The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview
Abstract
:Simple Summary
Abstract
1. Introduction
2. Extended-Spectrum β-Lactamase-Producing E. coli
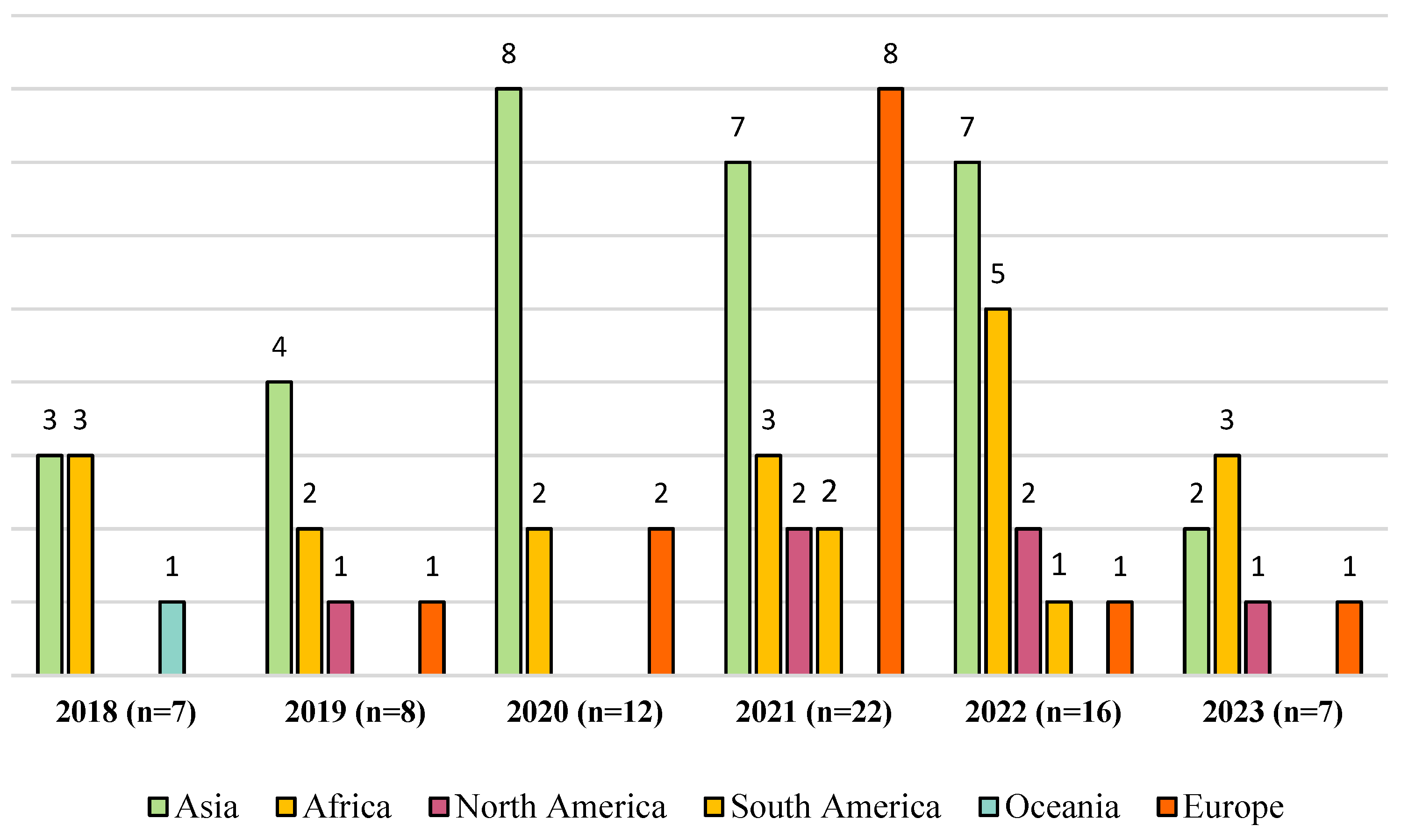
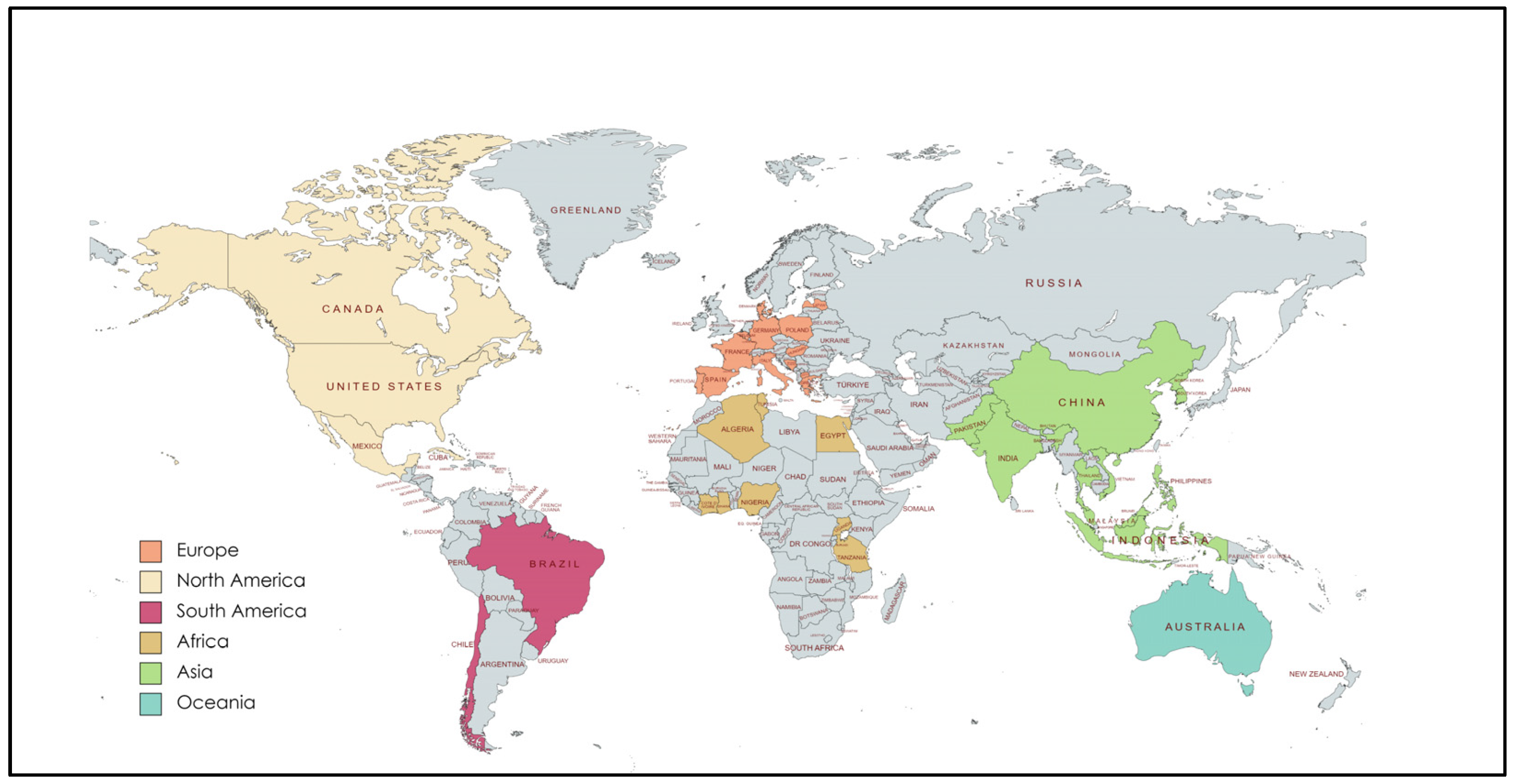
3. Publications Analyzed
4. Antibiotic Resistance in Livestock
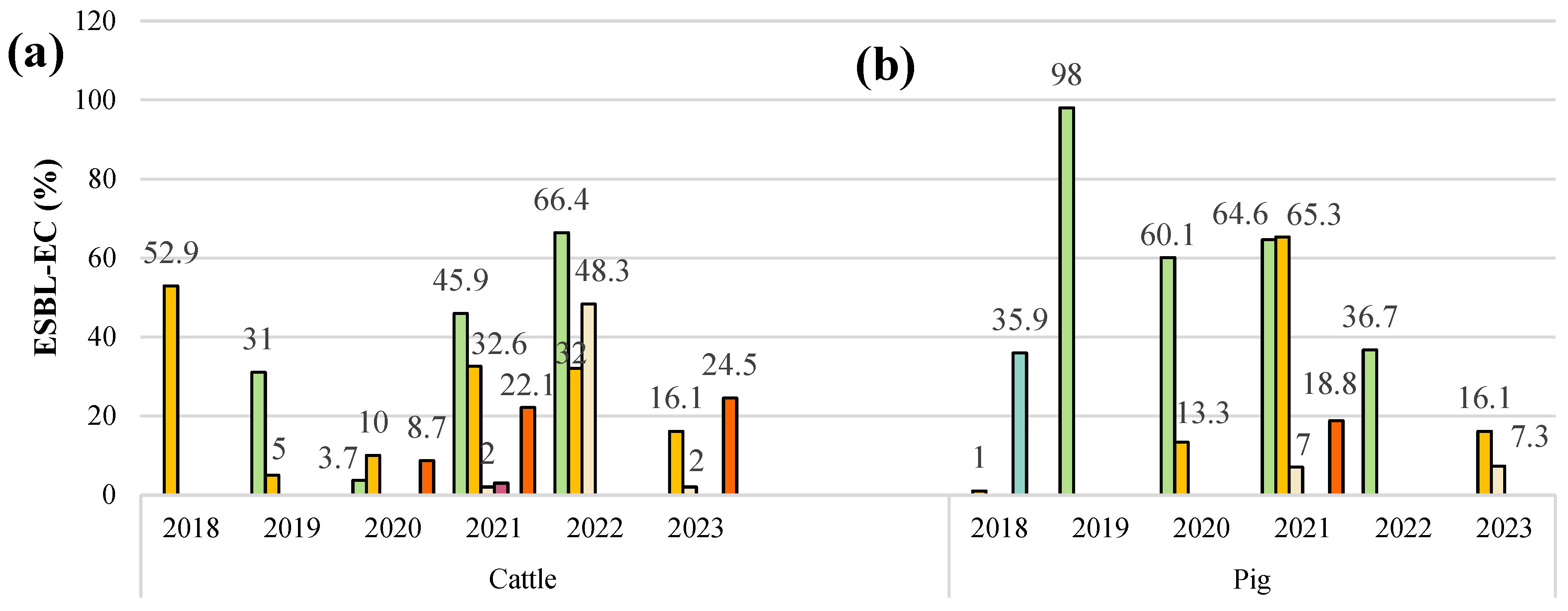
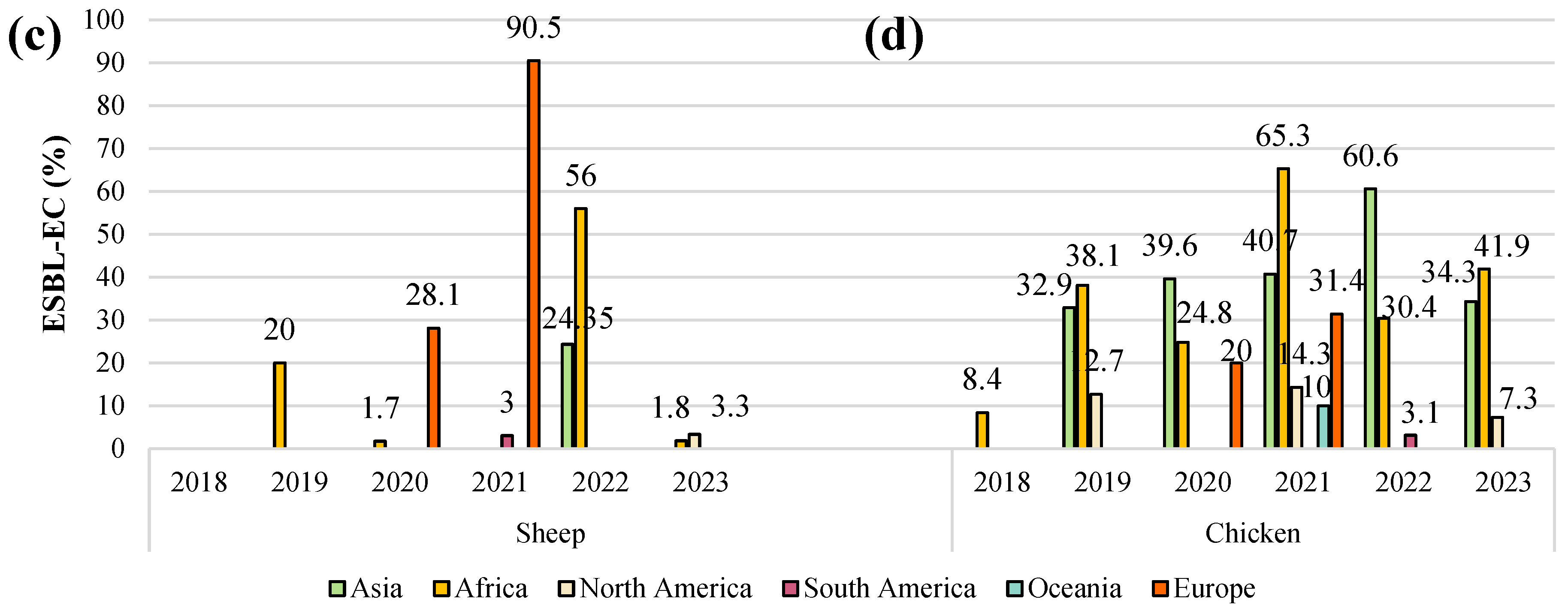
5. Prevalence of ESBL-E. coli in Livestock
6. Distribution of Genetically Variant ESBLs
7. Circulation of Non-β-Lactam Resistance Genes
8. Virulence Factors
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Lima, L.M.; Silva, B.N.M.D.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gāliņa, D.; Balins, A.; Valdovska, A. The Prevalence and Characterization of Fecal Extended-Spectrum-Beta-Lactamase-Producing Escherichia coli Isolated from Pigs on Farms of Different Sizes in Latvia. Antibiotics 2021, 10, 1099. [Google Scholar] [CrossRef]
- Nossair, M.A.; Abd El Baqy, F.A.; Rizk, M.S.Y.; Elaadli, H.; Mansour, A.M.; El-Aziz, A.H.A.; Alkhedaide, A.; Soliman, M.M.; Ramadan, H.; Shukry, M. Prevalence and Molecular Characterization of Extended-Spectrum β-Lactamases and AmpC β-lactamase-Producing Enterobacteriaceae among Human, Cattle, and Poultry. Pathogens 2022, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Bergšpica, I.; Kaprou, G.; Alexa, E.A.; Prieto, M.; Alvarez-Ordóñez, A. Extended Spectrum β-Lactamase (ESBL) Producing Escherichia coli in Pigs and Pork Meat in the European Union. Antibiotics 2020, 9, 678. [Google Scholar] [CrossRef]
- Majdinasab, M.; Kumar-Mishra, R.; Tang, X.; Louis-Maty, J. Detection of antibiotics in food: New achievements in the development of biosensors. TrAC 2020, 127, 115883. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 1 February 2024).
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Ogunkola, I.O. The global antimicrobial resistance response effort must not exclude marginalised populations. Trop. Med. Health 2023, 51, 33. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, 6459. [Google Scholar] [CrossRef] [PubMed]
- Haulisah, N.A.; Hassan, L.; Bejo, S.K.; Jajere, S.M.; Ahmad, N.I. High Levels of Antibiotic Resistance in Isolates from Diseased Livestock. Front. Vet. Sci. 2021, 8, 652351. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.V.; Padungtod, P.; Thwaites, G.; Carrique-Mas, J.J. Antimicrobial Usage in Animal Production: A Review of the Literature with a Focus on Low- and Middle-Income Countries. Antibiotics 2018, 7, 75. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating Antibiotic Resistance at the Livestock-Environment Interface: A Review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef]
- Bennani, H.; Mateus, A.; Mays, N.; Eastmure, E.; Stärk, K.D.C.; Häsler, B. Overview of Evidence of Antimicrobial Use and Antimicrobial Resistance in the Food Chain. Antibiotics 2020, 9, 49. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://remed.org/wp-content/uploads/2017/03/lobal-priority-list-of-antibiotic-resistant-bacteria-2017.pdf (accessed on 3 February 2024).
- Abraham, S.; Kirkwood, R.N.; Laird, T.; Saputra, S.; Mitchell, T.; Singh, M.; Linn, B.; Abraham, R.J.; Pang, S.; Gordon, D.M.; et al. Dissemination and persistence of extended-spectrum cephalosporin-resistance encoding IncI1-blaCTXM-1 plasmid among Escherichia coli in pigs. ISME J. 2018, 12, 2352–2362. [Google Scholar] [CrossRef]
- Benavides, J.A.; Salgado-Caxito, M.; Opazo-Capurro, A.; González Muñoz, P.; Piñeiro, A.; Otto Medina, M.; Rivas, L.; Munita, J.; Millán, J. ESBL-Producing Escherichia coli Carrying CTX-M Genes Circulating among Livestock, Dogs, and Wild Mammals in Small-Scale Farms of Central Chile. Antibiotics 2021, 10, 510. [Google Scholar] [CrossRef]
- Giufrè, M.; Mazzolini, E.; Cerquetti, M.; Brusaferro, S.; CCM2015 One-Health ESBL-producing Escherichia coli Study Group. Extended-spectrum β-lactamase-producing Escherichia coli from extraintestinal infections in humans and from food-producing animals in Italy: A ‘One Health’ study. Int. J. Antimicrob. Agents 2021, 58, 106433. [Google Scholar] [CrossRef]
- Ibekwe, A.; Durso, L.; Ducey, T.F.; Oladeinde, A.; Jackson, C.R.; Frye, J.G.; Dungan, R.; Moorman, T.; Brooks, J.P.; Obayiuwana, A.; et al. Diversity of Plasmids and Genes Encoding Resistance to Extended-Spectrum β-Lactamase in Escherichia coli from Different Animal Sources. Microorganisms 2021, 9, 1057. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Huang, F.Y.; Gan, L.L.; Yu, X.; Cai, D.J.; Fang, J.; Zhong, Z.J.; Guo, H.R.; Xie, Y.; Yi, J.; et al. High prevalence of blaCTX-M and blaSHV among ESBL producing E. coli isolates from beef cattle in China’s Sichuan-Chongqing Circle. Sci. Rep. 2021, 11, 13725. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.; Reda, R.M.; Hagag, N.M.; Kamel, E.; Elnomrosy, S.M.; Mansour, A.I.; Shahein, M.A.; Ali, S.F.; Ali, H.R. Multidrug-Resistant and Genetic Characterization of Extended-Spectrum Beta-Lactamase-Producing E. coli Recovered from Chickens and Humans in Egypt. Animals 2022, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Katz, D.E.; Marchaim, D. The Continuing Plague of Extended-Spectrum β-Lactamase Producing Enterbacterales Infections: An Update. Infect. Dis. Clin. N. Am. 2020, 34, 677–708. [Google Scholar] [CrossRef] [PubMed]
- Alsamawi, M.; Joudeh, A.I.; Eldeeb, Y.; Al-Dahshan, A.; Khan, F.; Ghadban, W.; Almaslamani, M.; Alkhal, A. Epidemiology of extended-spectrum beta-lactamase producing Enterobacteriaceae in Qatar: A 3-year hospital-based study. Front. Antibiot. 2022, 1, 980686. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Tang, S.S.; Apisarnthanarak, A.; Hsu, L.Y. Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv. Drug Deliv. Rev. 2014, 78, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Ali, T.; Ali, I.; Khan, N.A.; Han, B.; Gao, J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. BioMed Res. Int. 2018, 2018, 9519718. [Google Scholar] [CrossRef]
- De Angelis, G.; Del Giacomo, P.; Posteraro, B.; Sanguinetti, M.; Tumbarello, M. Molecular Mechanisms, Epidemiology, and Clinical Importance of β-Lactam Resistance in Enterobacteriaceae. Int. J. Mol. Sci. 2020, 21, 5090. [Google Scholar] [CrossRef]
- Gundran, R.S.; Cardenio, P.A.; Villanueva, M.A.; Sison, F.B.; Benigno, C.C.; Kreausukon, K.; Pichpol, D.; Punyapornwithaya, V. Prevalence and distribution of blaCTX-M, blaSHV, blaTEM genes in extended- spectrum β- lactamase- producing E. coli isolates from broiler farms in the Philippines. BMC Vet. Res. 2019, 15, 227. [Google Scholar] [CrossRef]
- Atlaw, N.A.; Keelara, S.; Correa, M.; Foster, D.; Gebreyes, W.; Aidara-Kane, A.; Harden, L.; Thakur, S.; Cray, P.J.F. Identification of CTX-M Type ESBL E. coli from Sheep and Their Abattoir Environment Using Whole-Genome Sequencing. Pathogens 2021, 10, 1480. [Google Scholar] [CrossRef]
- Chai, M.H.; Sukimana, M.Z.; Jasmya, N.; Zulkiflya, N.A.; Mohd-Yusofa, N.A.S.; Mohamadb, N.M.; Ariffinc, S.M.Z.; Ghazali, M.F. Molecular Detection and Antibiogram of ESBL-Producing and Carbapenem-Resistant Escherichia coli from Rabbit, Swine, and Poultry in Malaysia. Trop. Anim. Sci. J. 2022, 45, 16. [Google Scholar] [CrossRef]
- Bastidas-Caldes, C.; Romero-Alvarez, D.; Valdez-Vélez, V.; Morales, R.D.; Montalvo-Hernández, A.; Gomes-Dias, C.; Calvopiña, M. Extended-Spectrum Beta-Lactamases Producing Escherichia coli in South America: A Systematic Review with a One Health Perspective. Infect. Drug Resist. 2022, 15, 5759–5779. [Google Scholar] [CrossRef] [PubMed]
- Briñas, L.; Moreno, M.A.; Zarazaga, M.; Porrero, C.; Sáenz, Y.; García, M.; Dominguez, L.; Torres, C. Detection of CMY-2, CTX-M-14, and SHV-12 beta-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob. Agents Chemother. 2003, 47, 2056–2058. [Google Scholar] [CrossRef]
- Tello, M.; Ocejo, M.; Oporto, B.; Hurtado, A. Prevalence of Cefotaxime-Resistant Escherichia coli Isolates from Healthy Cattle and Sheep in Northern Spain: Phenotypic and Genome-Based Characterization of Antimicrobial Susceptibility. Appl. Environ. Microbiol. 2020, 86, e00742-20. [Google Scholar] [CrossRef] [PubMed]
- Alegría, Á.; Arias-Temprano, M.; Fernández-Natal, I.; Rodríguez-Calleja, J.M.; García-López, M.L.; Santos, J.A. Molecular Diversity of ESBL-Producing Escherichia coli from Foods of Animal Origin and Human Patients. Int. J. Environ. Res. Public Health 2020, 17, 1312. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Li, X.; Ma, L.; Cao, X.; Hu, W.; Zhao, L.; Jing, W.; Lan, X.; Li, Y.; et al. Genetic diversity, antimicrobial resistance and extended-spectrum β-lactamase type of Escherichia coli isolates from chicken, dog, pig and yak in Gansu and Qinghai Provinces, China. J. Glob. Antimicrob. Resist. 2020, 22, 726–732. [Google Scholar] [CrossRef]
- Song, J.; Oh, S.S.; Kim, J.; Park, S.; Shin, J. Clinically Relevant Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolates From Food Animals in South Korea. Front. Microbiol. 2020, 11, 604. [Google Scholar] [CrossRef]
- Chah, K.F.; Ugwu, I.C.; Okpala, A.; Adamu, K.Y.; Alonso, C.A.; Ceballos, S.; Nwanta, J.N.; Torres, C. Detection and molecular characterisation of extended-spectrum β-lactamase-producing enteric bacteria from pigs and chickens in Nsukka, Nigeria. J. Glob. Antimicrob. Resist. 2018, 15, 36–40. [Google Scholar] [CrossRef]
- Adeluwoye-Ajayi, O.A.; Thomas, F.M.; Awoniyi, R.R. Detection of Extended Spectrum Beta-Lactamase Production in Escherichia coli Isolated from Cattle Faeces in Owo Metropolis. Int. J. Pat. Res. 2021, 8, 32–39. [Google Scholar] [CrossRef]
- Martínez-Vázquez, A.V.; Vázquez-Villanueva, J.; Leyva-Zapata, L.M.; Barrios-García, H.; Rivera, G.; Bocanegra-García, V. Multidrug Resistance of Escherichia coli Strains Isolated From Bovine Feces and Carcasses in Northeast Mexico. Front. Vet. Sci. 2021, 8, 643802. [Google Scholar] [CrossRef]
- Martínez-Vázquez, A.V.; Mandujano, A.; Cruz-Gonzalez, E.; Guerrero, A.; Vazquez, J.; Cruz-Pulido, W.L.; Rivera, G.; Bocanegra-García, V. Evaluation of Retail Meat as a Source of ESBL Escherichia coli in Tamaulipas, Mexico. Antibiotics 2022, 11, 1795. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Joji, R.M.; Shahid, M. Evolution and implementation of One Health to control the dissemination of antibiotic-resistant bacteria and resistance genes: A review. Front. Cell. Infect. Microbiol. 2023, 12, 1065796. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Tekiner, İ.H.; Özpınar, H. Occurrence and characteristics of extended spectrum beta-lactamases-producing Enterobacteriaceae from foods of animal origin. Braz. J. Microbiol. 2016, 47, 444–451. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An enzyme from bacteria able to destroy penicillin. 1940. Rev. Infect. Dis. 1988, 10, 677–678. [Google Scholar]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Sghaier, S.; Abbassi, M.S.; Pascual, A.; Serrano, L.; Díaz-De-Alba, P.; Said, M.B.; Hassen, B.; Ibrahim, C.; Hassen, A.; López-Cerero, L. Extended-spectrum β-lactamase-producing Enterobacteriaceae from animal origin and wastewater in Tunisia: First detection of O25b-B23-CTX-M-27-ST131 Escherichia coli and CTX-M-15/OXA-204-producing Citrobacter freundii from wastewater. J. Glob. Antimicrob. Resist. 2019, 17, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Adefioye, O.J.; Weinreich, J.; Rödiger, S.; Schierack, P.; Olowe, O.A. Phylogenetic Characterization and Multilocus Sequence Typing of Extended-Spectrum Beta Lactamase-Producing Escherichia coli from Food-Producing Animals, Beef, and Humans in Southwest Nigeria. Microb. Drug Resist. 2021, 27, 111–120. [Google Scholar]
- Salem, G.A.; Abdelaziz, E.A.; Kamel, M.A.; Rhouma, N.R.; Ali, R.I. Prevalence of multidrug-resistant and extended-spectrum β-lactamase-producing Escherichia coli from chicken farms in Egypt. Vet. World 2023, 16, 1001–1007. [Google Scholar] [CrossRef]
- Carattoli, A. Animal reservoirs for extended spectrum beta-lactamase producers. Clin. Microbiol. Infect. 2008, 14, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef]
- Widodo, A.; Effendi, M.H.; Khairullah, A.R. Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli from livestock. Sys. Rev. Pharm. 2020, 11, 382–392. [Google Scholar]
- Lalruatdiki, A.; Dutta, T.K.; Roychoudhury, P.; Subudhi, P.K. Extended-spectrum β-lactamases producing multidrug resistance Escherichia coli, Salmonella and Klebsiella pneumoniae in pig population of Assam and Meghalaya, India. Vet. World 2018, 11, 868–873. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Wang, L.; Peng, Q.; Li, Y.; Zhou, H.; Li, Q. Molecular Characterization of Extended-Spectrum β-Lactamase-Producing Multidrug Resistant Escherichia coli From Swine in Northwest China. Front. Microbiol. 2018, 9, 1756. [Google Scholar] [CrossRef]
- Nuangmek, A.; Rojanasthien, S.; Chotinun, S.; Yamsakul, P.; Tadee, P.; Thamlikitkul, V.; Tansakul, N.; Patchanee, P. Antimicrobial Resistance in ESBL-Producing Escherichia coli Isolated from Layer and Pig Farms in Thailand. Acta Sci. Vet. 2018, 46, 8. [Google Scholar] [CrossRef]
- Seenama, C.; Thamlikitkul, V.; Ratthawongjirakul, P. Multilocus sequence typing and blaESBL characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolated from healthy humans and swine in Northern Thailand. Infect. Drug Resist. 2019, 12, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Mohsin, M.; Ali, Q.; Qamar, M.U.; Raza, S.; Ali, A.; Guenther, S.; Schierack, P. Prevalence and Genetic Relatedness of Extended Spectrum-β-Lactamase-Producing Escherichia coli Among Humans, Cattle, and Poultry in Pakistan. Microb. Drug Resist. 2019, 25, 1374–1381. [Google Scholar] [CrossRef]
- Tansawai, U.; Walsh, T.R.; Niumsup, P.R. Extended spectrum ß-lactamase-producing Escherichia coli among backyard poultry farms, farmers, and environments in Thailand. Poult. Sci. 2019, 98, 2622–2631. [Google Scholar] [CrossRef]
- Kamaruzzaman, E.A.; Abdul Aziz, S.; Bitrus, A.A.; Zakaria, Z.; Hassan, L. Occurrence and Characteristics of Extended-Spectrum β-Lactamase-Producing Escherichia coli from Dairy Cattle, Milk, and Farm Environments in Peninsular Malaysia. Pathogens 2020, 9, 1007. [Google Scholar] [CrossRef]
- Liu, X.; Wei, X.; Liu, L.; Feng, X.; Shao, Z.; Han, Z.; Li, Y. Prevalence and characteristics of extended-spectrum β-lactamases-producing Escherichia coli from broiler chickens at different day-age. Poult. Sci. 2020, 99, 3688–3696. [Google Scholar] [CrossRef] [PubMed]
- Mandakini, R.; Roychoudhury, P.; Subudhi, P.K.; Kylla, H.; Samanta, I.; Bandyopadhayay, S.; Dutta, T.K. Higher prevalence of multidrug-resistant extended-spectrum β-lactamases producing Escherichia coli in unorganized pig farms compared to organized pig farms in Mizoram, India. Vet. World 2020, 13, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Dey, S.; Batabyal, K.; Banerjee, A.; Joardar, S.N.; Samanta, I.; Isore, D.P. Prevalence and Characterization of Extended Spectrum Beta Lactamase Producing Escherichia coli from Broilers. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 594–602. [Google Scholar] [CrossRef]
- Tamta, S.; Kumar, O.R.V.; Singh, S.V.; Pruthvishree, B.S.; Karthikeyan, R.; Rupner, R.; Sinha, D.K.; Singh, B.R. Antimicrobial resistance pattern of extended-spectrum β-lactamase-producing Escherichia coli isolated from fecal samples of piglets and pig farm workers of selected organized farms of India. Vet. World 2020, 13, 360–363. [Google Scholar] [CrossRef]
- Wibisono, F.J.; Sumiarto, B.; Untari, T.; Effendi, M.H.; Permatasari, D.A.; Witaningrum, A.M. CTX gene of extended-spectrum beta-lactamase (ESBL) producing Escherichia coli on Broilers in Blitar, Indonesia. Sys. Rev. Pharm. 2020, 11, 396–403. [Google Scholar]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial Resistance Genes in ESBL-Producing Escherichia coli Isolates from Animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Balázs, B.; Nagy, J.B.; Tóth, Z.; Nagy, F.; Károlyi, S.; Turcsányi, I.; Bistyák, A.; Kálmán, A.; Sárközi, R.; Kardos, G. Occurrence of Escherichia coli producing extended spectrum β-lactamases in food-producing animals. Acta Vet. Hung. 2021, 69, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dantas Palmeira, J.; Haenni, M.; Madec, J.Y.; Ferreira, H.M.N. First Global Report of Plasmid-Mediated mcr-1 and Extended-Spectrum Beta-Lactamase-Producing Escherichia coli from Sheep in Portugal. Antibiotics 2021, 10, 1403. [Google Scholar] [CrossRef]
- Ewers, C.; de Jong, A.; Prenger-Berninghoff, E.; El Garch, F.; Leidner, U.; Tiwari, S.K.; Semmler, T. Genomic Diversity and Virulence Potential of ESBL- and AmpC-β-Lactamase-Producing Escherichia coli Strains From Healthy Food Animals Across Europe. Front. Microbiol. 2021, 12, 626774. [Google Scholar] [CrossRef]
- Fetahagić, M.; Ibrahimagić, A.; Uzunović, S.; Beader, N.; Elveđi-Gašparović, V.; Luxner, J.; Gladan, M.; Bedenić, B. Detection and characterisation of extended-spectrum and plasmid-mediated AmpC β-lactamase produced by Escherichia coli isolates found at poultry farms in Bosnia and Herzegovina. Arh. Hig. Rada Toksikol. 2021, 72, 305–314. [Google Scholar] [CrossRef]
- Gruel, G.; Sellin, A.; Riveiro, H.; Pot, M.; Breurec, S.; Guyomard-Rabenirina, S.; Talarmin, A.; Ferdinand, S. Antimicrobial use and resistance in Escherichia coli from healthy food-producing animals in Guadeloupe. BMC Vet. Res. 2021, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Effendi, M.H.; Hartadi, E.B.; Witaningrum, A.M.; Permatasari, D.A.; Ugbo, E.N. Molecular identification of blaTEM gene of extended-spectrum beta-lactamase-producing Escherichia coli from healthy pigs in Malang district, East Java, Indonesia. J. Adv. Vet. Anim. Res. 2022, 9, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, K.; Zhang, Y.; Xia, L.; Zhao, L.; Guo, C.; Liu, X.; Qin, L.; Hao, Z. High Prevalence and Diversity Characteristics of blaNDM, mcr, and blaESBLs Harboring Multidrug-Resistant Escherichia coli From Chicken, Pig, and Cattle in China. Front. Cell. Infect. Microbiol. 2022, 11, 755545. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Pandey, A.; Patel, A.C.; Patel, S.S.; Chauhan, H.C.; Shrimali, M.D.; Patel, P.A.; Mohapatra, S.K.; Chandel, B.S. Whole genome sequencing and characteristics of extended-spectrum beta-lactamase producing Escherichia coli isolated from poultry farms in Banaskantha, India. Front. Microbiol. 2022, 13, 996214. [Google Scholar] [CrossRef]
- Shafiq, M.; Rahman, S.U.; Bilal, H.; Ullah, A.; Noman, S.M.; Zeng, M.; Yuan, Y.; Xie, Q.; Li, X.; Jiao, X. Incidence and molecular characterization of ESBL-producing and colistin-resistant Escherichia coli isolates recovered from healthy food-producing animals in Pakistan. J. Appl. Microbiol. 2022, 133, 1169–1182. [Google Scholar] [CrossRef]
- D Trongjit, S.; Assavacheep, P.; Samngamnim, S.; My, T.H.; An, V.T.T.; Simjee, S.; Chuanchuen, R. Plasmid-mediated colistin resistance and ESBL production in Escherichia coli from clinically healthy and sick pigs. Sci. Rep. 2022, 12, 2466. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Zhou, Z.; Miao, Y.; Li, R.; Yang, B.; Cao, C.; Xiao, S.; Wang, X.; Liu, H.; et al. Characterization of Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolates That Cause Diarrhea in Sheep in Northwest China. Microbiol. Spectr. 2022, 10, e0159522. [Google Scholar] [CrossRef]
- Benlabidi, S.; Raddaoui, A.; Lengliz, S.; Cheriet, S.; Hynds, P.; Achour, W.; Ghrairi, T.; Abbassi, M.S. Occurrence of High-Risk Clonal Lineages ST58, ST69, ST224, and ST410 among Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolated from Healthy Free-Range Chickens (Gallus gallus domesticus) in a Rural Region in Tunisia. Genes 2023, 14, 875. [Google Scholar] [CrossRef]
- Muleme, J.; Kankya, C.; Munyeme, M.; Musoke, D.; Ssempebwa, J.C.; Isunju, J.B.; Wambi, R.; Balugaba, B.E.; Sekulima, T.; Mugambe, R.K.; et al. Phenotypic Characterization and Antibiograms of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Isolated at the Human-Animal-Environment Interface Using a One Health Approach Among Households in Wakiso District, Uganda. Infect. Drug Resist. 2023, 16, 2203–2216. [Google Scholar] [CrossRef]
- Yao, R.K.; Coulibaly, J.K.; Tiekoura, B.K.; Yapi, F.H.; Djaman, J.A. Molecular Characterisation of Extended-Spectrum Beta-lactamase Producing Escherichia coli Isolated from Cattle Faeces in Abidjan District, Ivory Coast. Microbiol. Res. J. Int. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Aworh, M.K.; Ekeng, E.; Nilsson, P.; Egyir, B.; Owusu-Nyantakyi, C.; Hendriksen, R.S. Extended-Spectrum ß-Lactamase-Producing Escherichia coli Among Humans, Beef Cattle, and Abattoir Environments in Nigeria. Front. Cell. Infect. Microbiol. 2022, 12, 869314. [Google Scholar] [CrossRef]
- Olorunleke, S.O.; Kirchner, M.; Duggett, N.; AbuOun, M.; Okorie-Kanu, O.J.; Stevens, K.; Card, R.M.; Chah, K.F.; Nwanta, J.A.; Brunton, L.A.; et al. Molecular characterization of extended spectrum cephalosporin resistant Escherichia coli isolated from livestock and in-contact humans in Southeast Nigeria. Front. Microbiol. 2022, 13, 937968. [Google Scholar] [CrossRef] [PubMed]
- Kimera, Z.I.; Mgaya, F.X.; Misinzo, G.; Mshana, S.E.; Moremi, N.; Matee, M.I.N. Multidrug-Resistant, Including Extended-Spectrum Beta Lactamase-Producing and Quinolone-Resistant, Escherichia coli Isolated from Poultry and Domestic Pigs in Dar es Salaam, Tanzania. Antibiotics 2021, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Mandujano, A.; Cortés-Espinosa, D.V.; Vásquez-Villanueva, J.; Guel, P.; Rivera, G.; Juárez-Rendón, K.; Cruz-Pulido, W.L.; Aguilera-Arreola, G.; Guerrero, A.; Bocanegra-García, V.; et al. Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolated from Food-Producing Animals in Tamaulipas, Mexico. Antibiotics 2023, 12, 1010. [Google Scholar] [CrossRef]
- Ilyas, S.; Rasool, M.H.; Arshed, M.J.; Qamar, M.U.; Aslam, B.; Almatroudi, A.; Khurshid, M. The Escherichia coli Sequence Type 131 Harboring Extended-Spectrum Beta-Lactamases and Carbapenemases Genes from Poultry Birds. Infect. Drug Resist. 2021, 14, 805–813. [Google Scholar] [CrossRef]
- Lemlem, M.; Aklilu, E.; Mohammed, M.; Kamaruzzaman, F.; Zakaria, Z.; Harun, A.; Devan, S.S. Molecular detection and antimicrobial resistance profiles of Extended-Spectrum Beta-Lactamase (ESBL) producing Escherichia coli in broiler chicken farms in Malaysia. PLoS ONE 2023, 18, e0285743. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.; Okolocha, E.; Harden, L.; Hull, D.; Hendriksen, R.S.; Thakur, S. Extended-spectrum ß-lactamase-producing Escherichia coli among humans, chickens and poultry environments in Abuja, Nigeria. One Health Outlook 2020, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Baez, M.; Espinosa, I.; Collaud, A.; Miranda, I.; Montano, D.L.N.; Feria, A.L.; Hernández-Fillor, R.E.; Obregón, D.; Alfonso, P.; Perreten, V. Genetic Features of Extended-Spectrum β-Lactamase-Producing Escherichia coli from Poultry in Mayabeque Province, Cuba. Antibiotics 2021, 10, 107. [Google Scholar] [CrossRef]
- Patricio, T.C.D.C.; Farias, B.O.; Santiago, G.S.; Souza, V.R.S.; Pimenta, R.L.; de Oliveira, C.C.; Coelho, I.S.; de Souza, M.M.S.; Coelho, S.M.O. Production of extended-spectrum beta-lactamases in Escherichia coli isolated from poultry in Rio de Janeiro, Brazil. Braz. J. Vet. Med. 2022, 44, e001722. [Google Scholar] [CrossRef]
- Carey, A.M.; Capik, S.F.; Giebel, S.; Nickodem, C.; Piñeiro, J.M.; Scott, H.M.; Vinasco, J.; Norman, K.N. Prevalence and Profiles of Antibiotic Resistance Genes mph(A) and qnrB in Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli Isolated from Dairy Calf Feces. Microorganisms 2022, 10, 411. [Google Scholar] [CrossRef]
- Kerluku, M.; Jankuloski, D.; Manovska, M.; Prodanov, M.; Dimzoska, B.; Dodovski, A.; Blagoevska, K. β-Lactamase genes (blaCTX-M, blaSHV, blaTEM, blaOXA1 AND blaOXA2) and phylogenetic groups in ESBL producing commensal Escherichia coli isolated from faecal samples from dairy farm in the Municipality of Debar. Mac. Vet. Rev. 2023, 46, 89–97. [Google Scholar] [CrossRef]
- Rizal, S.; Nurhapsari, I.; Fauziah, I.; Masrukhin, M.; Jatmiko, Y.D. Prevalence of multidrug-resistant and extended-spectrum β-lactamase producing Escherichia coli from local and broiler chickens at Cibinong market, West Java, Indonesia. Vet. World 2024, 17, 179–184. [Google Scholar] [CrossRef]
- Tseng, C.H.; Liu, C.W.; Liu, P.Y. Extended-Spectrum β-Lactamases (ESBL) Producing Bacteria in Animals. Antibiotics 2023, 12, 661. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, M.; Won, H.G.; Belaynehe, K.M.; Yoon, I.J.; Yoo, H.S. Characteristics of Transmissible CTX-M- and CMY-Type β-Lactamase-Producing Escherichia coli Isolates Collected from Pig and Chicken Farms in South Korea. J. Microbiol. Biotechnol. 2017, 27, 1716–1723. [Google Scholar] [CrossRef]
- Fashae, K.; Engelmann, I.; Monecke, S.; Braun, S.D.; Ehricht, R. Molecular characterisation of extended-spectrum ß-lactamase producing Escherichia coli in wild birds and cattle, Ibadan, Nigeria. BMC Vet. Res. 2021, 17, 33. [Google Scholar] [CrossRef]
- Day, M.J.; Hopkins, K.L.; Wareham, D.W.; Toleman, M.A.; Elviss, N.; Randall, L.; Teale, C.; Cleary, P.; Wiuff, C.; Doumith, M.; et al. Extended-spectrum β-lactamase-producing Escherichia coli in human-derived and foodchain-derived samples from England, Wales, and Scotland: An epidemiological surveillance and typing study. Lancet Infect. Dis. 2019, 19, 1325–1335. [Google Scholar] [CrossRef]
- Lay, K.K.; Jeamsripong, S.; Sunn, K.P.; Angkititrakul, S.; Prathan, R.; Srisanga, S.; Chuanchuen, R. Colistin Resistance and ESBL Production in Salmonella and Escherichia coli from Pigs and Pork in the Thailand, Cambodia, Lao PDR, and Myanmar Border Area. Antibiotics 2021, 10, 657. [Google Scholar] [CrossRef]
- Truong, D.T.Q.; Hounmanou, Y.M.G.; Dang, S.T.T.; Olsen, J.E.; Truong, G.T.H.; Tran, N.T.; Scheutz, F.; Dalsgaard, A. Genetic Comparison of ESBL-Producing Escherichia coli from Workers and Pigs at Vietnamese Pig Farms. Antibiotics 2021, 10, 1165. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Larsen, J.; Andersen, V.D.; Lester, C.H.; Skovgaard Skytte, T.S.; Hansen, F.; Olsen, S.S.; Mordhorst, H.; Skov, R.L.; Aarestrup, F.M.; et al. Characterization of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third- or fourth-generation cephalosporins. J. Antimicrob. Chemother. 2014, 69, 2650–2657. [Google Scholar] [CrossRef]
- Tsekouras, N.; Athanasakopoulou, Z.; Diezel, C.; Kostoulas, P.; Braun, S.D.; Sofia, M.; Monecke, S.; Ehricht, R.; Chatzopoulos, D.C.; Gary, D.; et al. Cross-Sectional Survey of Antibiotic Resistance in Extended Spectrum β-Lactamase-Producing Enterobacteriaceae Isolated from Pigs in Greece. Animals 2022, 12, 1560. [Google Scholar] [CrossRef]
- von Salviati, C.; Laube, H.; Guerra, B.; Roesler, U.; Friese, A. Emission of ESBL/AmpC-producing Escherichia coli from pig fattening farms to surrounding areas. Vet. Microbiol. 2015, 175, 77–84. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, J.; Gu, J.; Liu, Z.; Hu, J.; Li, X.; Chen, X.; Sun, Z.; Li, J. Prevalence and antimicrobial susceptibility of CTX-M-type-producing Escherichia coli from a wildlife zoo in China. Vet. Med. Sci. 2022, 8, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Falgenhauer, L.; Imirzalioglu, C.; Oppong, K.; Akenten, C.W.; Hogan, B.; Krumkamp, R.; Poppert, S.; Levermann, V.; Schwengers, O.; Sarpong, N.; et al. Detection and Characterization of ESBL-Producing Escherichia coli From Humans and Poultry in Ghana. Front. Microbiol. 2019, 9, 3358. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Lebert, L.A.; McEwen, S.A.; Reid-Smith, R.J.; Deckert, A.E.; Agunos, A.; Reid, M.A.; Rubin, J.E. Extended-Spectrum β-Lactamase and AmpC β-Lactamase-Producing Escherichia coli Isolates from Chickens Raised in Small Flocks in Ontario, Canada. Microb. Drug Resist. 2019, 25, 1250–1256. [Google Scholar] [CrossRef]
- Cardozo, M.V.; Liakopoulos, A.; Brouwer, M.; Kant, A.; Pizauro, L.J.L.; Borzi, M.M.; Mevius, D.; de Ávila, F.A. Occurrence and Molecular Characteristics of Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Recovered From Chicken, Chicken Meat, and Human Infections in Sao Paulo State, Brazil. Front. Microbiol. 2021, 12, 628738. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Neubauer, H.; Ehricht, R.; Monecke, S.; Tomaso, H.; Hafez, H.M.; Roesler, U.; El-Adawy, H. Antimicrobial resistance in Enterobacteriaceae from healthy broilers in Egypt: Emergence of colistin-resistant and extended-spectrum β-lactamase-producing Escherichia coli. Gut. Pathog. 2018, 10, 39. [Google Scholar] [CrossRef]
- Belmahdi, M.; Chenouf, N.S.; Ait Belkacem, A.; Martinez-Alvarez, S.; Pino-Hurtado, M.S.; Benkhechiba, Z.; Lahrech, S.; Hakem, A.; Torres, C. Extended Spectrum β-Lactamase-Producing Escherichia coli from Poultry and Wild Birds (Sparrow) in Djelfa (Algeria), with Frequent Detection of CTX-M-14 in Sparrow. Antibiotics 2022, 11, 1814. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakongkarat, T.; Akeda, Y.; Ratthawongjirakul, P.; Chuanchuen, R.; Chaichanawongsaroj, N. Rapid detection of extended spectrum β-lactamase producing Escherichia coli isolated from fresh pork meat and pig cecum samples using multiplex recombinase polymerase amplification and lateral flow strip analysis. PLoS ONE 2021, 16, e0248536. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.A.; Seo, Y.H.; Lee, H.; Lee, K. Prevalence and Molecular Epidemiology of Extended-Spectrum-β-Lactamase (ESBL)-Producing Escherichia coli from Multiple Sectors of Poultry Industry in Korea. Antibiotics 2021, 10, 1050. [Google Scholar] [CrossRef]
- Seo, K.W.; Lee, Y.J. The occurrence of CTX-M-producing E. coli in the broiler parent stock in Korea. Poult. Sci. 2021, 100, 1008–1015. [Google Scholar] [CrossRef]
- Hussain, H.I.; Aqib, A.I.; Seleem, M.N.; Shabbir, M.A.; Hao, H.; Iqbal, Z.; Kulyar, M.F.; Zaheer, T.; Li, K. Genetic basis of molecular mechanisms in β-lactam resistant gram-negative bacteria. Microb. Pathog. 2021, 158, 105040. [Google Scholar] [CrossRef]
- Mathers, A.J.; Peirano, G.; Pitout, J.D. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef]
- Jensen, L.B.; Birk, T.; Borck Høg, B.; Stehr, L.; Aabo, S.; Korsgaard, H. Cross and co resistance among Danish porcine E. coli isolates. Res. Vet. Sci. 2018, 119, 247–249. [Google Scholar] [CrossRef]
- Özgen, E.K.; Yanmaz, B.; Bağatir, P.Ş. Investigation of virulence factors, phylogenetic grouping, multiple-locus variable number tandem repeat analysis, and antimicrobial susceptibility of E. coli isolated from aborted bovine fetal tissue. Lett. Appl. Microbiol. 2023, 76, ovad100. [Google Scholar] [CrossRef]
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Nunes, P.H.S.; Valiatti, T.B.; Santos, A.C.M.; Nascimento, J.A.D.S.; Santos-Neto, J.F.; Rocchetti, T.T.; Yu, M.C.Z.; Hofling-Lima, A.L.; Gomes, T.A.T. Evaluation of the Pathogenic Potential of Escherichia coli Strains Isolated from Eye Infections. Microorganisms 2022, 10, 1084. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Fan, R.; Fu, S.; Zhang, J.; Matussek, A.; Xiong, Y.; Bai, X. Genetic diversity of the intimin gene (eae) in non-O157 Shiga toxin-producing Escherichia coli strains in China. Sci. Rep. 2020, 10, 3275. [Google Scholar] [CrossRef]

| Ambler Scheme (Molecular Class) | Bush–Jacoby Group (Functional) | Characteristics | Substrate | Inhibited by | Enzyme Examples | |
|---|---|---|---|---|---|---|
| Clavulanic Acid | EDTA | |||||
| A | 2a | Penicillinases | Penicillins | + | - | PC1 |
| 2b | Broad-spectrum enzymes | Penicillins, early cephalosporins | + | - | TEM-1, TEM-2, TEM-13, SHV-1, SHV-11 | |
| 2be | Extended broad-spectrum enzymes | Penicillins, oxyimino-cephalosporins, monobactams | + | - | TEM-3, TEM-10, SHV-2, CTX-M, PER-1, VEB-1 | |
| 2br | Broad-spectrum enzymes | Penicillins | - | - | TEM-30, TEM-31, SHV-10 | |
| 2ber | Extended-spectrum enzymes | Penicillins, extended-spectrum cephalosporins, monobactams | - | - | TEM-50, TEM-158 | |
| 2c | Carbenicillin-hydrolyzing enzymes | Penicillins, carbenicillin | + | - | PSE-1, CARB-3 | |
| 2ce | Extended-spectrum carbenicillinase | Carbenicillin, cefepime | + | - | CARB-10 | |
| 2e | Cephalosporinases | Extended-spectrum cephalosporins | + | - | CepA | |
| 2f | Carbapenem-hydrolyzing nonmetallo-β-lactamases | Carbapenems | + | - | GES, KPC-2, SME-1, IMI-1 | |
| B | 3 | Metallo-β-lactamases | Carbapenems | - | + | IMP, VIM, IND |
| C | 1 | Cephalosporinases | Narrow- and extended-spectrum cephalosporins | - | - | MIR-1, ACT-1, FOX-1, CMY-47 |
| D | 2d | Cloxacillin-hydrolyzing enzymes | Cloxacilina, oxacilina | + | - | OXA-1, OXA-10 |
| 2de | Cloxacillin, oxacillin, oxyimino-cephalosporins, monobactams | + | - | OXA-11, OXA-15 | ||
| 2df | Carbapenems | + | - | OXA-23, OXA-51, OXA-58 | ||
| Continent | Country | Source | Detection Test | ESBL Genes | Other Antibiotic Resistance Genes | Virulence Genes | Reference |
|---|---|---|---|---|---|---|---|
| Africa | Nigeria | Poultry | PCR | CTX-M-1, CTX-M-55, TEM | tetA, tetB, aac(3)-II | - | [40] |
| Pig | PCR | CTX-M-15 | - | - | |||
| Egypt | Poultry | PCR, microarrays | TEM, SHV, OXA-1, CTX-M-1, CTX-M-15 | aadA1, sul2, floR, qnrS, qnrB, dfrA, sul3, tetA, tetB, tetC | lpfA, hemL, ireA, iroN, iss, tir | [109] | |
| Ivory Coast | Cattle | PCR | CTX-M, TEM, SHV | - | - | [82] | |
| Ghana | Poultry | WGS | CTX-M-15, SHV-12 | - | - | [106] | |
| Tunisia | Poultry | PCR | CTX-M-1 | - | - | [50] | |
| Cow | CTX-M-15 | ||||||
| Sheep | CTX-M-1, CTX-M-15, TEM-1 | ||||||
| Nigeria | Pig, sheep, cow, poultry | PCR | TEM, CTX-M-15, SHV | aac(6’)-lb | - | [51] | |
| Nigeria | Poultry | WGS | CTX-M, CTX-M-15, TEM | tetA, sul2, mdfA, aph(3)-Ib, aph(6)-Id, dfrA14 | - | [89] | |
| Nigeria | Cow | Microarrays | CTX-M-15, CTX-M-9, TEM | strB, sul2 | hemL, iss, lpfA | [98] | |
| Tanzania | Poultry | PCR | CTX-M, TEM, SHV | aac(6)-Ib-cr, qnrB, qepA | - | [85] | |
| Pig | |||||||
| Nigeria | Cow | WGS | CTX-M-14, CTX-M-15, CTX-M-55 | qnrS1, aph(6)-Id, aph(3)-Ib, aadA2, aadA5, aph(3)-Id, sul2, dfrA14, dfrA17, mdfA, tetA | - | [83] | |
| Egypt | Poultry | PCR | CTX-M-9, TEM, OXA-2 | - | - | [24] | |
| Algeria | Poultry | PCR | CTX-M-1 | tetA, sul1 | - | [110] | |
| Egypt | Poultry | PCR | CTX-M, SHV, TEM | - | - | [5] | |
| Cow | |||||||
| Nigeria | Cow, poultry, pig, sheep | qPCR, WGS | CTX-M-15, CTX-M-55, CTX-M-64, CTX-M-65, TEM-1 | strA, strB, aac3-IId, aadA5, sul2, sul1, dfrA14, dfrA17, mphA | - | [84] | |
| Tunisia | Poultry | PCR | CTX-M-15, CTX-M-55, TEM, SHV-12 | aac(6’)-Ib-cr, sul1, tetB | fimH, fyuA, iutA, papGIII | [80] | |
| Egypt | Poultry | PCR | TEM, SHV | - | - | [52] | |
| America | Canada | Poultry | PCR | CTX-M-1 | - | - | [107] |
| Cuba | Poultry | PCR, microarrays | CTX-M-1, CTX-M-15 | tetA, tetB, mphA, sul2, dfrA17, strA, strB | - | [90] | |
| USA | Cow, pig, poultry | PCR, WGS | CTX-M-1, CTX-M-9, TEM | tetA, tetB, aph(6)-Id, sul1, sul2, sul3, strA, strB, aadA2, aph(3’)-Ia | - | [22] | |
| USA | Cow | PCR | CTX-M-1, CTX-M-9 | mphA, qnrB | - | [92] | |
| WGS | CTX-M-1, CTX-M-32, CTX-M-15, CTX-M-27, CTX-M-65 | aph(3’’)-Ib, aph(6)-Id, sul1, sul2, mphA, mdfA, tetA, floR | |||||
| Mexico | Cow, poultry, pig, sheep | PCR | CTX-M, TEM | tetA, tetB, aadA1, strA, strB, sul1,2,3, qnrB | hlyA | [86] | |
| Chile | Cow, poultry, pig, sheep | PCR | CTX-M, CTX-M-1, CTX-M-2, TEM, SHV | - | - | [20] | |
| Brazil | Poultry | Microarrays | CTX-M-1, CTX-M-2 | - | - | [108] | |
| WGS | CTX-M-2, CTX-M-15 | ||||||
| Brazil | Poultry | PCR | CTX-M, SHV | - | - | [91] | |
| Asia | China | Pig | PCR, WGS | CTX-M, TEM- SHV, OXA-48, NDM | qnrS, qnrA, aac(6’)-Ib-cr, qnrB, oqxAB, qnrD, qepA | - | [57] |
| Philippines | Poultry | PCR | CTX-M-1, CTX-M-15, CTX-M-25, CTX-M-2, CTX-M-8, CTX-M-9, TEM, SHV | - | - | [31] | |
| Thailand | Pig | PCR | CTX-M, TEM | - | - | [59] | |
| WGS | CTX-M-55, CTX-M-14, TEM-1B | sul1, sul2, sul3, qnrS1, tetA, tetD, aadA2, aph(3’)-Ia | |||||
| Pakistan | Cow | PCR | CTX-M-15, TEM | - | - | [60] | |
| Poultry | CTX-M-15, CTX-M-55, TEM | ||||||
| Thailand | Poultry | PCR, WGS | CTX-M-15, CTX-M-55, CTX-M-14, CTX-M-27, CTX-M-65 | - | - | [61]. | |
| Malaysia | Cow | PCR | CTX-M, TEM | - | - | [62] | |
| China | Poultry | PCR | CTX-M-14, CTX-M-9, CTX-M-55, CTX-M-15, CTX.M-1, CTX-M-65, CTX-M-74, CTX-M-25, TEM, SHV | qnrS, aac(6’)-Ib-cr, qnrB, qnrA | papC, iucD, iroN, iucD, iss, iutA, tsh, irp-2 | [63] | |
| India | Pig | PCR | TEM, CTX-M, CMY | tetA, tetB, sul1, sul2, aadA, dfrIa | - | [65] | |
| South Korea | Poultry | PCR | TEM-1, CTX-M-15, CTX-M-55, CTX-M-14, CTX-M-65 | - | - | [39] | |
| Pig | TEM-1, CTX-M-3, CTX-M-15, CTX-M-55, CTX-M-14, CTX-M-65 | ||||||
| Cow | CTX-M-15, CTX-M-55, CTX-M-65 | ||||||
| India | Poultry | PCR | CTX-M, TEM, SHV | - | - | [65] | |
| India | Pig | PCR | CTX-M | - | - | [66] | |
| China | Poultry | PCR | TEM | - | - | [38] | |
| Pig | CTX-M, TEM | ||||||
| Indonesia | Poultry | PCR | CTX-M | - | - | [67] | |
| Pakistan | Poultry | PCR | CTX-M-1, CTX-M-9, TEM | - | - | [87] | |
| Thailand | Pig | PCR | CTX-M-55, CTX-M-14, CTX-M-15, CTX-M-9, OXA-140, SHV-12 | - | - | [111] | |
| Korea | Pollo | PCR, WGS | CTX-M-55, CTX-M-14, CTX-M-65, CTX-M-1, CTX-M-27 | sul1, sul2, strA, strB, fosA, aac(3)-IId, mphA | - | [112] | |
| Thailand | Pig | PCR | CTX-M-55, CTX-M-14, TEM | mcr-1 | - | [100] | |
| Korea | Poultry | PCR | CTX-M-1, CTX-M-14, CTX-M-15, CTX-M-65, TEM-1 | dfrA1, aadA1 | - | [113] | |
| Vietnam | Pig | WGS | CTX-M-55, CTX-M-14, CTX-M-27, CTX-M-15, CTX-M-65, OXA-10 | mcr-1, mcr-3, qnrS1, qnrB19, aadA1, aph(3’)-Ia, aph(6)-Id, aac(3)-IId, aadA2, dfrA12, dfrA14, tetA, tetM, cmlA1, floR, mdfA, mefA, mefB, mphA, fosA3, aar2, aar3, sul1, sul2, chrA, merC, merE, merT | traT, ompT, sitA, lpfA, iss, terC, traT, chuA, fyuA | [101] | |
| China | Cow | PCR | CTX-M, TEM- SHV | - | - | [23] | |
| Malaysia | Pig, poultry | PCR | CTX-M | - | - | [33] | |
| Indonesia | Pig | PCR | TEM | - | - | [74] | |
| China | Poultry | PCR | TEM, CTX-M, OXA, SHV | - | - | [75] | |
| Cow | TEM, OXA, SHV | ||||||
| Pig | TEM, OXA, CTX-M, SHV | ||||||
| India | Poultry | PCR, WGS | TEM, SHV, OXA, CTX-M-1, CTX-M-2, CTX-M-9 | qnrS1, dfrA14, sul2, aph(3”)-Ib, aph(3’)-Ia, aph(6)-ld | cib, terC, traT | [76] | |
| Pakistan | Cow, poultry, sheep | PCR | CTX-M-1, CTX-M-9, CTX-M-2, TEM, SHV | mcr-1 | - | [77] | |
| Thailand | Pig | PCR | CTX-M-55, CTX-M-14, TEM-1 | - | - | [78] | |
| China | Sheep | WGS | CTX-M-55, CTX-M-15, CTX-M-14, CTX-M-65, CTX-M-17, TEM-1, TEM-150, TEM-235 | aph(6’)-Id aph(3’)-Ia, aph(3’’)-Ib, aadA1, aadA2, tetA, tetB, sul1, sul2, sul3, dfrA12, dfrA14, dfrA17, oqxA, oqxB, cmlA1, cmlA5, catA1, catA2, catA3, floR, mphA, ermB, fosA, mcr-1 | - | [79] | |
| Indonesia | Poultry | PCR | CTX-M, TEM | - | - | [94] | |
| Malaysia | Poultry | PCR | CTX-M, TEM | mcr-1 | - | [88] | |
| Europe | Spain | Cow, sheep | WGS | CTX-M-14, CTX-M-1, CTX-M-15, CTX-M-32 SHV-12, TEM-1B, TEM-190 OXA-1, OXA-10 | tetA, tetB, aac(3)-IIa, aac(3)-IId, qnrS, cml1, catA1, floR, mphA, sul1, sul2, sul3, fosA7 | - | [36] |
| Greek | Cow, pig | Microarrays | CTX-M-15, TEM | aadA1, aphA, strA, strB, qnrS, sul1, sul2, sul3, dfrA7, dfrA1, mph | - | [68] | |
| Portugal | Sheep | PCR | CTX-M-15, CTX-M-32, CTX-M-1, CTX-M-14, CTX-M-98, SHV-12 | aac(6’)-Ib-cr, qnrS, aac(3’)-II, tetA, tetB, sul1, sul2, sul3 | - | [70] | |
| Denmark, France, Germany, Hungary, Poland, Spain, Netherlands, UK | Poultry | WGS | CTX-M-1, CTX-M-14, SHV-12, TEM-52 | aadA1, aadA2, aadA5, tetA, tetB, dfrA1, dfrA7, sul1, sul2, sul3, catA1, floR, mphA, mphB | entABCDEFS, fimABCDEFGHI, csgABCDEFG | [71] | |
| Cow | CTX-M-1, CTX-M-2, SHV-12, TEM-52 | ||||||
| Pig | CTX-M-1, CTX-M-15 | ||||||
| Bosnia | Poultry | Microarrays | CTX-M-1, CTX-M-15, TEM, SHV | - | - | [72] | |
| Latvia | Pig | PCR | CTX-M, TEM, SHV | - | - | [4] | |
| France | Cow, pig, poultry | PCR | CTX-M-1, CTX-M-15, TEM-1B, TEM-1C | tetA | - | [73] | |
| Italy | Cow | PCR | CTX-M-1, CTX-M-9 | mcr-1, mcr-3 | - | [21] | |
| Pig | CTX-M-1, CTX-M-9 | mcr-1, mcr-4 | |||||
| Poultry | CTX-M-1, CTX-M-2, CTX-M-9, SHV-12 | mcr-1 | |||||
| Greece | Pig | Microarrays | CTX-M-15, CTX-M-9, CTX-M-8, SHV, TEM, OXA-1, OXA-60 | aadA1, aadA2, aadA4, aac(6’)-Ib, qnrS, qnrA, qnrB, sul1, sul2, sul3, dfrA1, dfrA5, dfrA7, dfrA12, mcr-1, mcr-2, mcr-4, mcr-8, mph, oqxA, oqxB | - | [103] | |
| Macedonia | Cow | PCR | CTX-M, SHV, TEM, OXA-1 | - | - | [93] | |
| Oceania | Australia | Pig | RT-PCR | CTX-M-1, TEM-1B | aadA5, dfrA17, dfrA5, sul2, tetA, strA, strB | eae, ehxA, paa | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandujano-Hernández, A.; Martínez-Vázquez, A.V.; Paz-González, A.D.; Herrera-Mayorga, V.; Sánchez-Sánchez, M.; Lara-Ramírez, E.E.; Vázquez, K.; de Jesús de Luna-Santillana, E.; Bocanegra-García, V.; Rivera, G. The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview. Animals 2024, 14, 2490. https://doi.org/10.3390/ani14172490
Mandujano-Hernández A, Martínez-Vázquez AV, Paz-González AD, Herrera-Mayorga V, Sánchez-Sánchez M, Lara-Ramírez EE, Vázquez K, de Jesús de Luna-Santillana E, Bocanegra-García V, Rivera G. The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview. Animals. 2024; 14(17):2490. https://doi.org/10.3390/ani14172490
Chicago/Turabian StyleMandujano-Hernández, Antonio, Ana Verónica Martínez-Vázquez, Alma D. Paz-González, Verónica Herrera-Mayorga, Mario Sánchez-Sánchez, Edgar E. Lara-Ramírez, Karina Vázquez, Erick de Jesús de Luna-Santillana, Virgilio Bocanegra-García, and Gildardo Rivera. 2024. "The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview" Animals 14, no. 17: 2490. https://doi.org/10.3390/ani14172490







