Diversity and Interactions between Picobiine Mites and Starlings
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Sampling
2.2. Mites Preparation, Identification, and Depository
2.3. Bird Systematics and Zoogeographical Regions
2.4. Statistical Analyses
3. Results
3.1. Systematics
3.1.1. Picobia malayi Patan and Skoracki sp. n. (Figure 1 and Figure 2)

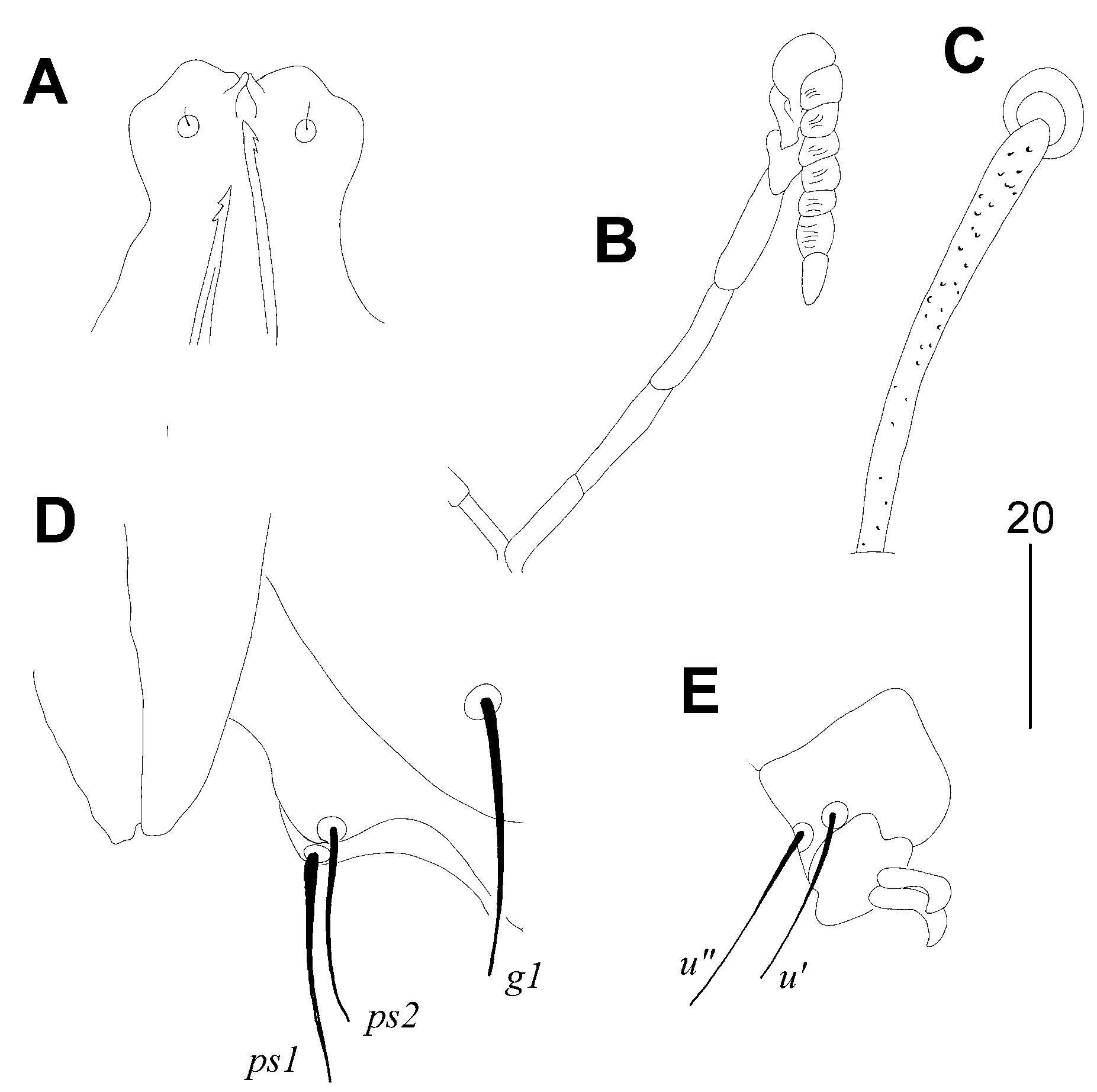
Type Material
Type Material Deposition
Additional Material
Differential Diagnosis
Etymology
3.1.2. Picobia indonesiana Skoracki and Glowska, 2008
3.1.3. Picobia lamprotornis Klimovicova, Skoracki, Wamiti and Hromada, 2014
3.1.4. Picobia sturni Skoracki, Bochkov and Wauthy, 2004
3.1.5. Picobia wisniewskii Patan, Skoracki and Marcisova, 2024
3.1.6. Key to the Species of the Picobiine Mites (Females) Associated with Starlings
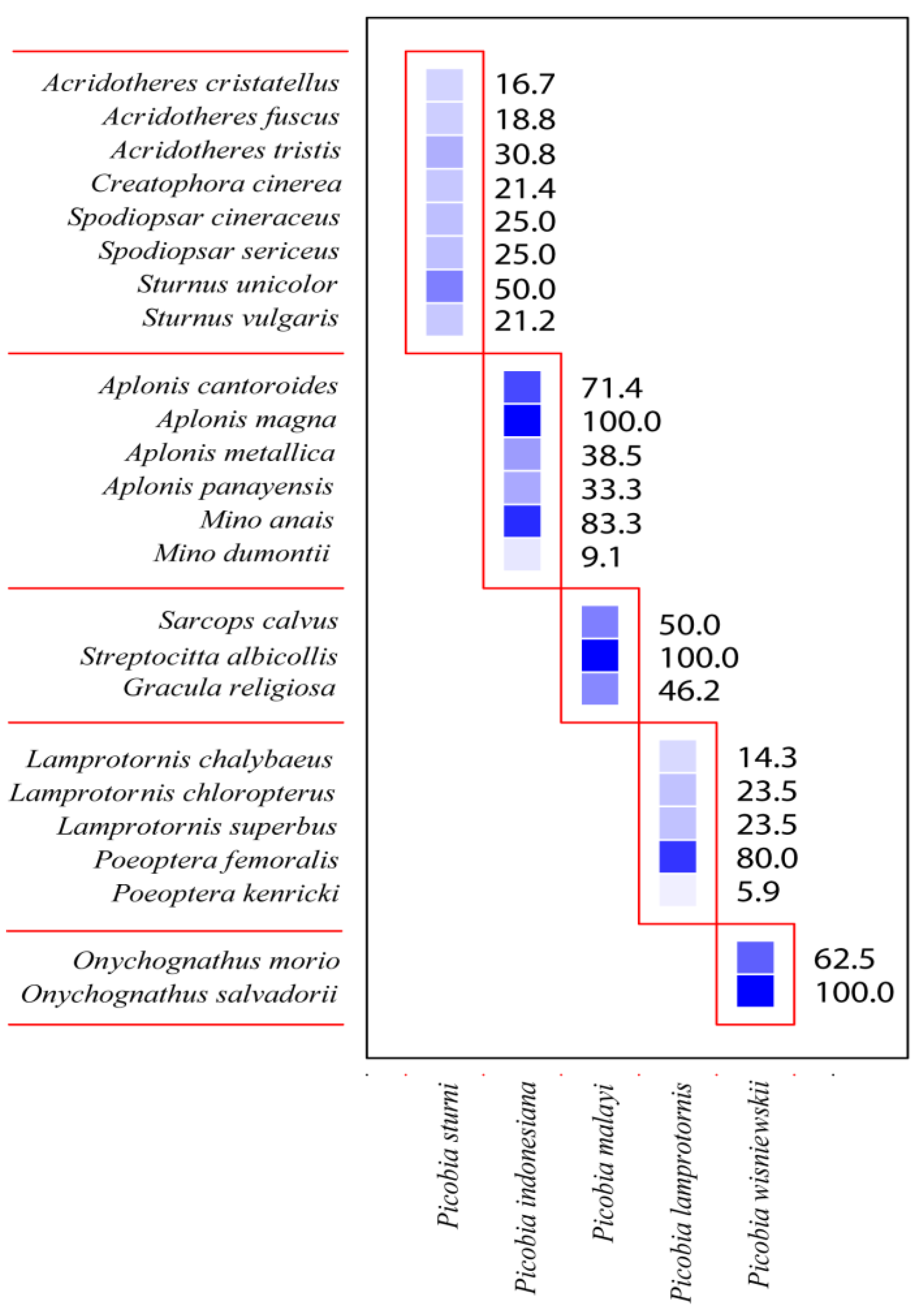
3.2. Prevalence
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kethley, J.B. A revision of the family Syringophilidae (Prostigmata: Acarina). Contrib. Am. Entomol. Inst. 1970, 6, 1–76. [Google Scholar]
- Johnston, D.E.; Kethley, J.B. A numerical phenetic study of the quill mites of the family Syringophilidae (Acari). J. Parasitol. 1973, 59, 520–530. [Google Scholar] [CrossRef]
- Casto, S.D. Cuculiphilus lobatus gen. n., sp. n. representing a new subfamily of quill mites (Acarina: Syringophilidae) from the Groove-billed ani Crotophaga sulcirostris (Cuculiformes: Cuculidae). Southwest Nat. 1977, 22, 169–176. [Google Scholar] [CrossRef]
- Kethley, J.B. Population regulation in quill mites (Acari: Syringophilidae). Ecology 1971, 52, 1113–1118. [Google Scholar] [CrossRef]
- Filimonova, S.A.; Mironov, S.V. Functional morphology of the gnathosoma in the quill mite Syringophilopsis fringilla Fritsch (Acari: Prostigmata: Syringohpilidae). Zool. Anz. 2010, 249, 165–180. [Google Scholar] [CrossRef]
- Casto, S.D. Quill wall thickness and feeding of Syringophiloidus minor (Berlese) (Acarina: Syringophilidae). Ann. Entomol. Soc. Am. 1974, 67, 824. [Google Scholar] [CrossRef]
- Casto, S.D. Entry and exit of syringophilid mites (Acarina: Syringophilidae) from the lumen of the quill. Wilson Bull. 1974, 86, 272–278. [Google Scholar]
- Skoracki, M.; Sikora, B.; Spicer, G.S. A review of the subfamily Picobiinae Johnston and Kethley, 1973 (Acariformes: Prostigmata: Syringophilidae). Zootaxa 2016, 4113, 1–95. [Google Scholar] [CrossRef]
- Skoracki, M.; Sikora, B.; Jerzak, L.; Hromada, M. Tanopicobia gen. nov., a new genus of quill mites, its phylogenetic placement in the subfamily Picobiinae (Acariformes: Syringophilidae) and picobiine relationships with avian hosts. PLoS ONE 2020, 15, e0225982. [Google Scholar] [CrossRef]
- Fain, A.; Bochkov, A.V.; Mironov, S.V. New genera and species of quill mites of the family Syringophilidae (Acari: Prostigmata). Bull. Inst. R. Sci. Nat. Belg. 2000, 70, 33–70. [Google Scholar]
- Skoracki, M. Quill mites (Acari: Syringophilidae) of the Palaearctic region. Zootaxa 2011, 2840, 1–414. [Google Scholar] [CrossRef]
- Glowska, E.; Schmidt, B.K. New quill mites (Cheyletoidea: Syringophilidae) parasitising the black-headed paradise flycatcher Terpsiphone rufiventer (Passeriformes: Monarchidae) in Gabon. Zootaxa 2014, 3786, 57–64. [Google Scholar] [CrossRef]
- Skoracki, M.; Hromada, M. A review of picobiine mites (Acari: Syringophilidae: Picobiinae) parasitising African birds. Folia Parasitol. 2013, 60, 192–212. [Google Scholar] [CrossRef]
- Marciniak-Musial, N.; Skoracki, M.; Kosicki, J.Z.; Unsöld, M.; Sikora, B. Host-parasite relationships of quill mites (Syringophilidae) and parrots (Psittaciformes). Diversity 2023, 15, 1. [Google Scholar] [CrossRef]
- Zmudzinski, M.; Skoracki, M.; Sikora, B. An Updated Checklist of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata). 2023. Available online: https://figshare.com/articles/dataset/An_updated_checklist_of_quill_mites_of_the_family_Syringophilidae_Acariformes_Prostigmata_/16529574 (accessed on 3 July 2024).
- Fjeldså, J.; Christidis, L.; Ericson, P.G.P. The Largest Avian Radiation: The Evolution of Perching Birds, or the Order Passeriformes; Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Starlings (Sturnidae), version 1.0. In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Clements, J.F.; Rasmussen, P.C.; Schulenberg, T.S.; Iliff, M.J.; Fredericks, T.A.; Gerbracht, J.A.; Lepage, D.; Spencer, A.; Billerman, S.M.; Sullivan, B.L.; et al. The eBird/Clements Checklist of Birds of the World. 2023. Available online: https://www.birds.cornell.edu/clementschecklist/download/ (accessed on 3 July 2024).
- Klimovicova, M.; Skoracki, M.; Wamiti, W.; Hromada, M. Quill mites of the subfamily Picobiinae (Acari: Syringophilidae) parasitising African birds, with description of two new species. Folia Parasitol. 2014, 61, 394–400. [Google Scholar] [CrossRef]
- Skoracki, M.; Bochkov, A.V.; Wauthy, G. Revision of the quill mites of the genus Picobia Haller, 1878 (Acari: Syringophilidae) with notes on their host-parasites relationships. Insect Syst. Evol. 2004, 35, 155–176. [Google Scholar] [CrossRef]
- Skoracki, M.; Patan, M.; Unsoeld, M.; Hromada, M.; Kwieciński, Z.; Marcisova, I. Diversity of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Starlings of the Genus Lamprotornis (Passeriformes: Sturnidae). Diversity 2024, 16, 51. [Google Scholar] [CrossRef]
- Walter, D.E.; Krantz, G.W. Collecting, rearing, and preparing specimens. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 83–96. [Google Scholar]
- Grandjean, F. Les segments postlarvaires de l’hysterosoma chez les oribates (Acariens). Bull. Soc. Zool. Fr. 1939, 64, 273–284. [Google Scholar]
- Kethley, J.B. Acarina: Prostigmata (Actinedida). In Soil Biology Guide; Dindal, D.L., Ed.; John Wiley & Sons: New York, NY, USA, 1990; pp. 667–756. [Google Scholar]
- Grandjean, F. Observations sure les Acariens de la famille des Stigmaeidae. Archiv. Sci. Phys. Nat. 1944, 26, 103–131. [Google Scholar]
- Holt, B.G.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [Google Scholar] [CrossRef]
- Ficetola, G.; Mazel, F.; Thuiller, W. Global determinants of zoogeographical boundaries. Nat. Ecol. Evol. 2017, 1, 0089. [Google Scholar] [CrossRef] [PubMed]
- Dormann, F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. Interaction 2008, 1, 8–11. [Google Scholar]
- Dormann, C.F.; Strauss, R. A method for detecting modules in quantitative bipartite networks. Methods Ecol. Evol. 2014, 5, 90–98. [Google Scholar] [CrossRef]
- Reiczigel, J.; Rozsa, L.; Reiczigel, A.; Fabian, I. Quantitative Parasitology (QPweb). 2013. Available online: https://www2.univet.hu/qpweb/qp10/index.php (accessed on 3 July 2024).
- Rozsa, L.; Reiczigel, J.; Majoros, G. Quantifying parasites in samples of hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analysing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Skoracki, M.; Glowska, E. Two new species of the genus Picobia Haller (Acari: Syringophilidae) from Australian and Indonesian passeriform birds. N. Z. J. Zool. 2008, 35, 281–286. [Google Scholar] [CrossRef]
- Patan, M.; Skoracki, M.; Marcisova, I.; Hromada, M.; Sikora, B. Picobiinae mites (Acariformes: Syringophilidae) parasitising the Starlings (Passeriformes: Sturnidae) in the Afrotropical region. Ann. Zool. 2024, 3. (in press). [Google Scholar] [CrossRef]
- Skoracki, M.; Michalik, J.; Sikora, B. Prevalence and habitat preference of quill mites (Acari, Syringophilidae) parasitising forest passerine birds in Poland. Acta Parasitol. 2010, 55, 188–193. [Google Scholar] [CrossRef]
- Lovette, I.J.; Rubenstein, D.R. A comprehensive molecular phylogeny of the starlings (Aves: Sturnidae) and mockingbirds (Aves: Mimidae): Congruent mtDNA and nuclear trees for a cosmopolitan avian radiation. Mol. Phylogenet. Evol. 2007, 44, 1031–1056. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.K.; Cibois, A.; Schikler, P.; Cracraft, J. Phylogeny and diversification of the largest avian radiation. Proc. Natl. Acad. Sci. USA 2004, 101, 11040–11045. [Google Scholar] [CrossRef] [PubMed]
- Voelker, G.; Spellman, G.M. Nuclear and mitochondrial DNA evidence of polyphyly in the avian superfamily Muscicapoidea. Mol. Phylogenet. Evol. 2004, 30, 386–394. [Google Scholar] [CrossRef]
- Ericson, P.G.P.; Johansson, U.S. Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data. Mol. Phylogenet. Evol. 2003, 29, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Cibois, A.; Cracraft, J. Assessing the passerine ‘‘Tapestry’’: Phylogenetic relationships of the Muscicapoidea inferred from nuclear DNA sequences. Mol. Phylogenet. Evol. 2004, 32, 264–273. [Google Scholar] [CrossRef]
- Sibley, C.G.; Ahlquist, J.E. The relationships of the starlings (Sturnidae: Sturnini) and the mockingbirds (Sturnidae: Mimini). Auk 1984, 101, 230–243. [Google Scholar] [CrossRef]
- Sibley, C.G.; Monroe, B.L. Distribution and Taxonomy of Birds of the World; Yale University Press: New Haven, CT, USA, 1990. [Google Scholar]
- Zuccon, D.; Cibois, A.; Pasquet, E.; Ericson, P.G.P. Nuclear and Mitochondrial Sequence Data Reveal the Major Lineages of Starlings, Mynas and Related Taxa. Mol. Phylo. Evol. 2006, 41, 333–344. [Google Scholar] [CrossRef] [PubMed]
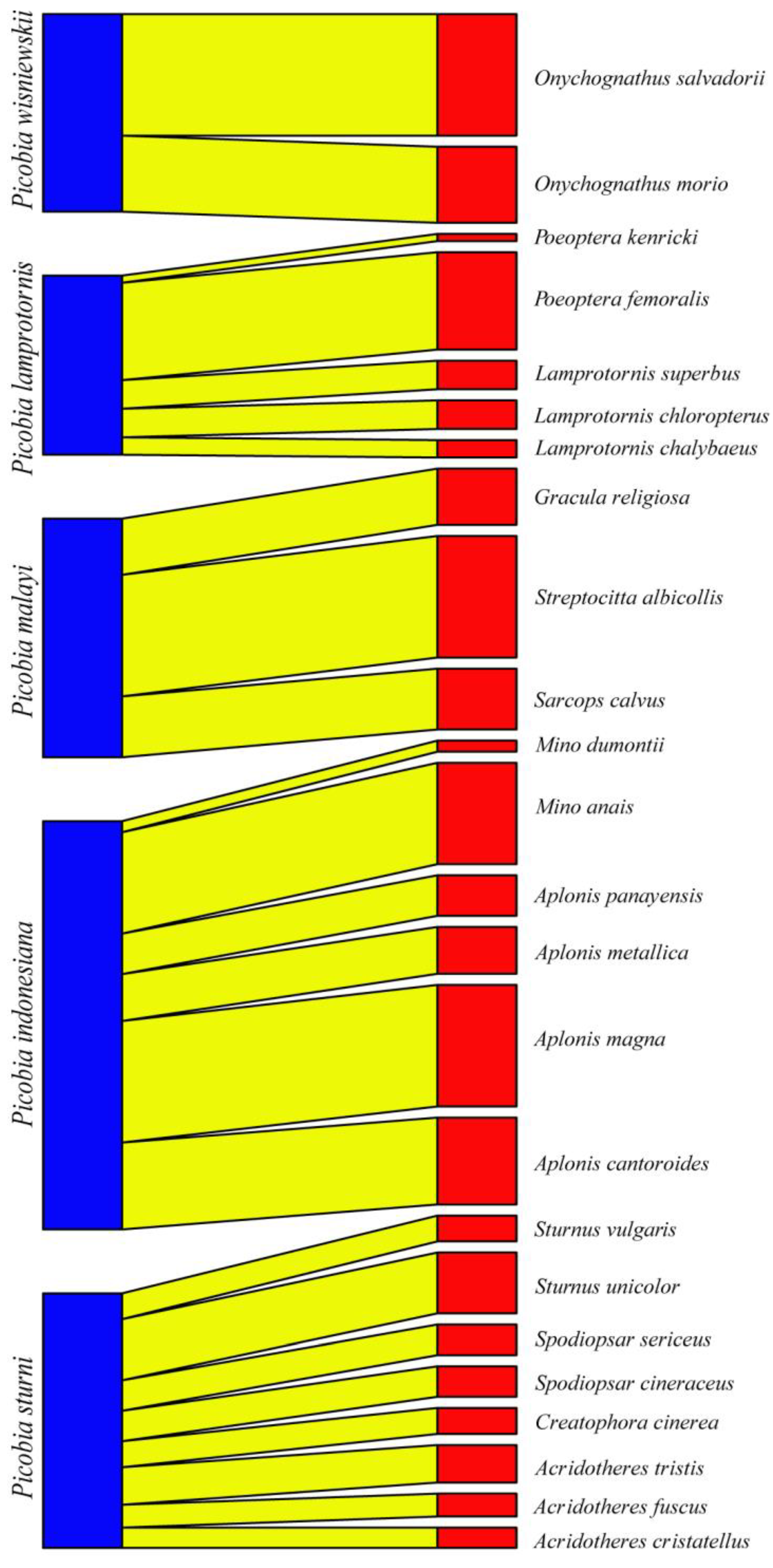
| Mite Species | Host Species | Locality | References |
|---|---|---|---|
| Picobia indonesiana Skoracki and Glowska, 2008 | Aplonis panayensis (Scopoli) * | Orie: Indonesia, India | [34]; current study |
| Aplonis metallica (Temminck) | Orie: Indonesia, Ocea: Papua New Guinea | current study | |
| Aplonis magna (Schlegel) | Orie: Indonesia | current study | |
| Aplonis cantoroides (Gray) | Orie: Indonesia | current study | |
| Enodes erythrophris (Temminck) | Orie: Indonesia | [34] | |
| Mino dumontii (Lesson) | Orie: Indonesia, Ocea: Papua New Guinea | [34]; current study | |
| Mino anais (Lesson) | Ocea: Papua New Guinea | current study | |
| Picobia lamprotornis Klimovičová, Skoracki, Wamiti and Hromada, 2014 | Lamprotornis superbus Rüppell * | Afro: Kenya | [19,21] |
| Lamprotornis chalybaeus Hemprich and Ehrenberg | Afro: Tanzania, Kenya | [21] | |
| Lamprotornis chloropterus Swainson | Afro: Tanzania | [21] | |
| Poeoptera femoralis (Richmond) | Afro: Tanzania | [35] | |
| Poeoptera kenricki Shelley | Afro: Tanzania | [35] | |
| Picobia malayi Patan and Skoracki sp. n. | Gracula religiosa Linnaeus * | Orie: Indonesia | current study |
| Streptocitta albicollis (Vieillot) | Orie: Indonesia | current study | |
| Sarcops calvus (Linnaeus) | Orie: Philippines | current study | |
| Picobia sturni Skoracki, Bochkov and Wauthy, 2004 | Sturnus vulgaris Linnaeus * | Pala: Poland, Moldova, Slovakia, Iceland, Kazakhstan, Kyrgyzstan, Uzbekistan, China | [11,20,36]; current study |
| Sturnus unicolor Temminck | Pala: Italy, Spain, Morocco | [8]; current study | |
| Spodiopsar cineraceus (Temminck) | Pala: China, Japan | [11]; current study | |
| Spodiopsar sericeus (Gmelin) | Pala: China | current study | |
| Acridotheres cristatellus (Linnaeus) | Pala: China | current study | |
| Acridotheres fuscus (Wagler) | Pala: Nepal | current study | |
| Acridotheres tristis (Linnaeus) | Orie: India | current study | |
| Creatophora cinerea (Meuschen) | Afro: Kenya, Tanzania | [35] | |
| Picobia wisniewskii Patan, Skoracki and Marcisova, 2024 | Onychognathus morio (Linnaeus) * | Afro: Tanzania | [35] |
| Onychognathus salvadorii (Sharpe) | Afro: Ethiopia | current study |
| Host Species | No. Examined | No. Infested | Prevalence (CI) | Mite Species |
|---|---|---|---|---|
| Acridotheres cristatellus | 6 | 1 | 16.7% (0.9–58.9) | Picobia sturni |
| Acridotheres fuscus | 16 | 3 | 18.8% (5.3–43.6) | Picobia sturni |
| Acridotheres tristis | 13 | 4 | 30.8% (11.3–58.7) | Picobia sturni |
| Aplonis cantoroides | 7 | 5 | 71.4% (34.1–94.7) | Picobia indonesiana |
| Aplonis magna | 1 | 1 | 100% (5–100) | Picobia indonesiana |
| Aplonis metallica | 26 | 10 | 38.5% (21.2–57.8) | Picobia indonesiana |
| Aplonis panayensis | 18 | 6 | 33.3% (15.6–58.6) | Picobia indonesiana |
| Creatophora cinerea | 14 | 3 | 21.4% (6.1–50) | Picobia sturni |
| Gracula religiosa | 13 | 6 | 46.2% (22.4–74) | Picobia malayi |
| Lamprotornis chalybaeus | 14 | 2 | 14.3% (2.6–42.6) | Picobia lamprotornis |
| Lamprotornis chloropterus | 17 | 4 | 23.5% (8.5–48.9) | Picobia lamprotornis |
| Lamprotornis superbus | 17 | 4 | 23.5% (8.5–48.9) | Picobia lamprotornis |
| Mino anais | 6 | 5 | 83.3% (41.1–99.2) | Picobia indonesiana |
| Mino dumontii | 11 | 1 | 9.1% (0.5–40.5) | Picobia indonesiana |
| Onychognathus morio | 8 | 5 | 62.5% (28.9–88.9) | Picobia wisniewskii |
| Onychognathus salvadorii | 1 | 1 | 100% (5–100) | Picobia wisniewskii |
| Poeoptera femoralis | 5 | 4 | 80% (34.3–99) | Picobia lamprotornis |
| Poeoptera kenricki | 17 | 1 | 5.9% (0.3–28.7) | Picobia lamprotornis |
| Sarcops calvus | 4 | 2 | 50% (9.8–90.2) | Picobia malayi sp. n. |
| Spodiopsar cineraceus | 16 | 4 | 25% (9–50) | Picobia sturni |
| Spodiopsar sericeus | 8 | 2 | 25% (4.6–63.5) | Picobia sturni |
| Streptocitta albicollis | 1 | 1 | 100% (5–100) | Picobia malayi sp. n. |
| Sturnus unicolor | 12 | 6 | 50% (24.3–75.7) | Picobia sturni |
| Sturnus vulgaris | 104 | 22 | 21.2% (14.3–30.2) | Picobia sturni |
| Starling Species | N | Starling Species | N | Starling Species | N |
|---|---|---|---|---|---|
| Acridotheres melanopterus | 1 | Aplonis minor | 6 | Aplonis opaca | 2 |
| Hartlaubius auratus | 1 | Hylopsar cupreocauda | 1 | Hylopsar purpureiceps | 1 |
| Lamprotornis australis | 1 | Lamprotornis caudatus | 2 | Lamprotornis fischeri | 5 |
| Lamprotornis nitens | 6 | Lamprotornis purpureus | 3 | Lamprotornis purpuroptera | 5 |
| Lamprotornis regius | 3 | Lamprotornis splendidus | 4 | Onychognathus tristramii | 1 |
| Poeoptera sharpii | 11 | Poeoptera stuhlmanni | 1 | Saroglossa spilopterus | 2 |
| Speculipastor bicolor | 1 | Sturnia sinensis | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, B.; Kosicki, J.Z.; Patan, M.; Marcisova, I.; Hromada, M.; Skoracki, M. Diversity and Interactions between Picobiine Mites and Starlings. Animals 2024, 14, 2517. https://doi.org/10.3390/ani14172517
Sikora B, Kosicki JZ, Patan M, Marcisova I, Hromada M, Skoracki M. Diversity and Interactions between Picobiine Mites and Starlings. Animals. 2024; 14(17):2517. https://doi.org/10.3390/ani14172517
Chicago/Turabian StyleSikora, Bozena, Jakub Z. Kosicki, Milena Patan, Iva Marcisova, Martin Hromada, and Maciej Skoracki. 2024. "Diversity and Interactions between Picobiine Mites and Starlings" Animals 14, no. 17: 2517. https://doi.org/10.3390/ani14172517





