Thriving or Striving: Comparing Intra-Uterine Growth Restricted, Low Birth Weight and Normal Birth Weight Piglets within the First 24 Hours
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Piglet Selection
2.4. Observational Study
2.5. Statistical Analysis
3. Results
3.1. Piglet Characteristics
3.2. Vitality Score and Umbilical Cord Condition
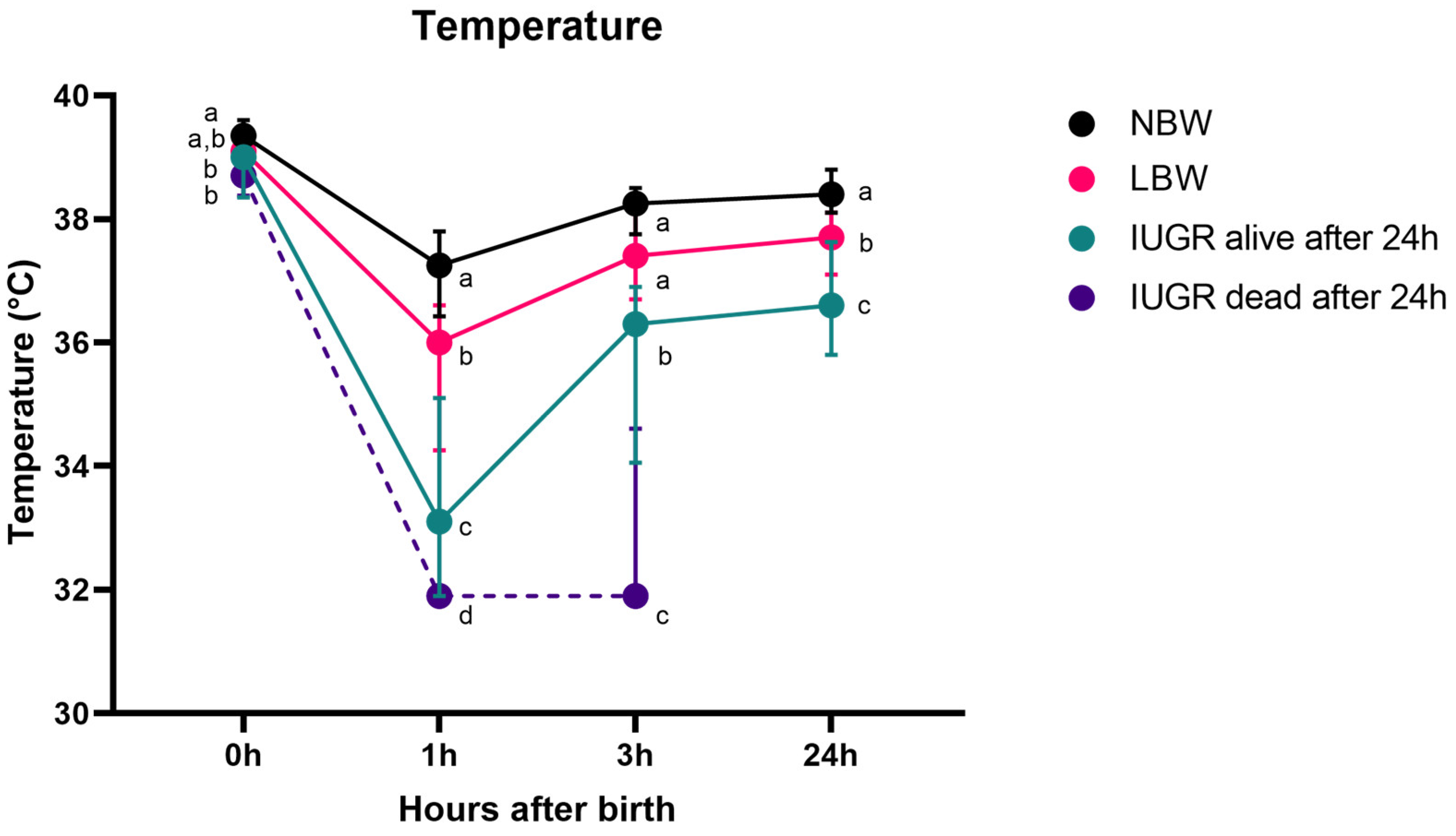
3.3. Body Temperature
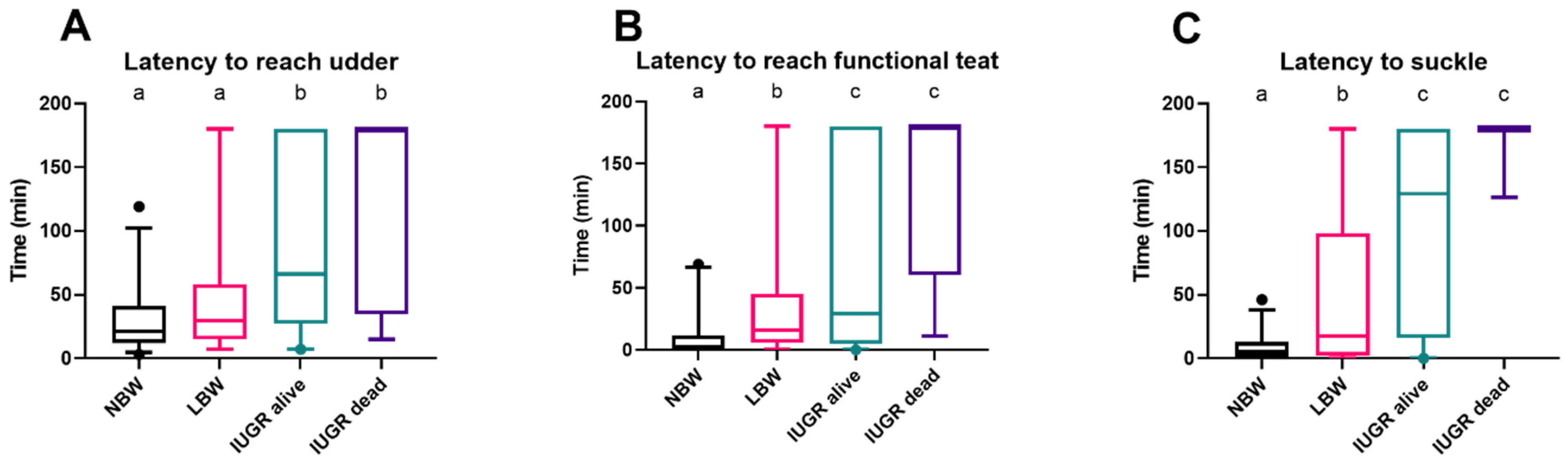
3.4. Latency
3.5. Weight Gain and Colostrum Intake
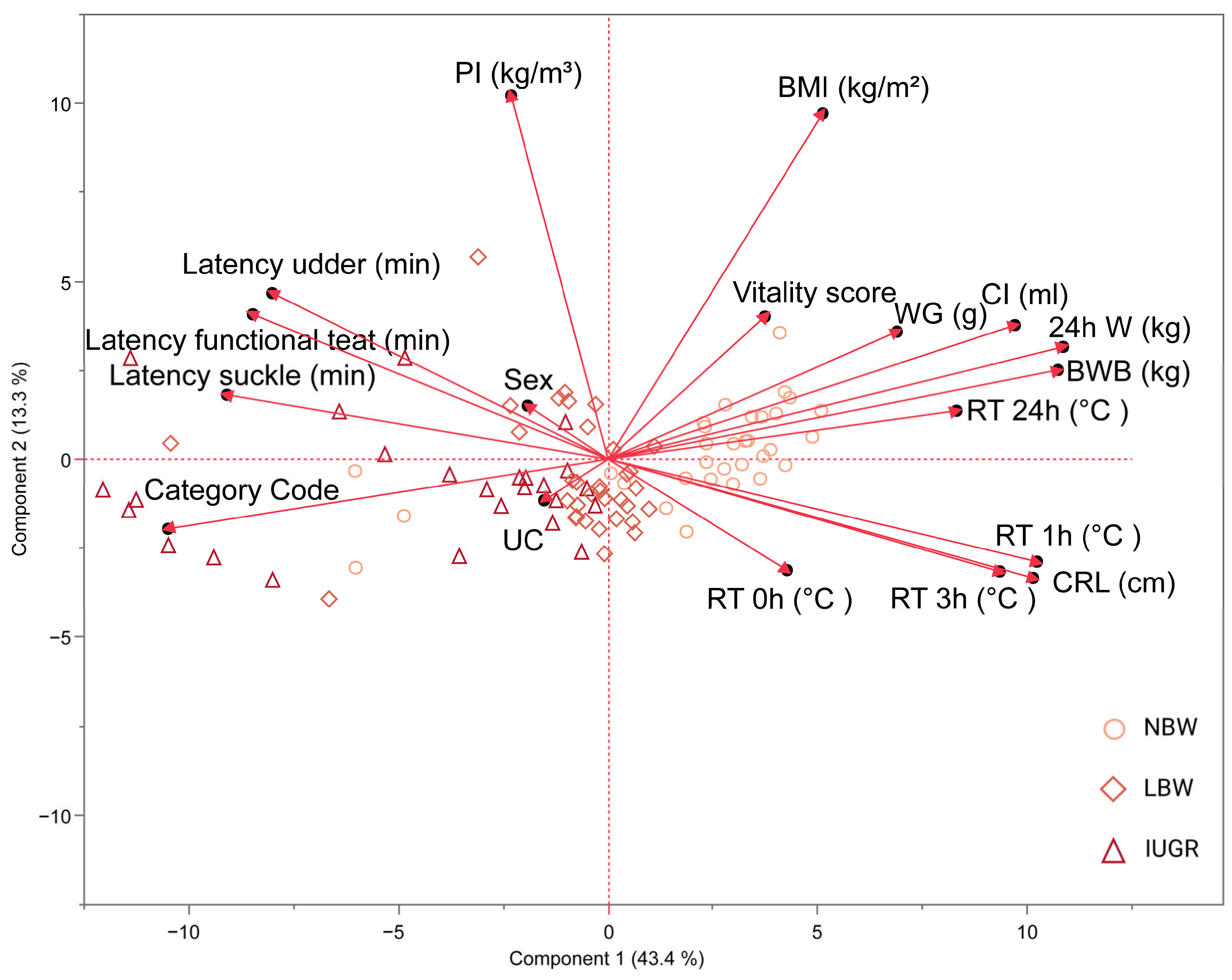
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huting, A.M.S.; Sakkas, P.; Wellock, I.; Almond, K.; Kyriazakis, I. Once small always small? To what extent morphometric characteristics and post-weaning starter regime affect pig lifetime growth performance. Porc. Health Manag. 2018, 4, 21. [Google Scholar] [CrossRef]
- Foxcroft, G.R.; Dixon, W.T.; Novak, S.; Putman, C.T.; Town, S.C.; Vinsky, M.D. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 2006, 84, E105–E112. [Google Scholar] [CrossRef] [PubMed]
- Riddersholm, K.V.; Bahnsen, I.; Bruun, T.S.; de Knegt, L.V.; Amdi, C. Identifying Risk Factors for Low Piglet Birth Weight, High Within-Litter Variation and Occurrence of Intrauterine Growth-Restricted Piglets in Hyperprolific Sows. Animals 2021, 11, 2731. [Google Scholar] [CrossRef]
- Oliviero, C. Offspring of hyper prolific sows: Immunity, birthweight, and heterogeneous litters. Mol. Reprod. Dev. 2023, 90, 580–584. [Google Scholar] [CrossRef]
- Amdi, C.; Krogh, U.; Flummer, C.; Oksbjerg, N.; Hansen, C.F.; Theil, P.K. Intrauterine growth restricted piglets defined by their head shape ingest insufficient amounts of colostrum. J. Anim. Sci. 2013, 91, 5605–5613. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Hales, J.; Amdi, C.; Moustsen, V. Intrauterine growth-restricted piglets defined by their head shape have impaired survival and growth during the suckling period. Anim. Prod. Sci. 2018, 59, 1056–1062. [Google Scholar] [CrossRef]
- Wientjes, J.G.; Soede, N.M.; Knol, E.F.; van den Brand, H.; Kemp, B. Piglet birth weight and litter uniformity: Effects of weaning-to-pregnancy interval and body condition changes in sows of different parities and crossbred lines. J. Anim. Sci. 2013, 91, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.P.; Jansman, A.J.M.; Verstegen, M.W.A.; den Hartog, L.A.; van Hees, H.M.J.; Bolhuis, J.E.; van Kempen, T.A.T.G.; Gerrits, W.J.J. Identifying the limitations for growth in low performing piglets from birth until 10 weeks of age. Animal 2014, 8, 923–930. [Google Scholar] [CrossRef]
- Beaulieu, A.D.; Aalhus, J.L.; Williams, N.H.; Patience, J.F. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 2010, 88, 2767–2778. [Google Scholar] [CrossRef]
- Matheson, S.M.; Walling, G.A.; Edwards, S.A. Genetic selection against intrauterine growth retardation in piglets: A problem at the piglet level with a solution at the sow level. Genet. Sel. Evol. 2018, 50, 46. [Google Scholar] [CrossRef]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Edwards, S.; Matheson, S.; Baxter, E. Genetic influences on intra-uterine growth retardation of piglet and management interventions for low birth weight piglets. In Nutrition of Hyperprolific Sows; Novus International, Inc.: Chesterfield, UK, 2019; pp. 207–235. [Google Scholar]
- Douglas, S.L.; Edwards, S.A.; Sutcliffe, E.; Knap, P.W.; Kyriazakis, I. Identification of risk factors associated with poor lifetime growth performance in pigs. J. Anim. Sci. 2013, 91, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Are all piglets born lightweight alike? Morphological measurements as predictors of postnatal performance1. J. Anim. Sci. 2016, 94, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.M.; Jarvis, S.; D’Eath, R.B.; Ross, D.W.; Robson, S.K.; Farish, M.; Nevison, I.M.; Lawrence, A.B.; Edwards, S.A. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 2008, 69, 773–783. [Google Scholar] [CrossRef]
- Baxter, E.M.; Jarvis, S.; Palarea-Albaladejo, J.; Edwards, S.A. The Weaker Sex? The Propensity for Male-Biased Piglet Mortality. PLoS ONE 2012, 7, e30318. [Google Scholar] [CrossRef]
- Baxter, E.M.; Jarvis, S.; Sherwood, L.; Robson, S.K.; Ormandy, E.; Farish, M.; Smurthwaite, K.M.; Roehe, R.; Lawrence, A.B.; Edwards, S.A. Indicators of piglet survival in an outdoor farrowing system. Livest. Sci. 2009, 124, 266–276. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Tiemann, U. Early identification of neonates at risk: Traits of newborn piglets with respect to survival. Theriogenology 2000, 54, 371–388. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- InterPIG. Pig Cost of Production in Selected Countries 2022. Available online: https://ahdb.org.uk/cost-of-production-in-selected-countries (accessed on 9 April 2024).
- Feldpausch, J.; Jourquin, J.; Bergstrom, J.; Bargen, J.; Bokenkroger, C.; Davis, D.; Gonzalez, J.; Nelssen, J.; Puls, C.; Trout, W.; et al. Birth weight threshold for identifying piglets at risk for preweaning mortality. Transl. Anim. Sci. 2019, 3, 633–640. [Google Scholar] [CrossRef]
- Huting, A.M.S.; Almond, K.; Wellock, I.; Kyriazakis, I. What is good for small piglets might not be good for big piglets: The consequences of cross-fostering and creep feed provision on performance to slaughter. J. Anim. Sci. 2017, 95, 4926–4944. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Degroote, J.; Van Ginneken, C.; Van Poucke, M.; Vergauwen, H.; Dam, T.M.T.; Vanrompay, D.; Peelman, L.J.; De Smet, S.; Michiels, J. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J. 2016, 30, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. BOARD-INVITED REVIEW: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Van Ginneken, C.; Ayuso, M.; Van Bockstal, L.; Van Cruchten, S. Preweaning performance in intrauterine growth-restricted piglets: Characteristics and interventions. Mol. Reprod. Dev. 2022, 90, 697–707. [Google Scholar] [CrossRef]
- D’Inca, R.; Gras-Le Guen, C.; Che, L.; Sangild, P.T.; Le Huërou-Luron, I. Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 2011, 99, 208–216. [Google Scholar] [CrossRef]
- Chevaux, E.; Sacy, A.; Le Treut, Y.; Martineau, G.P. IntraUterine Growth Retardation (IUGR): Morphological and behavioral description. In Proceedings of the 21st IPVS Congress, Vancouver, BC, Canada, 18–21 July 2010. [Google Scholar]
- Hales, J.; Moustsen, V.A.; Nielsen, M.B.F.; Hansen, C.F. Individual physical characteristics of neonatal piglets affect preweaning survival of piglets born in a noncrated system. J. Anim. Sci. 2013, 91, 4991–5003. [Google Scholar] [CrossRef]
- Balzani, A.; Cordell, H.J.; Edwards, S.A. Relationship of sow udder morphology with piglet suckling behavior and teat access. Theriogenology 2016, 86, 1913–1920. [Google Scholar] [CrossRef]
- Schmitt, O.; O’Driscoll, K. Use of infrared thermography to noninvasively assess neonatal piglet temperature. Transl. Anim. Sci. 2021, 5, txaa208. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.S.; Petrovski, K.R.; Craig, J.R.; Morrison, R.S.; Smits, R.J.; Kirkwood, R.N. Associations between Surface and Rectal Temperature Profiles of Low-Birth-Weight Piglets. Animals 2023, 13, 3259. [Google Scholar] [CrossRef]
- Herpin, P.; Le Dividich, J.; Berthon, D.; Hulin, J.C. Assessment of thermoregulatory and postprandial thermogenesis over the first 24 hours after birth in pigs. Exp. Physiol. 1994, 79, 1011–1019. [Google Scholar] [CrossRef]
- Van Tichelen, K.; Prims, S.; Ayuso, M.; Van Kerschaver, C.; Vandaele, M.; Degroote, J.; Van Cruchten, S.; Michiels, J.; Van Ginneken, C. Drenching Bovine Colostrum, Quercetin or Fructo-Oligosaccharides Has No Effect on Health or Survival of Low Birth Weight Piglets. Animals 2021, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Van Tichelen, K.; Prims, S.; Ayuso, M.; Van Bockstal, L.; Van Kerschaver, C.; Vandaele, M.; Degroote, J.; Van Cruchten, S.; Michiels, J.; Van Ginneken, C. The Effect of Drenching (Very) Low Birth Weight Piglets with a Dense, Concentrated Milk Replacer at Farms with Differing Farrowing Management. Animals 2023, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Rootwelt, V.; Reksen, O.; Farstad, W.; Framstad, T. Associations between intrapartum death and piglet, placental, and umbilical characteristics. J. Anim. Sci. 2012, 90, 4289–4296. [Google Scholar] [CrossRef] [PubMed]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef]
- Barnett, H.; Geys, H.; Jacobs, T.; Jaki, T. Methods for Non-Compartmental Pharmacokinetic Analysis With Observations Below the Limit of Quantification. Stat. Biopharm. Res. 2020, 13, 59–70. [Google Scholar] [CrossRef]
- Croghan, W.; Egeghy, P.P. Methods of Dealing with Values Below the Limit of Detection using SAS Carry. In Proceedings of the SouthEast SAS Users Group, St. Petersburg, FL, USA, 22–24 September 2003. [Google Scholar]
- Finkelstein, D.M. A Proportional Hazards Model for Interval-Censored Failure Time Data. Biometrics 1986, 42, 845–854. [Google Scholar] [CrossRef]
- Tucker, B.S.; Craig, J.R.; Morrison, R.S.; Smits, R.J.; Kirkwood, R.N. Piglet Viability: A Review of Identification and Pre-Weaning Management Strategies. Animals 2021, 11, 2902. [Google Scholar] [CrossRef]
- Rootwelt, V.; Reksen, O.; Farstad, W.; Framstad, T. Postpartum deaths: Piglet, placental, and umbilical characteristics. J. Anim. Sci. 2013, 91, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Edwards, S.A. 1. The neonatal pig: Developmental influences on vitality. In The Suckling and Weaned Piglet; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020; pp. 9–39. [Google Scholar]
- Baxter, E.; Schmitt, O.; Pedersen, L. 3. Managing the litter from hyperprolific sows. In The Suckling and Weaned Piglet; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 71–106. [Google Scholar]
- Farmer, C.; Edwards, S.A. Review: Improving the performance of neonatal piglets. Animal 2022, 16, 100350. [Google Scholar] [CrossRef]
- Tucker, B.S.; Petrovski, K.R.; Craig, J.R.; Morrison, R.S.; Smits, R.J.; Kirkwood, R.N. Piglet Morphology: Indicators of Neonatal Viability? Animals 2022, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Vanden Hole, C.; Ayuso, M.; Aerts, P.; Prims, S.; Van Cruchten, S.; Van Ginneken, C. Glucose and glycogen levels in piglets that differ in birth weight and vitality. Heliyon 2019, 5, e02510. [Google Scholar] [CrossRef] [PubMed]
- Devillers, N.; van Milgen, J.; Prunier, A.; Le Dividich, J. Estimation of colostrum intake in the neonatal pig. Anim. Sci. 2004, 78, 305–313. [Google Scholar] [CrossRef]
- Ward, S.A.; Kirkwood, R.N.; Plush, K.J. Are Larger Litters a Concern for Piglet Survival or an Effectively Manageable Trait? Animals 2020, 10, 309. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Dividich, J.L.; Rooke, J.A.; Herpin, P. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 2005, 143, 469–485. [Google Scholar] [CrossRef]
- Klobasa, F.; Werhahn, E.; Butler, J.E. Composition of sow milk during lactation. J. Anim. Sci. 1987, 64, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Rooke, J.A.; Bland, I.M. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 2002, 78, 13–23. [Google Scholar] [CrossRef]
- Vanden Hole, C.; Aerts, P.; Prims, S.; Ayuso, M.; Van Cruchten, S.; Van Ginneken, C. Does intrauterine crowding affect locomotor development? A comparative study of motor performance, neuromotor maturation and gait variability among piglets that differ in birth weight and vitality. PLoS ONE 2018, 13, e0195961. [Google Scholar] [CrossRef]
- Vanden Hole, C.; Goyens, J.; Prims, S.; Fransen, E.; Hernando, M.A.; Van Cruchten, S.; Aerts, P.; Van Ginneken, C. How innate is locomotion in precocial animals? A study on the early development of spatio-temporal gait variables and gait symmetry in piglets. J. Exp. Biol. 2017, 220, 2706–2716. [Google Scholar] [CrossRef]
- Wixey, J.A.; Lee, K.M.; Miller, S.M.; Goasdoue, K.; Colditz, P.B.; Tracey Bjorkman, S.; Chand, K.K. Neuropathology in intrauterine growth restricted newborn piglets is associated with glial activation and proinflammatory status in the brain. J. Neuroinflamm. 2019, 16, 5. [Google Scholar] [CrossRef]
- Mielke, F.; Van Ginneken, C.; Aerts, P. A workflow for automatic, high precision livestock diagnostic screening of locomotor kinematics. Front. Vet. Sci. 2023, 10, 1111140. [Google Scholar] [CrossRef] [PubMed]
- Aerts, P.; Mielke, F.; Vanden Hole, C.; Van Gorp, M.J.W.; Van Ginneken, C. Early Development of Locomotion in the Term Piglet Model: Does Size Matter? Integr. Comp. Biol. 2023, 63, 610–624. [Google Scholar] [CrossRef]
- Engelsmann, M.N.; Hansen, C.F.; Nielsen, M.N.; Kristensen, A.R.; Amdi, C. Glucose Injections at Birth, Warmth and Placing at a Nurse Sow Improve the Growth of IUGR Piglets. Animals 2019, 9, 519. [Google Scholar] [CrossRef]
- Tucker, B.S.; Petrovski, K.R.; Kirkwood, R.N. Neonatal Piglet Temperature Changes: Effect of Intraperitoneal Warm Saline Injection. Animals 2022, 12, 1312. [Google Scholar] [CrossRef]
- Van Tichelen, K. The Impact of Drenching on the Pre-Weaning Resilience of Low Birth Weight Piglets; University of Antwerp, Faculty of Pharmaceutical, Biomedical and Veterinary Sciences: Antwerp, Belgium, 2024; p. 149. [Google Scholar]
- Geiping, L.; Hartmann, M.; Kreienbrock, L.; grosse Beilage, E. Killing underweighted low viable newborn piglets: Which health parameters are appropriate to make a decision? Porc. Health Manag. 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.M.; Rutherford, K.M.D.; D’Eath, R.B.; Arnott, G.; Turner, S.P.; SandØe, P.; Moustsen, V.A.; Thorup, F.; Edwards, S.A.; Lawrence, A.B. The welfare implications of large litter size in the domestic pig II: Management factors. Anim. Welf. 2013, 22, 219–238. [Google Scholar] [CrossRef]
- Ramirez, B.C.; Hayes, M.D.; Condotta, I.C.F.S.; Leonard, S.M. Impact of housing environment and management on pre-/post-weaning piglet productivity. J. Anim. Sci. 2022, 100, skac142. [Google Scholar] [CrossRef] [PubMed]
- Kobek-Kjeldager, C.; Moustsen, V.A.; Theil, P.K.; Pedersen, L.J. Effect of litter size, milk replacer and housing on production results of hyper-prolific sows. Animal 2020, 14, 824–833. [Google Scholar] [CrossRef]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Management strategies to improve the performance of low birth weight pigs to weaning and their long-term consequences. J. Anim. Sci. 2014, 92, 2280–2288. [Google Scholar] [CrossRef]
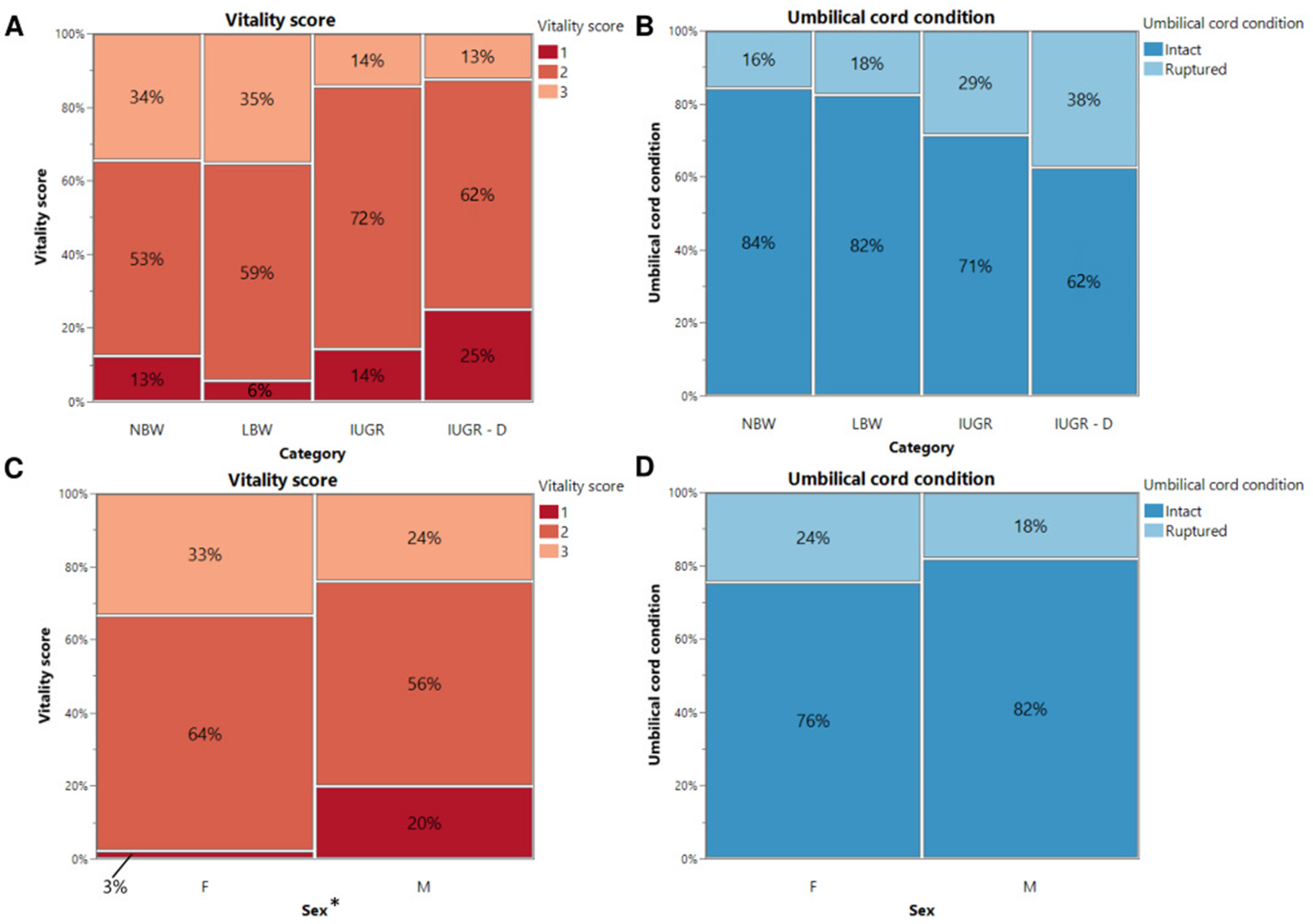

| Vitality Score | Description |
|---|---|
| 0 | Piglet shows no movement and no breathing after 15 s (stillborn). |
| 1 | Piglet shows no movement after 15 s, is breathing or attempts are made to breath (coughing, spluttering, clearing its lungs). |
| 2 | Piglet shows some movement and attempts are made to right themselves onto the sternum within 15 s, is breathing or attempting to breath. |
| 3 | Piglet shows good movement and good breathing. Piglet rights itself onto sternum and attempts are made to stand within 15 s. |
| 4 | Piglet shows good movement and good breathing. Piglet stands within 1 min. |
| NBW | LBW | IUGR | p-Value | |
|---|---|---|---|---|
| n | 32 (16 ♂–16 ♀) | 34 (18 ♂–16 ♀) | 29 (16 ♂–13 ♀) | |
| BWB, kg | 1.30 a (1.12–1.44) | 0.75 b (0.70–0.78) | 0.57 c (0.49–0.63) | <0.0001 |
| CRL, cm | 26.0 a (25.0–28.0) | 22.0 b (21.0–23.5) | 20.0 c (18.0–21.8) | <0.0001 |
| BMI, kg/m2 | 18.4 a (17.0–21.1) | 15.3 b (14.0–17.5) | 14.3 c (12.7–15.3) | <0.0001 |
| PI, kg/m3 | 68.0 a (63.1–80.5) | 69.9 a (58.0–85.2) | 71.3 a (56.3–83.2) | 0.805 |
| Mortality, % | 0 a | 0 a | 31 b | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loyens, M.; Van Bockstal, L.; Prims, S.; Van Cruchten, S.; Van Ginneken, C. Thriving or Striving: Comparing Intra-Uterine Growth Restricted, Low Birth Weight and Normal Birth Weight Piglets within the First 24 Hours. Animals 2024, 14, 2508. https://doi.org/10.3390/ani14172508
Loyens M, Van Bockstal L, Prims S, Van Cruchten S, Van Ginneken C. Thriving or Striving: Comparing Intra-Uterine Growth Restricted, Low Birth Weight and Normal Birth Weight Piglets within the First 24 Hours. Animals. 2024; 14(17):2508. https://doi.org/10.3390/ani14172508
Chicago/Turabian StyleLoyens, Marlotte, Lieselotte Van Bockstal, Sara Prims, Steven Van Cruchten, and Chris Van Ginneken. 2024. "Thriving or Striving: Comparing Intra-Uterine Growth Restricted, Low Birth Weight and Normal Birth Weight Piglets within the First 24 Hours" Animals 14, no. 17: 2508. https://doi.org/10.3390/ani14172508





