Distribution of Bovine Mastitis Pathogens in Quarter Milk Samples from Bavaria, Southern Germany, between 2014 and 2023—A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteriological Analysis
2.2. Statistical Analysis
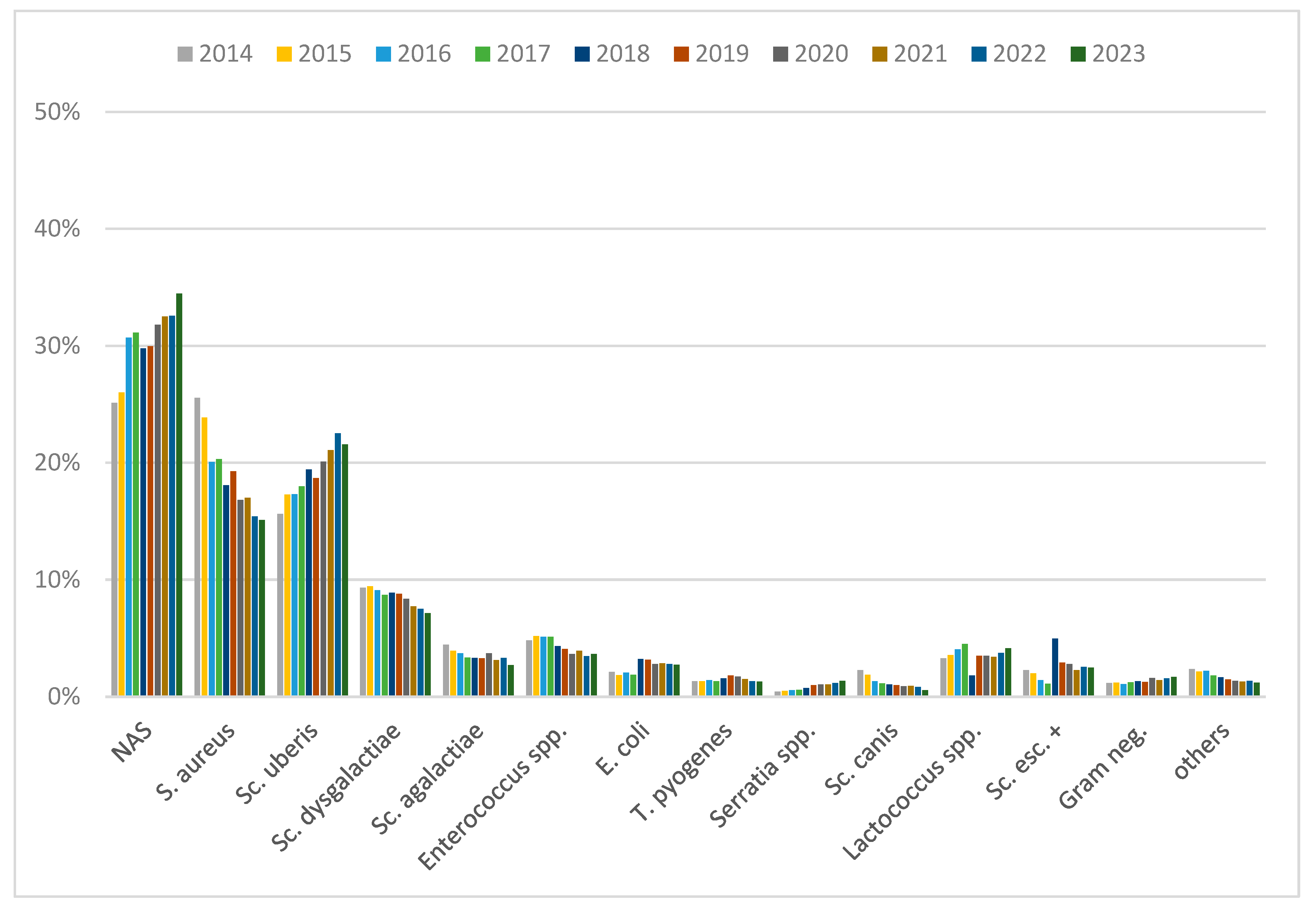
3. Results
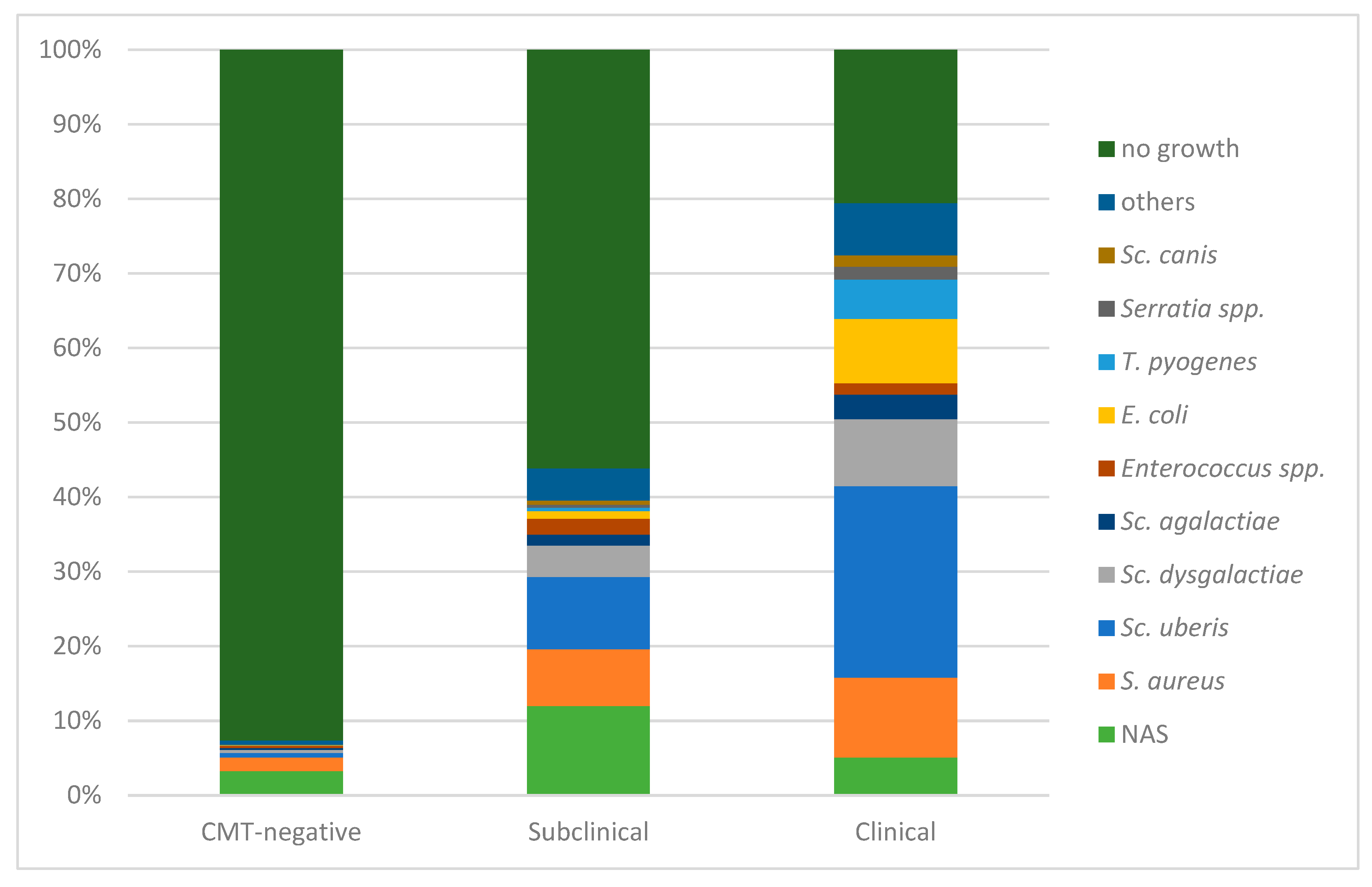
3.1. Distribution in Dependence of Mastitis Status
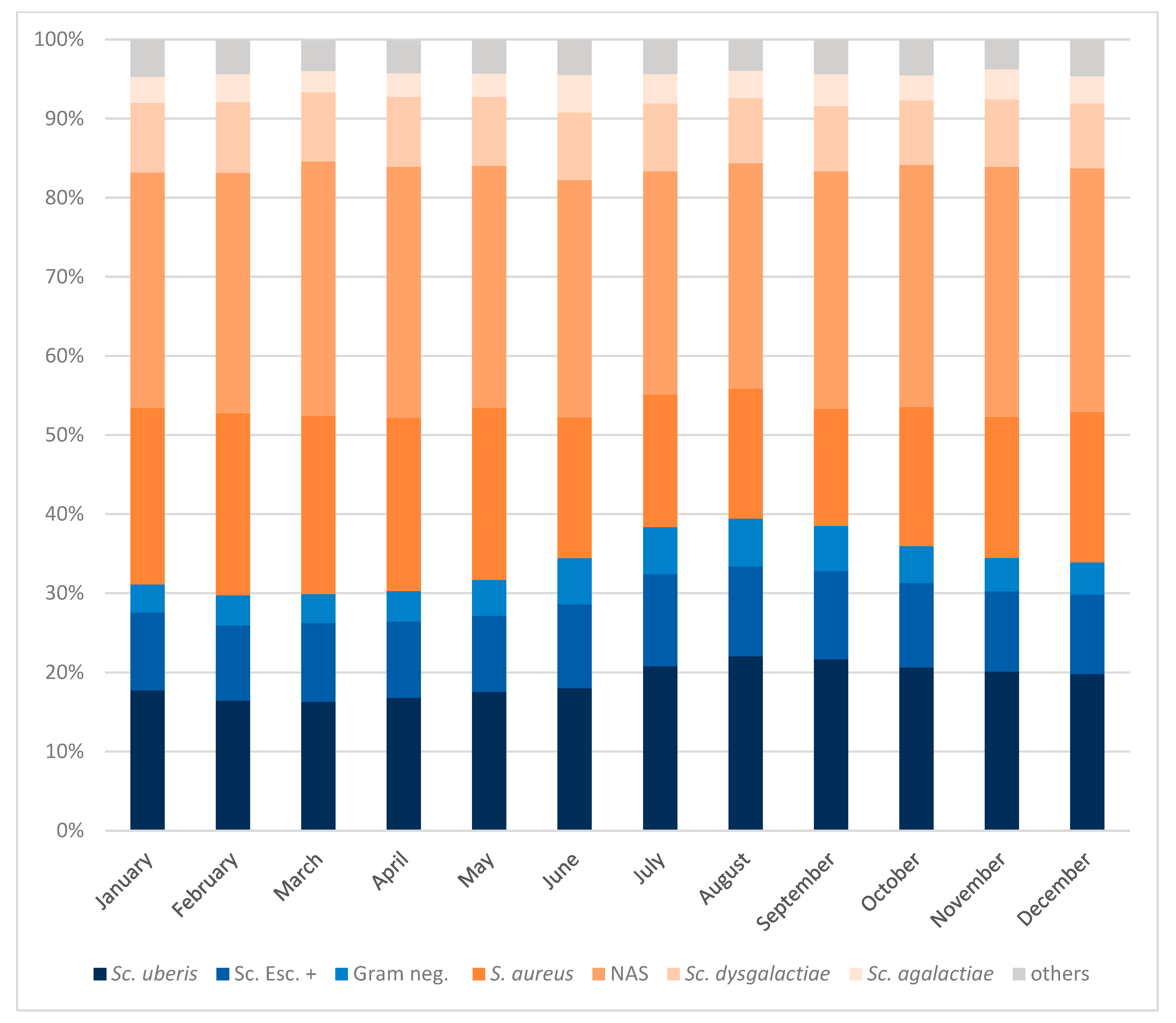
3.2. Seasonal Distribution of Mastitis Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Heikkilä, A.M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-specific production losses in bovine mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef]
- Kitila, G.; Kebede, B.; Wakgari, M. Prevalence, aetiology and risk factors of mastitis of dairy cows kept under extensive management system in west Wollega, western Oromia, Ethiopia. Vet. Med. Sci. 2021, 7, 1593–1599. [Google Scholar] [CrossRef]
- Meçaj, R.; Muça, G.; Koleci, X.; Sulçe, M.; Turmalaj, L.; Zalla, P.; Koni, A.; Tafaj, M. Bovine environmental mastitis and their control: An overview. Int. J. Agric. Biosci. 2023, 12, 216–221. [Google Scholar] [CrossRef]
- Sharif, A.; Umer, M.; Muhammad, G. Mastitis control in dairy production. J. Agric. Soc. Sci 2009, 5, 102–105. [Google Scholar]
- Khasa, V.; Chaudhary, V.; Singh, P. Mastitis: A review on disease affecting livestock and its control. J. Entomol. Zool. Stud. 2020, 8, 1393–1395. [Google Scholar]
- Reyher, K.K.; Dohoo, I.R.; Scholl, D.T.; Keefe, G.P. Evaluation of minor pathogen intramammary infection, susceptibility parameters, and somatic cell counts on the development of new intramammary infections with major mastitis pathogens. J. Dairy Sci. 2012, 95, 3766–3780. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, S.; Wente, N.; Zhang, Y.; Leimbach, S.; Gussmann, M.K.; Kirkeby, C.; Krömker, V. Strain diversity and infection durations of Staphylococcus spp. and Streptococcus spp. causing intramammary infections in dairy cows. J. Dairy Sci. 2023, 106, 4214–4231. [Google Scholar] [CrossRef]
- Middleton, J.R.; Saeman, A.; Fox, L.K.; Lombard, J.; Hogan, J.S.; Smith, K.L. The National Mastitis Council: A Global Organization for Mastitis Control and Milk Quality, 50 Years and Beyond. J. Mammary Gland Biol. Neoplasia 2014, 19, 241–251. [Google Scholar] [CrossRef]
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef]
- Hamel, J.; Zhang, Y.; Wente, N.; Krömker, V. Heat stress and cow factors affect bacteria shedding pattern from naturally infected mammary gland quarters in dairy cattle. J. Dairy Sci. 2021, 104, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Schmenger, A.; Krömker, V. Characterization, Cure Rates and Associated Risks of Clinical Mastitis in Northern Germany. Vet. Sci. 2020, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Huber-Schlenstedt, R.; Gey, A.; Schierling, K.; Sorge, U. Mastitis? TGD checkt die Erreger. Milchpur 2017, 1, 18–24. [Google Scholar]
- Bayerische Landesamt für Statistik. Zum Tag der Milch am 1. Juni in Bayern. Available online: https://www.statistik.bayern.de/presse/mitteilungen/2023/pm134/index.html (accessed on 20 August 2024).
- Bayerisches Landwirtschaftliches Wochenblatt. Bayern ist Fleckviehland. Available online: https://www.wochenblatt-dlv.de/feld-stall/tierhaltung/bayern-fleckviehland-571662 (accessed on 20 August 2024).
- Tergast, H.; Hansen, H.; Weber, E.-C. Steckbriefe zur Tierhaltung in Deutschland: Milchkühe; Thünen-Institut für Betriebswirtschaft: Braunschweig, Germany, 2022; pp. 1–17. [Google Scholar]
- DVG. Leitlinien zur Labordiagnostik der Mastitis—Probenahme und Mikrobiologische Untersuchung, 3rd ed.; DVG Service GmbH: Gießen, Germany, 2018. [Google Scholar]
- Zadoks, R.; Fitzpatrick, J. Changing trends in mastitis. Ir. Vet. J. 2009, 62 (Suppl. 4), S59–S70. [Google Scholar] [CrossRef] [PubMed]
- Goulart, D.B.; Mellata, M. Escherichia coli mastitis in dairy cattle: Etiology, diagnosis, and treatment challenges. Front. Microbiol. 2022, 13, 928346. [Google Scholar] [CrossRef]
- Wente, N.; Leimbach, S.; Woudstra, S.; Krömker, V. Trueperella Pyogenes—Strain Diversity and Occurrence in Dairy Herds. Pathogens 2024, 13, 534. [Google Scholar] [CrossRef]
- Tenhagen, B.A.; Koster, G.; Wallmann, J.; Heuwieser, W. Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Brandenburg, Germany. J. Dairy Sci. 2006, 89, 2542–2551. [Google Scholar] [CrossRef]
- DVG. Zur Prävalenz von Mastitiserregern in Milchproben in Deutschland—Update 2022. Available online: https://www.dvg.de/wp-content/uploads/2024/04/24-03-19_AG-Euter_Praevalenz-Mastitserreger2022.pdf (accessed on 20 August 2024).
- Smistad, M.; Bakka, H.C.; Sølverød, L.; Jørgensen, H.J.; Wolff, C. Prevalence of udder pathogens in milk samples from Norwegian dairy cows recorded in a national database in 2019 and 2020. Acta Vet. Scand. 2023, 65, 19. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Al-Harbi, H.; Ranjbar, S.; Moore, R.J.; Alawneh, J.I. Bacteria isolated from milk of dairy cows with and without clinical mastitis in different regions of Australia and their AMR profiles. Front. Vet. Sci. 2021, 8, 743725. [Google Scholar] [CrossRef] [PubMed]
- Condas, L.A.Z.; De Buck, J.; Nobrega, D.B.; Carson, D.A.; Naushad, S.; De Vliegher, S.; Zadoks, R.N.; Middleton, J.R.; Dufour, S.; Kastelic, J.P.; et al. Prevalence of non-aureus staphylococci species causing intramammary infections in Canadian dairy herds. J. Dairy Sci. 2017, 100, 5592–5612. [Google Scholar] [CrossRef]
- De Buck, J.; Ha, V.; Naushad, S.; Nobrega, D.B.; Luby, C.; Middleton, J.R.; De Vliegher, S.; Barkema, H.W. Non-aureus Staphylococci and Bovine Udder Health: Current Understanding and Knowledge Gaps. Front. Vet. Sci. 2021, 8, 658031. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, J.; Piepers, S.; Supré, K.; De Vliegher, S. Pathogen-specific incidence rate of clinical mastitis in Flemish dairy herds, severity, and association with herd hygiene. J. Dairy Sci. 2014, 97, 6926–6934. [Google Scholar] [CrossRef] [PubMed]
- Koivula, M.; Pitkälä, A.; Pyörälä, S.; Mäntysaari, E.A. Distribution of bacteria and seasonal and regional effects in a new database for mastitis pathogens in Finland. Acta Agric. Scand. A Anim. Sci. 2007, 57, 89–96. [Google Scholar] [CrossRef]
- Zigo, F.; Vasil, M.; Ondrašovičová, S.; Výrostková, J.; Bujok, J.; Pecka-Kielb, E. Maintaining Optimal Mammary Gland Health and Prevention of Mastitis. Front. Vet. Sci. 2021, 8, 607311. [Google Scholar] [CrossRef]
- Kortstegge, J.; Krömker, V. Prevalence of Contagious Mastitis Pathogens in Bulk Tank Milk from Dairy Farms in Lower Saxony, Germany. Hygiene 2024, 4, 122–134. [Google Scholar] [CrossRef]
- Fadlelmoula, A.; Fahr, R.D.; Anacker, G.; Swalve, H.H. The Management Practices Associated with Prevalence and Risk Factors of Mastitis in Large Scale Dairy Farms in Thuringia-Germany 1: Environmental Factors Associated With Prevalence of mastitis. Aust. J. Basic Appl. Sci. 2007, 1, 619–624. [Google Scholar]
- Wang, K.; Cha, J.; Liu, K.; Deng, J.; Yang, B.; Xu, H.; Wang, J.; Zhang, L.; Gu, X.; Huang, C.; et al. The prevalence of bovine mastitis-associated Staphylococcus aureus in China and its antimicrobial resistance rate: A meta-analysis. Front. Vet. Sci. 2022, 9, 1006676. [Google Scholar] [CrossRef]
- Munoz, B.; Lakner, S.; Brümmer, B. Determinants of Economic Growth in Organic Farming: The Case of Bavaria and Baden-Wuerttemberg. In Proceedings of the 2011 International Congress, Zurich, Switzerland, 30 August–2 September 2011. [Google Scholar]
- Acharya, K.R.; Brankston, G.; Slavic, D.; Greer, A.L. Spatio-temporal variation in the prevalence of major mastitis pathogens isolated from bovine milk samples between 2008 and 2017 in Ontario, Canada. Front. Vet. Sci. 2021, 8, 742696. [Google Scholar] [CrossRef]
- Karell, J.; Petzl, W.; Gangl, A.; Huber-Schlenstedt, R.; Sorge, U.S. Changes in antimicrobial resistance of Staphylococcus aureus in bovine quarter milk samples from southern Germany between 2012 and 2022. J. Dairy Sci. 2024, 107, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.F.; Bonsaglia, E.C.R.; Castilho, I.G.; Dantas, S.T.A.; Salina, A.; Langoni, H.; Pantoja, J.C.F.; Budri, P.E.; Fitzgerald-Hughes, D.; Júnior, A.F.; et al. Genotyping of long term persistent Staphylococcus aureus in bovine subclinical mastitis. Microb. Pathog. 2019, 132, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, K.R.; Heuer, C.; Parkinson, T.J.; Williamson, N.B. The incidence and aetiology of clinical bovine mastitis on 14 farms in Northland, New Zealand. N. Z. Vet. J. 2009, 57, 109–115. [Google Scholar] [CrossRef]
- Phuektes, P.; Mansell, P.D.; Dyson, R.S.; Hooper, N.D.; Dick, J.S.; Browning, G.F. Molecular epidemiology of Streptococcus uberis isolates from dairy cows with mastitis. J. Clin. Microbiol. 2001, 39, 1460–1466. [Google Scholar] [CrossRef]
- Schukken, Y.; Chuff, M.; Moroni, P.; Gurjar, A.; Santisteban, C.; Welcome, F.; Zadoks, R. The “Other” Gram-Negative Bacteria in Mastitis: Klebsiella, Serratia, and More. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 239–256. [Google Scholar] [CrossRef]
- Bundestierärztekammer. Die neue TÄHAV ist in Kraft. Dtsch. Tierärzteblatt 2018, 66, 484–489. [Google Scholar]
- Pirner, L.H.; Petzl, W.; Gangl, A.; Huber-Schlenstedt, R.; Sorge, U.S. In vitro antimicrobial resistance of Escherichia coli, Serratia marcescens, Klebsiella oxytoca and Klebsiella pneumoniae on Bavarian dairy farms between 2014–2022. J. Dairy Sci. 2024, in press. [Google Scholar] [CrossRef]
- Campos, F.C.; Castilho, I.G.; Rossi, B.F.; Bonsaglia É, C.R.; Dantas, S.T.A.; Dias, R.C.B.; Fernandes Júnior, A.; Hernandes, R.T.; Camargo, C.H.; Ribeiro, M.G.; et al. Genetic and Antimicrobial Resistance Profiles of Mammary Pathogenic E. coli (MPEC) Isolates from Bovine Clinical Mastitis. Pathogens 2022, 11, 1435. [Google Scholar] [CrossRef] [PubMed]
- Krebs, I.; Zhang, Y.; Wente, N.; Leimbach, S.; Krömker, V. Severity of Clinical Mastitis and Bacterial Shedding. Pathogens 2023, 12, 1098. [Google Scholar] [CrossRef]
- Bradley, A.J.; Leach, K.A.; Breen, J.E.; Green, L.E.; Green, M.J. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet. Rec. 2007, 160, 253–258. [Google Scholar] [CrossRef]
- Xu, T.; Cao, W.; Huang, Y.; Zhao, J.; Wu, X.; Yang, Z. The Prevalence of Escherichia coli Derived from Bovine Clinical Mastitis and Distribution of Resistance to Antimicrobials in Part of Jiangsu Province, China. Agriculture 2023, 13, 90. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine mastitis, a worldwide impact disease: Prevalence, antimicrobial resistance, and viable alternative approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef]
- Tomazi, T.; Ferreira, G.C.; Orsi, A.M.; Gonçalves, J.L.; Ospina, P.A.; Nydam, D.V.; Moroni, P.; Dos Santos, M.V. Association of herd-level risk factors and incidence rate of clinical mastitis in 20 Brazilian dairy herds. Prev. Vet. Med. 2018, 161, 9–18. [Google Scholar] [CrossRef]
- Gorniak, T.; Meyer, U.; Südekum, K.-H.; Dänicke, S. Impact of mild heat stress on dry matter intake, milk yield and milk composition in mid-lactation Holstein dairy cows in a temperate climate. Arch. Anim. Nutr. 2014, 68, 358–369. [Google Scholar] [CrossRef]
- Negri, R.; dos Santos Daltro, D.; Cobuci, J.A. Heat stress effects on somatic cell score of Holstein cattle in tropical environment. Livest. Sci. 2021, 247, 104480. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; El-Tarabany, M.S. Impact of three THI levels on somatic cell count, milk yield and composition of multiparous Holstein cows in a subtropical region. J. Therm. Biol. 2017, 64, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Gantner, V.; Popović, V.; Steiner, Z.; Gantner, R.; Potočnik, K. The differences in subclinical mastitis prevalence and effect on milk production due to cows’ breed and breeding region. In Proceedings of the 4th International Scientific Conference “Sustainable Agriculture and Rural Development”, Belgrade, Serbia, 14–15 December 2023. [Google Scholar]
- Olde Riekerink, R.G.M.; Barkema, H.W.; Stryhn, H. The Effect of Season on Somatic Cell Count and the Incidence of Clinical Mastitis. J. Dairy Sci. 2007, 90, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Ericsson Unnerstad, H.; Lindberg, A.; Persson Waller, K.; Ekman, T.; Artursson, K.; Nilsson-Öst, M.; Bengtsson, B. Microbial aetiology of acute clinical mastitis and agent-specific risk factors. Vet. Microbiol. 2009, 137, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rakib, M.R.H.; Zhou, M.; Xu, S.; Liu, Y.; Asfandyar Khan, M.; Han, B.; Gao, J. Effect of heat stress on udder health of dairy cows. J. Dairy Res. 2020, 87, 315–321. [Google Scholar] [CrossRef]
- Bokharaeian, M.; Toghdory, A.; Ghoorchi, T.; Ghassemi Nejad, J.; Esfahani, I.J. Quantitative Associations between Season, Month, and Temperature-Humidity Index with Milk Yield, Composition, Somatic Cell Counts, and Microbial Load: A Comprehensive Study across Ten Dairy Farms over an Annual Cycle. Animals 2023, 13, 3205. [Google Scholar] [CrossRef]
- Feliciano, R.J.; Boué, G.; Membré, J.-M. Overview of the Potential Impacts of Climate Change on the Microbial Safety of the Dairy Industry. Foods 2020, 9, 1794. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Hogan, J.S. Environmental Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 1993, 9, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.; Smith, K.L. Managing Environmental Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E. Impact and Mitigation of Heat Stress for Mastitis Control. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 473–478. [Google Scholar] [CrossRef]

| Year | QMS (n) | Herds (n) | Cows (n) | Herd (%) | Individual (%) | CMT 1 Negative (%) | Subclinical Mastitis (%) | Clinical Mastitis (%) |
|---|---|---|---|---|---|---|---|---|
| All | 3,886,162 | 15,609 | 634,022 | 83 | 17 | 71 | 27 | 2 |
| 2014 | 382,752 | 4957 | 97,364 | 87 | 13 | 74 | 25 | 2 |
| 2015 | 371,039 | 4820 | 94,878 | 86 | 14 | 72 | 27 | 2 |
| 2016 | 389,947 | 4646 | 99,713 | 83 | 17 | 72 | 27 | 2 |
| 2017 | 401,453 | 4719 | 103,335 | 81 | 19 | 71 | 27 | 2 |
| 2018 | 451,573 | 5767 | 116,397 | 81 | 19 | 71 | 27 | 2 |
| 2019 | 395,016 | 4899 | 101,980 | 82 | 18 | 73 | 25 | 2 |
| 2020 | 380,001 | 4689 | 98,385 | 82 | 18 | 67 | 31 | 2 |
| 2021 | 388,417 | 4479 | 99,747 | 82 | 18 | 69 | 29 | 2 |
| 2022 | 350,167 | 3773 | 89,689 | 82 | 18 | 70 | 27 | 2 |
| 2023 | 375,797 | 3774 | 96,993 | 83 | 17 | 72 | 26 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechtold, V.; Petzl, W.; Huber-Schlenstedt, R.; Sorge, U.S. Distribution of Bovine Mastitis Pathogens in Quarter Milk Samples from Bavaria, Southern Germany, between 2014 and 2023—A Retrospective Study. Animals 2024, 14, 2504. https://doi.org/10.3390/ani14172504
Bechtold V, Petzl W, Huber-Schlenstedt R, Sorge US. Distribution of Bovine Mastitis Pathogens in Quarter Milk Samples from Bavaria, Southern Germany, between 2014 and 2023—A Retrospective Study. Animals. 2024; 14(17):2504. https://doi.org/10.3390/ani14172504
Chicago/Turabian StyleBechtold, Verena, Wolfram Petzl, Reglindis Huber-Schlenstedt, and Ulrike S. Sorge. 2024. "Distribution of Bovine Mastitis Pathogens in Quarter Milk Samples from Bavaria, Southern Germany, between 2014 and 2023—A Retrospective Study" Animals 14, no. 17: 2504. https://doi.org/10.3390/ani14172504
APA StyleBechtold, V., Petzl, W., Huber-Schlenstedt, R., & Sorge, U. S. (2024). Distribution of Bovine Mastitis Pathogens in Quarter Milk Samples from Bavaria, Southern Germany, between 2014 and 2023—A Retrospective Study. Animals, 14(17), 2504. https://doi.org/10.3390/ani14172504





