The Influence of High-Concentrate Diet Supplemented with Tannin on Growth Performance, Rumen Fermentation, and Antioxidant Ability of Fattening Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Feeding Management
2.2. Determination of Growth Performance
2.3. Sample Collection of Blood, Ruminal Fluid, and Feces
2.4. Analysis of Rumen Fermentation Characteristics and Serum Indexes
2.5. Analysis of Apparent Digestibility and Microbial Count
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Ruminal Fermentation
3.3. Nutrient Digestibility
3.4. Biochemical Index of Serum
3.5. Immunoglobulin and Cytokine of Serum
3.6. Antioxidant Parameter of Serum
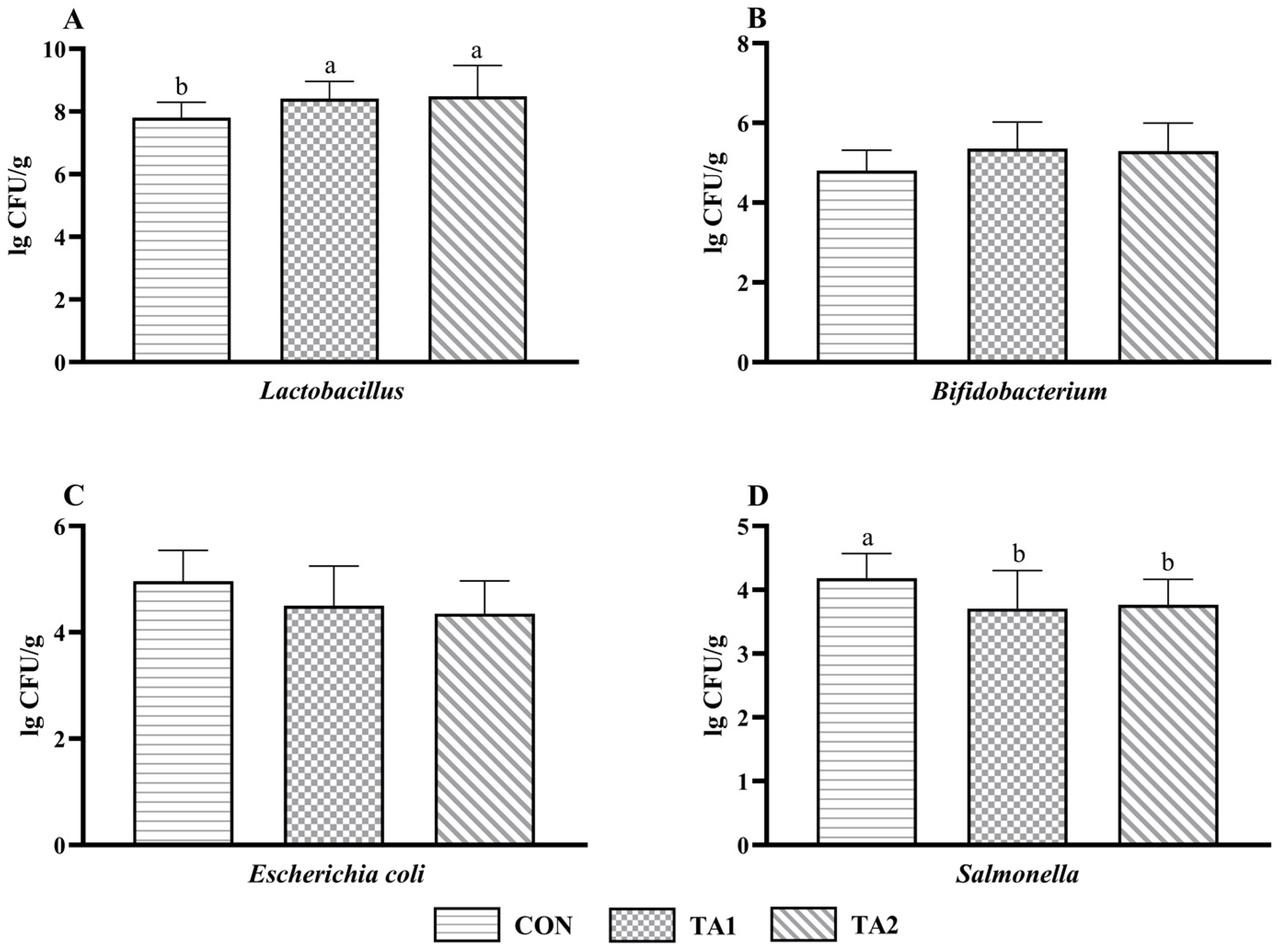
3.7. Microbial Count of Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanišić, N.; Ružić-Muslić, D.; Maksimović, N.; Cekić, B.; Caro, P.V.; Ćosić, I.; Lazarević, M. Assessing the impact of sustainable pasture systems on lamb meat quality. Processes 2024, 12, 1532. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, L.; Zhao, M.; Zhang, X.; Chen, J.; Zhang, Z.; Cheng, X.; Ren, C. Feeding regimens affecting carcass and quality attributes of sheep and goat meat—A comprehensive review. Anim. Biosci. 2023, 36, 1314–1326. [Google Scholar] [CrossRef]
- Ke, T.; Zhao, M.; Zhang, X.; Cheng, Y.; Sun, Y.; Wang, P.; Ren, C.; Cheng, X.; Zhang, Z.; Huang, Y. Review of feeding systems affecting production, carcass attributes, and meat quality of ovine and caprine species. Life 2023, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, W.; Wang, P.; Zhao, J.; Hao, X.; Zhang, J. Effects of high-concentrate diet supplemented with grape seed proanthocyanidins on growth performance, liver function, meat quality, and antioxidant activity in finishing lambs. Anim. Feed Sci. Technol. 2020, 266, 114518. [Google Scholar] [CrossRef]
- Ma, N.; Abaker, J.A.; Wei, G.; Chen, H.; Shen, X.; Chang, G. A high-concentrate diet induces an inflammatory response and oxidative stress and depresses milk fat synthesis in the mammary gland of dairy cows. J. Dairy Sci. 2022, 105, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, J.; Urrutia, O.; Lobón, S.; Ripoll, G.; Bertolín, J.R.; Joy, M. Insights into the role of major bioactive dietary nutrients in lamb meat quality: A review. J. Anim. Sci. Biotechnol. 2022, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fan, X.; Zhang, W.; Zhou, G.; Yin, F.; Zhao, Z.; Gan, S. Supplementation of grape seed extract improves the gastrointestinal development of weaned beef calves. Anim. Feed Sci. Technol. 2023, 305, 115788. [Google Scholar] [CrossRef]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Ren, S.; Shen, Y.; Chen, P.; Cui, Q.; Cao, Y.; Li, Q.; Xu, H.; Sun, F.; et al. Effects of quebracho–chestnut tannin extract supplementation on production performance, nitrogen partitioning, and rumen fermentation patterns in early-lactating Holstein cows. Anim. Feed Sci. Technol. 2024, 315, 116043. [Google Scholar] [CrossRef]
- Urrutia, I.; Maresca, S.; López-Valiente, S.O.; Canton, G.; Gomez, C.; Cabral, C.; Rodriguez, A.M.; Lorenzo, N.D. Effect of supplementation with tannins and saponins on animal performance of Holstein calves. J. Anim. Sci. 2023, 101, 605–606. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.; Mingbin, L.; Zhao, J.; Xiong, B. Effects of chestnut tannins on the meat quality, welfare, and antioxidant status of heat-stressed lambs. Meat Sci. 2016, 116, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Lv, L.; Liu, Y.; Chen, H.; Yang, H. Supplementation of feedlot lambs with magnesium oxide and sodium bicarbonate: Effects on performance, nutrient digestibility, rumen environment, serum biochemistry and antioxidant indices. Anim. Feed Sci. Technol. 2024, 311, 115951. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, M.; Ren, A.; Mao, H.; Long, D.; Yang, L. Effects of high-concentrate-induced SARA on antioxidant capacity, immune levels and rumen microbiota and function in goats. Animals 2024, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Akpensuen, T.T.; Wolffram, S.; Salminen, J.P.; Taube, F.; Blank, R.; Klub, C.; Malisch, C.S. Investigating the efficacy of purified tannin extracts from underutilized temperate forages in reducing enteric methane emissions in vitro. Sci. Rep. 2024, 14, 12578. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin-Valls, J.; Álvarez-Rodríguez, J.; Martín-Alonso, M.J.; Ramírez, G.A.; Baila, C.; Lobon, S.; Joy, M.; Serrano-Pérez, B. Effect of maternal dietary condensed tannins from sainfoin (Onobrychis viciifolia) on gut health and antioxidant-immune crosstalk in suckling lambs. Agriculture 2022, 12, 1694. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists Official Methods of Analysis; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Alimirzaei, M.; Alijoo, Y.A.; Dehghan-Banadaky, M.; Eslamizad, M. The effects of feeding high or low milk levels in early life on growth performance, fecal microbial count and metabolic and inflammatory status of Holstein female calves. Animal 2020, 14, 303–311. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. JoVE-J. Vis. Exp. 2012, 63, 3064. [Google Scholar]
- Seyedin, S.M.V.; Ghavipanje, N.; Mojtahedi, M.; Farhangfar, S.H.; Vargas-Bello-Pérez, E. Inclusion of Berberis vulgaris leaf in the diet of fattening lambs: Effects on performance, nutrient intake, rumen fermentation, and carcass traits. J. Anim. Sci. 2023, 101, 131. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Zhang, H.; Wang, H.R.; Elmhadi, M. Thiamine alleviates high-concentrate-diet-induced oxidative stress, apoptosis, and protects the rumen epithelial barrier function in goats. Front. Vet. Sci. 2021, 8, 663698. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Filho, J.P.; Menezes, D.; Caldas, A.C.; Cavalcante, I.; Oliveira, J.; Oliveira, R.; Júnior, J.S.; Cézar, M.; Bezerra, L. Carcass and meat quality in lambs receiving natural tannins from Mimosa tenuiflora hay. Small Rumin. Res. 2021, 198, 106362. [Google Scholar] [CrossRef]
- Carvalho, P.H.V.; Latack, B.C.; Ferraz, M.V.C.; Nolasco, L.J.R.P.; Meireles, W.R.; Oliveira, H.O.M.; Zinn, R.A. Influence of low-level tannin supplementation on comparative growth performance of Holstein and Angus × Holstein cross calf-fed concentrate-based finishing diets for 328d. J. Anim. Sci. 2024, 102, 087. [Google Scholar] [CrossRef] [PubMed]
- Andersone, A.; Janceva, S.; Lauberte, L.; Ramata-Stunda, A.; Nikolajeva, V.; Zaharova, N.; Rieksts, G.; Telysheva, G. Anti-Inflammatory, anti-bacterial, and anti-fungal activity of oligomeric proanthocyanidins and extracts obtained from lignocellulosic agricultural wast. Molecules 2023, 28, 863. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Xing, Y.; Wang, J.; Bai, L.; Xie, Y.; Zhu, S.; Sun, M.; Yang, J.; Li, D.; Liu, Y. Effects of Caragana korshinskii tannin on fermentation, methane emission, community of methanogens, and metabolome of rumen in sheep. Front. Microbiol. 2024, 15, 1334045. [Google Scholar]
- Pérez-Ruchel, A.; Britos, A.; Alvarado, A.; Fernández-Ciganda, S.; Gadeyne, F.; Bustos, M.; Zunino, P.; Cajarville, C. Impact of adding tannins or medium-chain fatty acids in a dairy cow diet on variables of in vitro fermentation using a rumen simulation technique (RUSITEC) system. Anim. Feed Sci. Technol. 2023, 305, 115763. [Google Scholar] [CrossRef]
- Gao, C.; Qi, M.; Zhou, Y. Chestnut tannin extract modulates growth performance and fatty acid composition in finishing Tan lambs by regulating blood antioxidant capacity, rumen fermentation, and biohydrogenation. BMC Vet. Res. 2024, 20, 23. [Google Scholar] [CrossRef]
- Aschenbacah, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Caldas, A.C.; Filho, J.P.; Menezes, D.; Cavalcante, I.; Fernandes, J.; Oliveira, J.; Oliveira, R.; Moura, J.F.; Bezerra, L. Tannins from Mimosa tenuiflora in the diet improves nutrient utilisation, animal performance, carcass traits and commercial cuts of lambs. Anim. Prod. Sci. 2021, 61, 1373–1384. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Lan, X.; He, J.; Wan, F.; Shen, W.; Tang, S.; Zhou, C.; Tan, Z.; Yang, Y. Tannic acid supplementation in the diet of Holstein bulls: Impacts on production performance, physiological and immunological characteristics, and ruminal microbiota. Front. Nutr. 2022, 9, 1066074. [Google Scholar] [CrossRef] [PubMed]
- Rira, M.; Morgavi, D.P.; Popova, M.; Maxin, G.; Doreau, M. Microbial colonisation of tannin-rich tropical plants: Interplay between degradability, methane production and tannin disappearance in the rumen. Animal 2022, 16, 100589. [Google Scholar] [CrossRef] [PubMed]
- Juráček, M.; Vašeková, P.; Massányi, P.; Kováčik, A.; Bíro, D.; Šimko, M.; Gálik, B.; Rolinec, M.; Hanušovský, O.; Kolláthová, R.; et al. The effect of dried grape pomace feeding on nutrients digestibility and serum biochemical profile of wethers. Agriculture 2021, 11, 1194. [Google Scholar] [CrossRef]
- Xie, F.; Yang, W.; Xing, M.; Zhang, H.; Ai, L. Natural polyphenols-gut microbiota interactions and effects on glycolipid metabolism via polyphenols-gut-brain axis: A state-of-the-art review. Trends Food Sci. Technol. 2023, 140, 104171. [Google Scholar] [CrossRef]
- Fonseca, N.V.B.; Cardoso, A.S.; Granja-Salcedo, Y.T.; Siniscalchi, D.; Camargo, K.D.V.; Dornellas, I.A.; Silva, M.L.C.; Vecchio, L.S.D.; Grizotto, R.K.; Reis, R.A. Effects of condensed tannin-enriched alternative energy feedstuff supplementation on performance, nitrogen utilization, and rumen microbial diversity in grazing beef cattle. Livest. Sci. 2024, 287, 105529. [Google Scholar] [CrossRef]
- Nuamah, E.; Poaty Ditengou, J.I.C.; Hirwa, F.; Cheon, I.; Chae, B.; Choi, N.-J. Dietary supplementation of tannins: Effect on growth performance, serum antioxidant capacity, and immunoglobins of weaned piglets—A systematic review with meta-analysis. Antioxidants 2024, 13, 236. [Google Scholar] [CrossRef]
- Hong, M.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, P.; Zhang, X. A natural plant source-tea polyphenols, a potential drug for improving immunity and combating virus. Nutrients 2022, 14, 550. [Google Scholar] [CrossRef]
- Chen, M.; Xie, W.; Zhou, S.; Ma, N.; Wang, Y.; Huang, J.; Shen, X.; Chang, G. A high-concentrate diet induces colonic inflammation and barrier damage in Hu sheep. J. Dairy Sci. 2023, 106, 9644–9662. [Google Scholar] [CrossRef]
- Song, D.; Zhao, J.; Deng, W.; Liao, Y.; Hong, X.; Hou, J. Tannic acid inhibits NLRP3 inflammasome-mediated IL-1β production via blocking NF-κB signaling in macrophages. Biochem. Biophys. Res. Commun. 2018, 503, 3078–3085. [Google Scholar] [CrossRef]
- Shah, T.; Malhi, M.; Kachiwal, A.B.; Bhutto, B.; Shah, Q.A.; Lei, Y.; Soomro, S.A.; Soomro, J.; Kalhoro, N.H.; Gui, H. Ameliorative effects of supranutritional selenium on TLR-4-NF-κB-TNF-α-mediated hepatic oxidative injury and inflammation in goats fed high concentrate diet. Food Sci. Nutr. 2022, 10, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed tannins as antioxidants in ruminants—Effectiveness and action mechanisms to improve animal antioxidant status and oxidative stability of products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef]
- Lin, L.; Lai, Z.; Zhang, J.; Zhu, W.; Mao, S. The gastrointestinal microbiome in dairy cattle is constrained by the deterministic driver of the region and the modified effect of diet. Microbiome 2023, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Batchu, P.; Hazard, T.; Lee, J.H.; Terrill, T.H.; Kouakou, B.; Kannan, G. High-condensed tannin diet and transportation stress in goats: Effects on physiological responses, gut microbial counts and meat quality. Animals 2021, 11, 2857. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biot. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]


| Ingredients, % | |
|---|---|
| Alfalfa hay | 22.36 |
| Corn straw | 7.64 |
| Corn | 40.87 |
| Wheat bran | 10.64 |
| Soybean meal | 8.37 |
| Cottonseed meal | 7.45 |
| NaCl | 0.50 |
| Limestone | 0.31 |
| NaHCO3 | 0.96 |
| CaHPO4 | 0.40 |
| Premix 1 | 0.50 |
| Nutrients levels | |
| DM (%) | 91.12 |
| NDF (%) | 29.32 |
| ADF (%) | 15.60 |
| CP (%) | 16.20 |
| EE (%) | 3.26 |
| Starch (%) | 28.16 |
| Ash (%) | 5.30 |
| Ca (%) | 0.84 |
| P (%) | 0.49 |
| ME 2 (MJ/kg) | 9.94 |
| Items | Groups | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | TA1 | TA2 | |||
| Initial BW (kg) | 17.40 | 17.36 | 17.53 | 0.117 | 0.841 |
| Final BW (kg) | 34.46 | 35.96 | 35.11 | 0.275 | 0.078 |
| ADG (g/d) | 284.31 b | 310.01 a | 293.06 ab | 4.319 | 0.042 |
| DMI (kg/d) | 1.33 | 1.30 | 1.34 | 0.021 | 0.753 |
| Feed efficiency | 4.69 a | 4.20 b | 4.59 a | 0.079 | 0.021 |
| Items | Groups | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | TA1 | TA2 | |||
| ALP (U/L) | 259.35 | 258.89 | 248.20 | 7.194 | 0.784 |
| ALT (U/L) | 16.97 | 17.36 | 17.72 | 0.347 | 0.689 |
| AST (U/L) | 86.66 | 88.65 | 86.89 | 1.559 | 0.857 |
| TP (g/L) | 56.39 | 59.56 | 56.74 | 1.056 | 0.418 |
| GLB (g/L) | 27.97 | 25.88 | 26.36 | 0.702 | 0.456 |
| ALB (g/L) | 28.42 | 33.67 | 30.38 | 1.330 | 0.272 |
| TG (mmol/L) | 0.374 b | 0.428 a | 0.419 a | 0.007 | 0.004 |
| NEFA (mmol/L) | 0.169 | 0.155 | 0.158 | 0.007 | 0.696 |
| GLU (mmol/L) | 4.02 | 4.05 | 3.89 | 0.082 | 0.732 |
| UN (mmol/L) | 3.51 a | 3.11 b | 3.03 b | 0.064 | 0.003 |
| Items | Groups | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | TA1 | TA2 | |||
| IgM (mg/mL) | 4.50 | 4.52 | 4.61 | 0.159 | 0.961 |
| IgA (mg/mL) | 23.29 b | 27.42 a | 25.70 ab | 0.592 | 0.013 |
| IgG (μg/mL) | 51.17 | 55.53 | 54.22 | 0.796 | 0.068 |
| IL-1β (ng/L) | 89.65 a | 83.60 b | 84.93 ab | 1.027 | 0.036 |
| IL-6 (ng/L) | 140.75 | 140.69 | 145.26 | 2.803 | 0.758 |
| IL-10 (ng/L) | 70.19 | 75.99 | 77.74 | 1.382 | 0.061 |
| TNF-α (ng/L) | 505.77 | 486.09 | 489.89 | 3.740 | 0.071 |
| Items | Groups | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | TA1 | TA2 | |||
| CAT (U/mL) | 45.82 b | 50.29 a | 48.58 ab | 0.652 | 0.014 |
| GSH-Px (U/mL) | 138.98 | 147.08 | 150.57 | 2.167 | 0.078 |
| SOD (U/mL) | 70.63 | 69.27 | 67.83 | 1.070 | 0.579 |
| T-AOC (U/mL) | 15.33 b | 19.11 a | 19.38 a | 0.531 | 0.001 |
| MDA (mmol/mL) | 4.44 a | 3.65 b | 3.63 b | 0.141 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Lu, Y.; Wang, W.; Luo, W.; Li, T.; Cao, G.; Du, C.; Wei, C.; Yin, F.; Gan, S.; et al. The Influence of High-Concentrate Diet Supplemented with Tannin on Growth Performance, Rumen Fermentation, and Antioxidant Ability of Fattening Lambs. Animals 2024, 14, 2471. https://doi.org/10.3390/ani14172471
Lin L, Lu Y, Wang W, Luo W, Li T, Cao G, Du C, Wei C, Yin F, Gan S, et al. The Influence of High-Concentrate Diet Supplemented with Tannin on Growth Performance, Rumen Fermentation, and Antioxidant Ability of Fattening Lambs. Animals. 2024; 14(17):2471. https://doi.org/10.3390/ani14172471
Chicago/Turabian StyleLin, Lu, Yuezhang Lu, Weiqian Wang, Wenjun Luo, Tao Li, Guang Cao, Chunmei Du, Chen Wei, Fuquan Yin, Shangquan Gan, and et al. 2024. "The Influence of High-Concentrate Diet Supplemented with Tannin on Growth Performance, Rumen Fermentation, and Antioxidant Ability of Fattening Lambs" Animals 14, no. 17: 2471. https://doi.org/10.3390/ani14172471
APA StyleLin, L., Lu, Y., Wang, W., Luo, W., Li, T., Cao, G., Du, C., Wei, C., Yin, F., Gan, S., & Ma, J. (2024). The Influence of High-Concentrate Diet Supplemented with Tannin on Growth Performance, Rumen Fermentation, and Antioxidant Ability of Fattening Lambs. Animals, 14(17), 2471. https://doi.org/10.3390/ani14172471






