAAACGGAG | 111 | XM_011608719.2 |
| (hypoxia-inducible factor 1-alpha-like) | GTGTGGGCGAGCTCATAAAAC | ||
| hif2α/epas1b | CACATGTGCAGAATCCCCCT | 191 | XM_011603052.2 |
| (endothelial PAS domain-containing protein 1) | ATGGGGTATGCTCTGTTGGC | ||
| hif1α | CCCCCTTCAGCTTACCAGAC | 157 | XM_003962474.3 |
| (hypoxia-inducible factor 1 subunit alpha) | CTTTTGCGTGGGGTGTTCTG | ||
| vegfa | CATATCACGATGCCGTTTGTG | 162 | XM_029849217.1 |
| (vascular endothelial growth factor A) | CACATTTGCAGGTCCGTTCG | ||
| gck | GAGGACTGTGGAACTGGTGG | 81 | XM_029829322.1 |
| (glucokinase) | TCTCCATTGTCCCCGAATGC | ||
| hk1 | GGTTGAGGACCACCATAGGC | 100 | XM_003969460.3 |
| (hexokinase 1) | ATATCCGGGACCAAACGACG | ||
| pfk | GAATGGGCATCTACGTGGGG | 140 | XM_029844357.1 |
| (ATP-dependent 6-phosphofructokinase, liver type) | ACCTATCATGGTCCCACCCT | ||
| ldha | GCGTCACCGCTAATTCCAAG | 193 | XM_003967364.3 |
| (L-lactate dehydrogenase A chain) | CAGGCCACGTAGGTCAGAAT | ||
| srebf1 | CGAGTGTGGAGCAGCCTAAA | 170 | XM_011603881.2 |
| (sterol regulatory element binding transcription factor 1) | AGGGCTCTGGGTCTGAATCT | ||
| fasn | GGAGCTGACTACAAGCTGGG | 81 | XM_011619859.2 |
| (fatty acid synthase) | CAGGAAGGTTCGGTGGTCTC | ||
| cpt1ab | CCTGATGGATGAAGAGCGGT | 111 | XM_011607269.2 |
| (carnitine O-palmitoyltransferase 1, liver isoform-like) | GAGGCCCACCAGGATTTGAG | ||
| cpt1b | TCTATCCCGCCAGTCCATCT | 115 | XM_029841851.1 |
| (carnitine O-palmitoyltransferase 1, muscle isoform) | GGCAGGTTCTCCTTCATTGC | ||
| atgl/pnpla2 | CGCCGTGGAACATTTCGTTT | 84 | XM_003967696.3 |
| (patatin-like phospholipase domain containing 2) | GCGCTTGTTCCAACAGACAG | ||
| uba1 | TTTCATTGGCGGTTTGGCTG | 156 | XM_029834111.1 |
| (ubiquitin-like modifier activating enzyme 1) | CGGCAGTTTCTAGGAGCACA | ||
| mtor | CGCCTTCCTCTCTTGTTGGT | 162 | XM_011621515.2 |
| (mechanistic target of rapamycin kinase) | AGGGGTAGAGGACCCTTGTC | ||
| ir | AGCAAGGACATCCGGAACAG | 113 | XM_003975383.3 |
| (insulin receptor) | CGGAAATCCTCTGGCTTGGT | ||
| akt1 | GGAGACGGACACGCGATATT | 139 | XM_029832808.1 |
| (AKT serine/threonine kinase 1) | ACTGGCGGAGTAGGAGAACT | ||
| gsk3β | ATCAAGGTTCTGGGCACACC | 126 | XM_029839479.1 |
| (glycogen synthase kinase-3 beta) | TGGTCGGAATACCTGCTGAC | ||
| rpl19 | GATCCCAACGAGACCAACGA | 191 | XM_003964816.3 |
| (60S ribosomal protein L19) | CGAGCATTGGCTGTACCCTT | ||
| β-actin | GGAAGATGAAATCGCCGCAC | 196 | XM_003964421.3 |
| (actin beta) | GGTCAGGATACCCCTCTTGC |
| Fatty Acid | NO | IH | CH |
|---|---|---|---|
| C14:0 | 3.68 ± 0.04 | 3.62 ± 0.09 | 3.63 ± 0.08 |
| C16:0 | 20.48 ± 0.32 | 19.34 ± 0.24 | 19.6 ± 0.46 |
| C17:0 | 0.40 ± 0.01 | 0.4 ± 0.02 | 0.41 ± 0.02 |
| C18:0 | 7.47 ± 0.20 | 7.36 ± 0.06 | 7.39 ± 0.20 |
| C20:0 | 0.26 ± 0.02 | 0.24 ± 0.01 | 0.26 ± 0.01 |
| ∑SFA | 32.29 ± 0.32 | 30.95 ± 0.29 | 31.77 ± 0.30 |
| C14:1n-5 | 0.43 ± 0.01 | 0.42 ± 0.02 | 0.42 ± 0.01 |
| C16:1n-7 | 7.89 ± 0.22 | 7.76 ± 0.07 | 7.75 ± 0.12 |
| C17:1n-7 | 0.38 ± 0.01 | 0.42 ± 0.01 | 0.4 ± 0.01 |
| C18:1n-9 | 24.71 ± 0.23 | 23.89 ± 0.32 | 24.66 ± 0.37 |
| C20:1n-9 | 1.61 ± 0.06 | 1.70 ± 0.08 | 1.56 ± 0.05 |
| C22:1n-9 | 0.16 ± 0.02 | 0.18 ± 0.01 | 0.20 ± 0.01 |
| C24:1n-9 | 0.29 ± 0.04 | 0.26 ± 0.02 | 0.30 ± 0.02 |
| ∑MUFA | 35.41 ± 0.13 a | 34.58 ± 0.27 b | 35.21 ± 0.24 ab |
| C18:2n-6 | 11.04 ± 0.18 | 11.27 ± 0.29 | 11.11 ± 0.15 |
| C18:3n-6 | 0.40 ± 0.02 | 0.39 ± 0.02 | 0.38 ± 0.03 |
| C20:2n-6 | 0.62 ± 0.02 | 0.72 ± 0.03 | 0.71 ± 0.04 |
| C20:3n-6 | 0.71 ± 0.04 | 0.65 ± 0.03 | 0.71 ± 0.04 |
| C22:2n-6 | 0.43 ± 0.02 | 0.47 ± 0.02 | 0.39 ± 0.07 |
| ∑n-6PUFA | 13.2 ± 0.16 | 13.5 ± 0.35 | 13.3 ± 0.26 |
| C20:3n-3 | 0.58 ± 0.02 b | 0.55 ± 0.02 b | 0.68 ± 0.03 a |
| C20:5n-3 | 5.50 ± 0.13 | 6.10 ± 0.13 | 5.95 ± 0.21 |
| C22:5n-3 | 4.05 ± 0.16 b | 4.63 ± 0.09 a | 4.00 ± 0.17 b |
| C22:6n-3 | 8.91 ± 0.12 b | 9.69 ± 0.09 a | 9.55 ± 0.24 ab |
| ∑n-3PUFA | 19.03 ± 0.33 b | 20.97 ± 0.23 a | 20.17 ± 0.58 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Zhang, R.; Wei, Y.; Liang, M.; Xu, H. Effects of Intermittent and Chronic Hypoxia on Fish Size and Nutrient Metabolism in Tiger Puffer (Takifugu rubripes). Animals 2024, 14, 2470. https://doi.org/10.3390/ani14172470
Ma Q, Zhang R, Wei Y, Liang M, Xu H. Effects of Intermittent and Chronic Hypoxia on Fish Size and Nutrient Metabolism in Tiger Puffer (Takifugu rubripes). Animals. 2024; 14(17):2470. https://doi.org/10.3390/ani14172470
Chicago/Turabian StyleMa, Qiang, Renxiao Zhang, Yuliang Wei, Mengqing Liang, and Houguo Xu. 2024. "Effects of Intermittent and Chronic Hypoxia on Fish Size and Nutrient Metabolism in Tiger Puffer (Takifugu rubripes)" Animals 14, no. 17: 2470. https://doi.org/10.3390/ani14172470
APA StyleMa, Q., Zhang, R., Wei, Y., Liang, M., & Xu, H. (2024). Effects of Intermittent and Chronic Hypoxia on Fish Size and Nutrient Metabolism in Tiger Puffer (Takifugu rubripes). Animals, 14(17), 2470. https://doi.org/10.3390/ani14172470










