Effect of Two Different Sperm Selection Methods on Boar Sperm Parameters and In Vitro Fertilisation Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Boar Sperm Preparation and Capacitation
2.1.1. Sperm Density Gradient Selection (DGS)
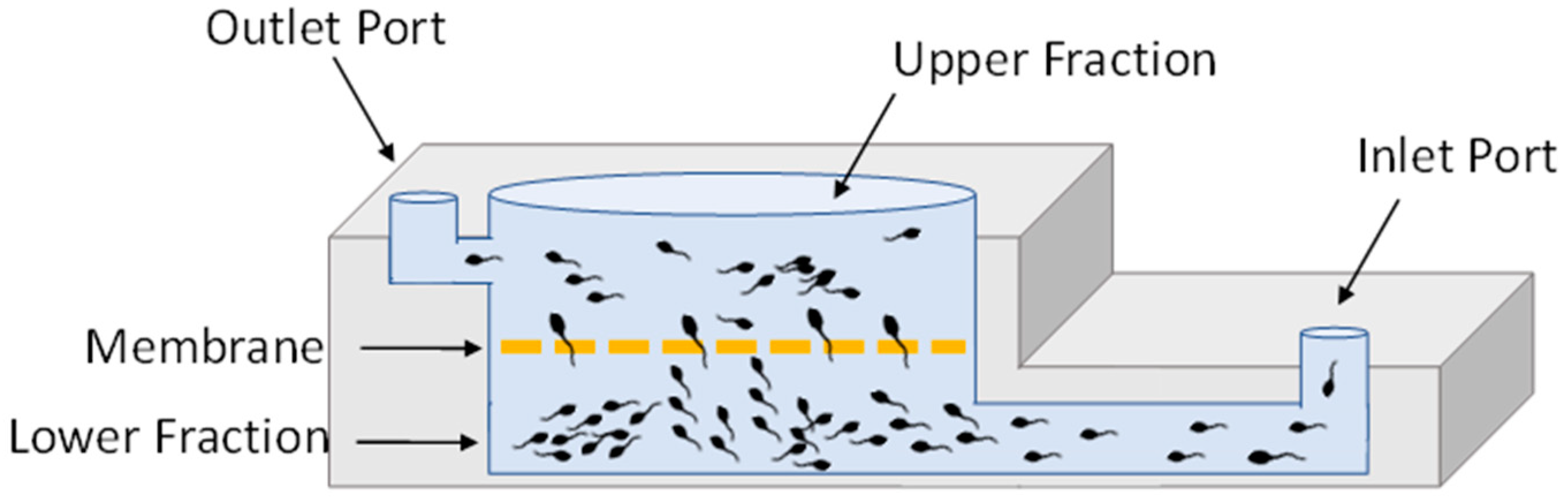
2.1.2. Microfluidics Chip-Based Sperm Separation (MCS)
2.2. Boar Sperm Evaluation
2.2.1. Sperm Morphology
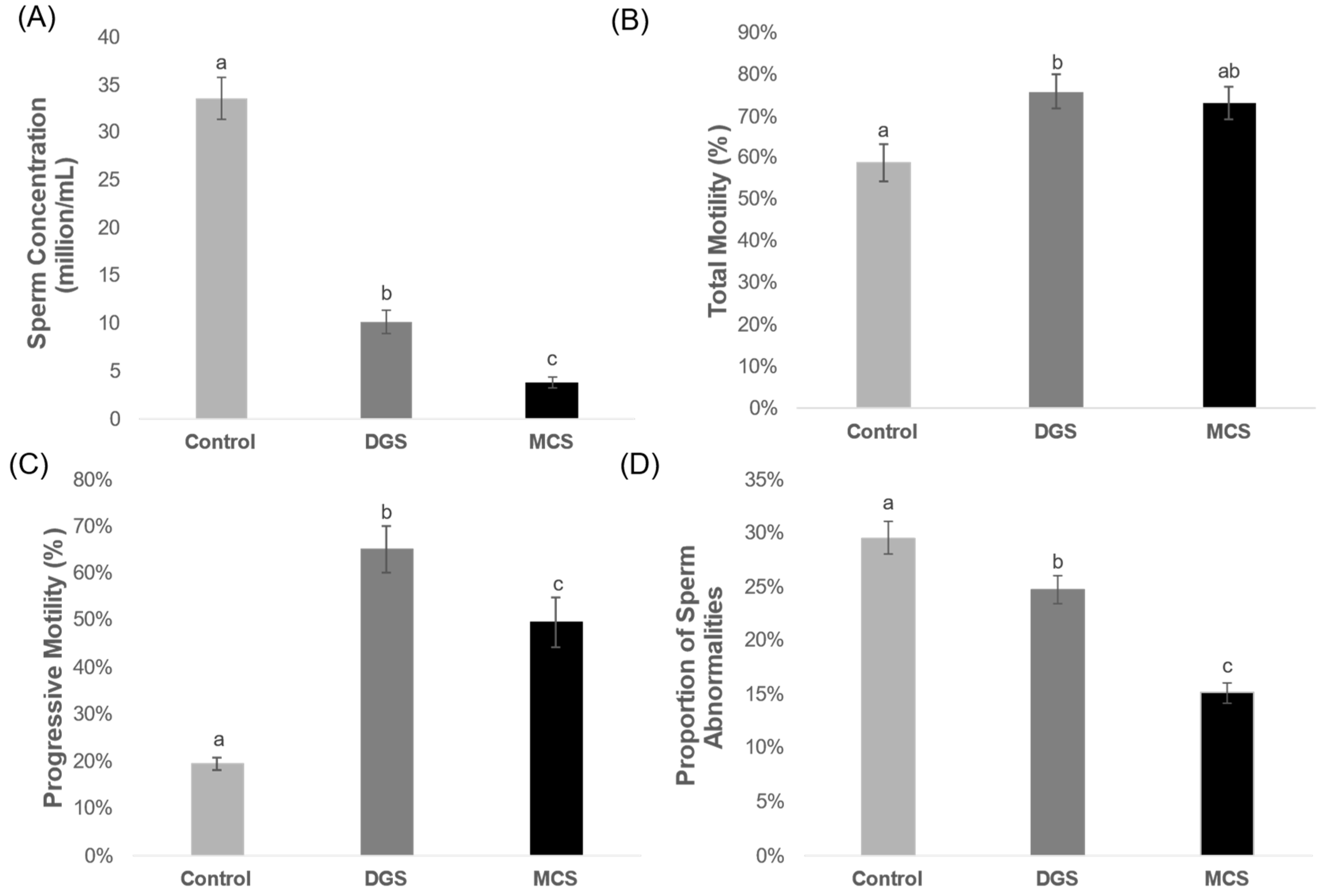
2.2.2. Total Motility
2.2.3. Sperm Concentration
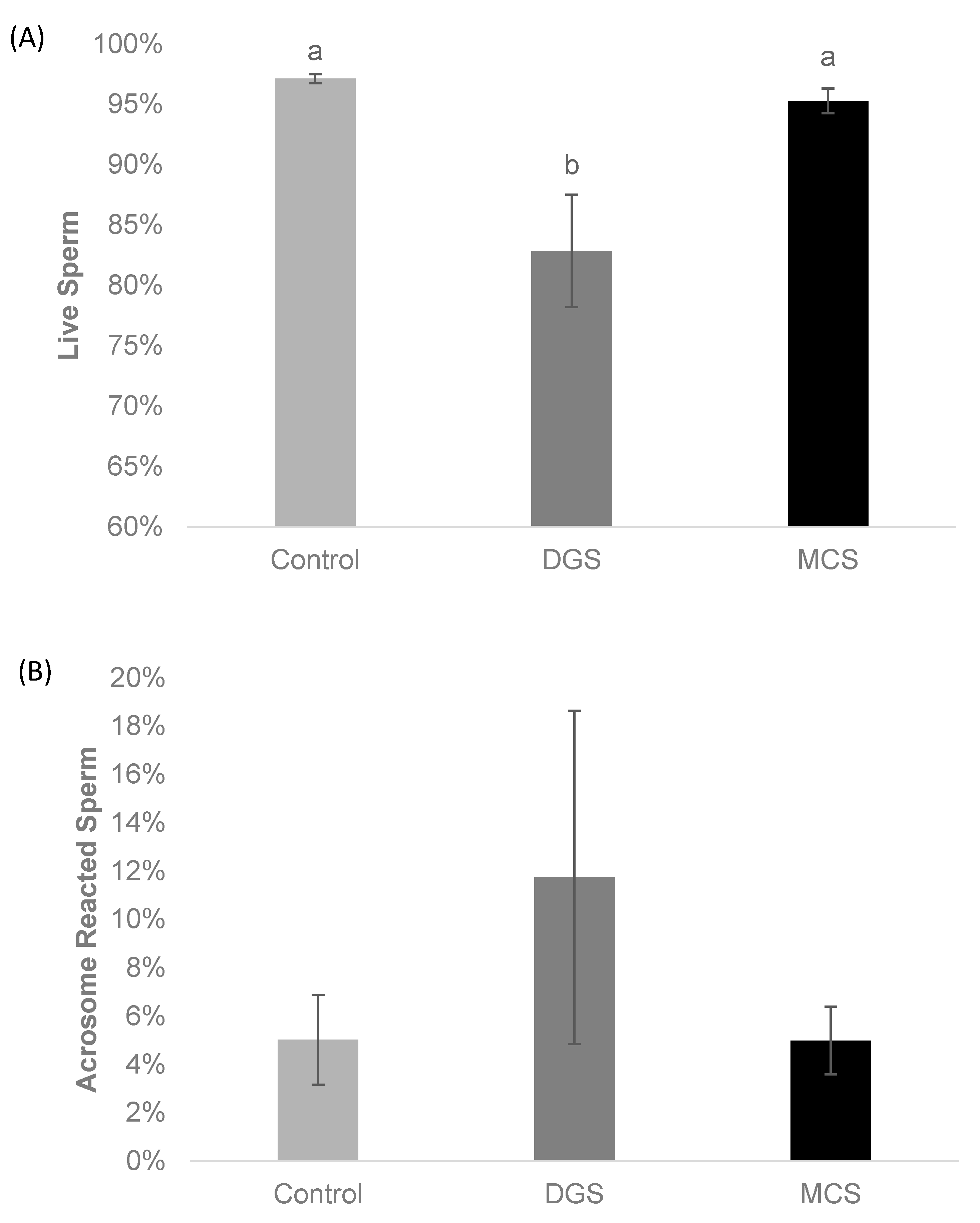
2.2.4. Sperm Viability and Acrosomal Integrity
2.2.5. Sperm DNA Damage Testing
2.3. Porcine Oocyte Collection and in Vitro Maturation (IVM)
2.4. Porcine in Vitro Fertilisation and Embryo Culture
2.5. Evaluation of Pig Blastocysts
2.6. Statistical Analysis
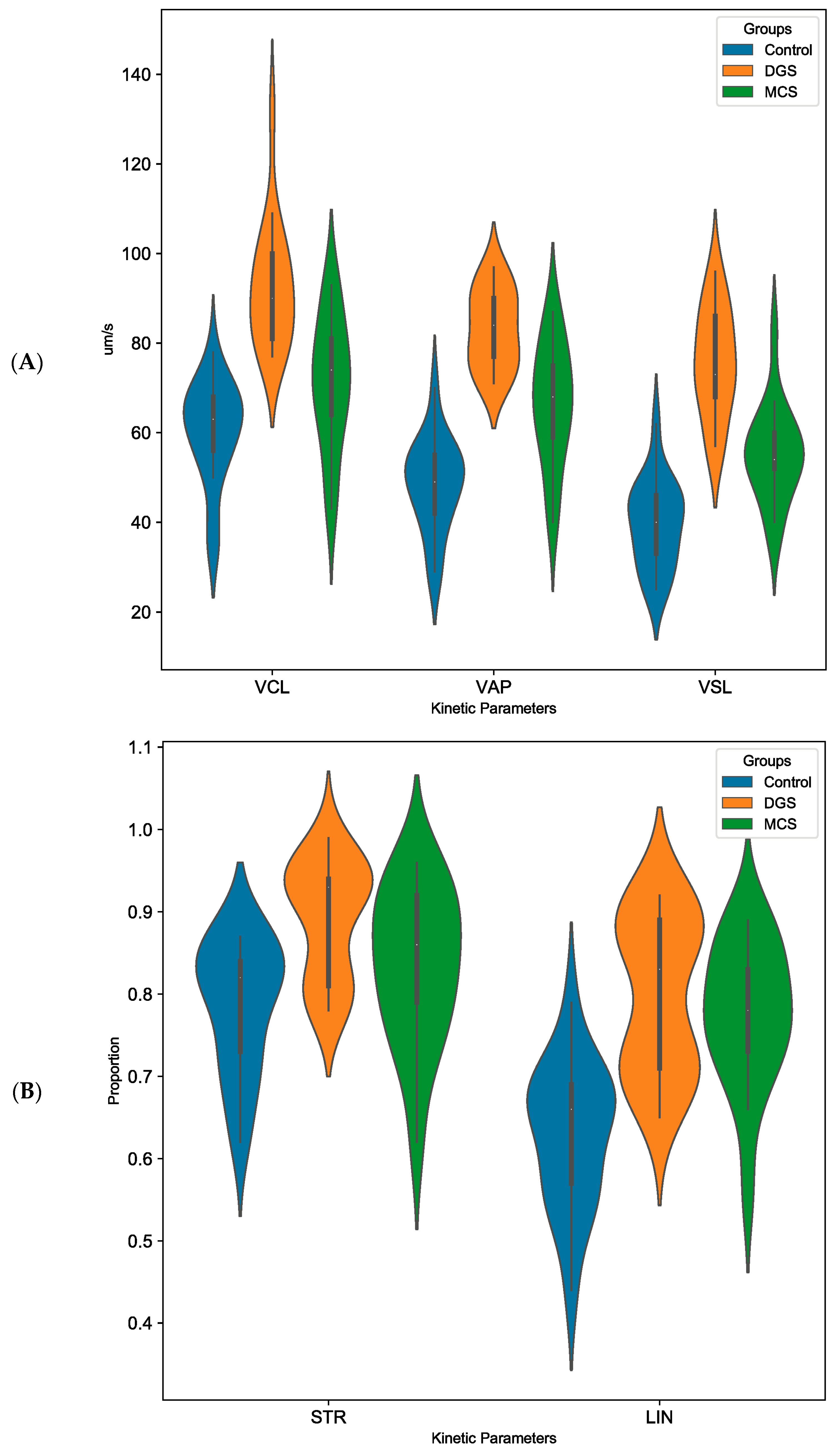
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Sharma, N.; Sharma, S.; Mehta, N.; Verma, A.K.; Chemmalar, S.; Sazili, A.Q. In-Vitro Meat: A Promising Solution for Sustainability of Meat Sector. J. Anim. Sci. Technol. 2021, 63, 693. [Google Scholar] [CrossRef] [PubMed]
- Macháty, Z.; Day, B.N.; Prather, R.S. Development of Early Porcine Embryos in Vitro and in Vivo. Biol. Reprod. 1998, 59, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.K.; Isom, S.C.; Spate, L.D.; Whitworth, K.M.; Spollen, W.G.; Blake, S.M.; Springer, G.K.; Murphy, C.N.; Prather, R.S. Transcriptional Profiling by Deep Sequencing Identifies Differences in MRNA Transcript Abundance in in Vivo-Derived versus in Vitro-Cultured Porcine Blastocyst Stage Embryos. Biol. Reprod. 2010, 83, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Salumets, A.; Suikkari, A.-M.; Möls, T.; Söderström-Anttila, V.; Tuuri, T. Influence of Oocytes and Spermatozoa on Early Embryonic Development. Fertil. Steril. 2002, 78, 1082–1087. [Google Scholar] [CrossRef]
- Cesari, A.; Kaiser, G.G.; Mucci, N.; Mutto, A.; Vincenti, A.; Fornés, M.W.; Alberio, R.H. Integrated Morphophysiological Assessment of Two Methods for Sperm Selection in Bovine Embryo Production in Vitro. Theriogenology 2006, 66, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Gadea, J.; Matas, C. Sperm Factors Related to in Vitro Penetration of Porcine Oocytes. Theriogenology 2000, 54, 1343–1357. [Google Scholar] [CrossRef]
- Gadea, J. Sperm Factors Related to in Vitro and in Vivo Porcine Fertility. Theriogenology 2005, 63, 431–444. [Google Scholar] [CrossRef]
- Gil, M.A.; Alminana, C.; Roca, J.; Vázquez, J.M.; Martinez, E.A. Boar Semen Variability and Its Effects on IVF Efficiency. Theriogenology 2008, 70, 1260–1268. [Google Scholar] [CrossRef]
- Thurston, L.M.; Watson, P.F.; Mileham, A.J.; Holt, W.V. Morphologically Distinct Sperm Subpopulations Defined by Fourier Shape Descriptors in Fresh Ejaculates Correlate with Variation in Boar Semen Quality Following Cryopreservation. J. Androl. 2001, 22, 382–394. [Google Scholar] [CrossRef]
- Quintero-Moreno, A.; Rigau, T.; Rodrıguez-Gil, J.E. Regression Analyses and Motile Sperm Subpopulation Structure Study as Improving Tools in Boar Semen Quality Analysis. Theriogenology 2004, 61, 673–690. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Tienthai, P.; Suzuki, K.; Funahashi, H.; Ekwall, H.; Johannisson, A. Involvement of Oviduct in Sperm Capacitation and Oocyte Development in Pigs. Reprod. Suppl. 2001, 58, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M. Update on Semen Technologies for Animal Breeding. Reprod. Domest. Anim. 2006, 41, 63–67. [Google Scholar] [CrossRef]
- Austin, C.R. The ‘Capacitation’of the Mammalian Sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef]
- Gadella, B.M. Sperm Membrane Physiology and Relevance for Fertilization. Anim. Reprod. Sci. 2008, 107, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L.R. Sperm Selection in Natural Conception: What Can We Learn from Mother Nature to Improve Assisted Reproduction Outcomes? Hum. Reprod. Update 2015, 21, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Martinez, C.A.; Wright, D.; Rodriguez-Martinez, H. Does the Act of Copulation per Se, without Considering Seminal Deposition, Change the Expression of Genes in the Porcine Female Genital Tract? Int. J. Mol. Sci. 2020, 21, 5477. [Google Scholar] [CrossRef]
- Fitzpatrick, J.L.; Lüpold, S. Sexual Selection and the Evolution of Sperm Quality. Mol. Hum. Reprod. 2014, 20, 1180–1189. [Google Scholar] [CrossRef]
- Avilés, M.; Coy, P.; Rizos Dimitrios, D. The Oviduct A Key Organ for the Success of Early Reproductive Events. Anim. Front. 2015, 5, 25–31. [Google Scholar] [CrossRef]
- Henkel, R.R.; Schill, W.-B. Sperm Preparation for ART. Reprod. Biol. Endocrinol. 2003, 1, 108. [Google Scholar] [CrossRef]
- Rappa, K.L.; Rodriguez, H.F.; Hakkarainen, G.C.; Anchan, R.M.; Mutter, G.L.; Asghar, W. Sperm Processing for Advanced Reproductive Technologies: Where Are We Today? Biotechnol. Adv. 2016, 34, 578–587. [Google Scholar] [CrossRef]
- Mortimer, D. Sperm Preparation Techniques and Iatrogenic Failures of In-Vitro Fertilization. Hum. Reprod. 1991, 6, 173–176. [Google Scholar] [CrossRef]
- Mortimer, D. Sperm Recovery Techniques to Maximize Fertilizing Capacity. Reprod. Fertil. Dev. 1994, 6, 25–31. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Sakkas, D. Sperm Selection Methods in the 21st Century. Biol. Reprod. 2019, 101, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.Z.; de Lima, V.F.M.H.; Levenhagen, M.A.; Dos Santos, R.M.; Assumpção, T.I.; Jacomini, J.O.; de Andrade, A.F.C.; de Arruda, R.P.; Beletti, M.E. Transmission Electron Microscopy for Characterization of Acrosomal Damage after Percoll Gradient Centrifugation of Cryopreserved Bovine Spermatozoa. J. Vet. Sci. 2011, 12, 267–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, H.; Saito, Y.; Kagawa, N.; Yang, X. In Vitro Fertilization and Polyspermy in the Pig: Factors Affecting Fertilization Rates and Cytoskeletal Reorganization of the Oocyte. Microsc. Res. Tech. 2003, 61, 327–334. [Google Scholar] [CrossRef]
- Suzuki, K.; Geshi, M.; Yamauchi, N.; Nagai, T. Functional Changes and Motility Characteristics of Japanese Black Bull Spermatozoa Separated by Percoll. Anim. Reprod. Sci. 2003, 77, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Matás, C.; Vieira, L.; García-Vázquez, F.A.; Avilés-López, K.; López-Úbeda, R.; Carvajal, J.A.; Gadea, J. Effects of Centrifugation through Three Different Discontinuous Percoll Gradients on Boar Sperm Function. Anim. Reprod. Sci. 2011, 127, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Yoshioka, K.; Hikono, H.; Iwagami, G.; Suzuki, C.; Kikuchi, K. Centrifugation on Percoll Density Gradient Enhances Motility, Membrane Integrity and in Vitro Fertilizing Ability of Frozen–Thawed Boar Sperm. Zygote 2015, 23, 68–75. [Google Scholar] [CrossRef]
- Jeong, B.; Yang, X. Cysteine, Glutathione, and Percoll Treatments Improve Porcine Oocyte Maturation and Fertilization in Vitro. Mol. Reprod. Dev. 2001, 59, 330–335. [Google Scholar] [CrossRef]
- Ohlweiler, L.U.; Mezzalira, J.C.; Mezzalira, A. Porcine IVF Embryo Development and Estrogen Receptors Are Influenced by the Concentration of Percoll Gradients during Sperm Selection. Mol. Reprod. Dev. 2020, 87, 135–141. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Lasso, J.L.; Blasco, L.; Nunez, R.C.; Heyner, S.; Caballero, P.P.; Storey, B.T. Centrifugation of Human Spermatozoa Induces Sublethal Damage; Separation of Human Spermatozoa from Seminal Plasma by a Dextran Swim-up Procedure without Centrifugation Extends Their Motile Lifetime. Hum. Reprod. 1993, 8, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S. Significance of Reactive Oxygen Species and Antioxidants in Defining the Efficacy of Sperm Preparation Techniques. J. Androl. 1988, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Tavalaee, M.; Deemeh, M.R.; Arbabian, M.; Nasr-Esfahani, M.H. Density Gradient Centrifugation before or after Magnetic-Activated Cell Sorting: Which Technique Is More Useful for Clinical Sperm Selection? J. Assist. Reprod. Genet. 2012, 29, 31–38. [Google Scholar] [CrossRef]
- Betarelli, R.P.; Rocco, M.; Yeste, M.; Fernández-Novell, J.M.; Placci, A.; Azevedo Pereira, B.; Castillo-Martín, M.; Estrada, E.; Peña, A.; Zangeronimo, M.G. The Achievement of Boar Sperm in Vitro Capacitation Is Related to an Increase of Disrupted Disulphide Bonds and Intracellular Reactive Oxygen Species Levels. Andrology 2018, 6, 781–797. [Google Scholar] [CrossRef]
- Smith, G.D.; Takayama, S. Application of Microfluidic Technologies to Human Assisted Reproduction. MHR Basic Sci. Reprod. Med. 2017, 23, 257–268. [Google Scholar] [CrossRef]
- Suarez, S.S.; Wu, M. Microfluidic Devices for the Study of Sperm Migration. MHR Basic Sci. Reprod. Med. 2017, 23, 227–234. [Google Scholar] [CrossRef][Green Version]
- Samuel, R.; Feng, H.; Jafek, A.; Despain, D.; Jenkins, T.; Gale, B. Microfluidic—Based Sperm Sorting & Analysis for Treatment of Male Infertility. Transl. Androl. Urol. 2018, 7, S336. [Google Scholar] [PubMed]
- Ahmadkhani, N.; Hosseini, M.; Saadatmand, M.; Abbaspourrad, A. The Influence of the Female Reproductive Tract and Sperm Features on the Design of Microfluidic Sperm-Sorting Devices. J. Assist. Reprod. Genet. 2022, 39, 19–36. [Google Scholar] [CrossRef]
- Asghar, W.; Velasco, V.; Kingsley, J.L.; Shoukat, M.S.; Shafiee, H.; Anchan, R.M.; Mutter, G.L.; Tüzel, E.; Demirci, U. Selection of Functional Human Sperm with Higher DNA Integrity and Fewer Reactive Oxygen Species. Adv. Healthc. Mater. 2014, 3, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Jalalian, L.; Ribeiro, S.; Ona, K.; Demirci, U.; Cedars, M.I.; Rosen, M.P. Microfluidic Sorting Selects Sperm for Clinical Use with Reduced DNA Damage Compared to Density Gradient Centrifugation with Swim-up in Split Semen Samples. Hum. Reprod. 2018, 33, 1388–1393. [Google Scholar] [CrossRef]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A Treatment Approach for Couples with Disrupted Sperm DNA Integrity and Recurrent ART Failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef]
- Nagata, M.P.B.; Endo, K.; Ogata, K.; Yamanaka, K.; Egashira, J.; Katafuchi, N.; Yamanouchi, T.; Matsuda, H.; Goto, Y.; Sakatani, M. Live Births from Artificial Insemination of Microfluidic-Sorted Bovine Spermatozoa Characterized by Trajectories Correlated with Fertility. Proc. Natl. Acad. Sci. USA 2018, 115, E3087–E3096. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.R.; Kasa, E.; Taylor, D.; Covington, D.; Williams, E.; Bhakta, S.A.; Balthazar, U. Euploidy rates and pregnancy outcomes using the zymōtTM device for sperm preparation. Fertil. Steril. 2020, 114, e43–e44. [Google Scholar] [CrossRef]
- Beyhan, Z.; Abboud, S.; Moliver, J.A.; Chuong, F.S.; Akerman, F.M.; Jacobs, M.H. Microfluidic device-based semen preparation influences euploidy rates of human blastocysts. Fertil. Steril. 2020, 114, e127–e128. [Google Scholar] [CrossRef]
- Orsolini, M.F.; Verstraete, M.H.; van Heule, M.; Meyers, S.A.; Dini, P. Efficacy of the Novel Microfluidic Chip as a Sperm Selection Method for Equine ICSI. J. Equine Vet. Sci. 2022, 113, 103966. [Google Scholar] [CrossRef]
- Yoshioka, K.; Suzuki, C.; Onishi, A. Defined System for in Vitro Production of Porcine Embryos Using a Single Basic Medium. J. Reprod. Dev. 2008, 54, 208–213. [Google Scholar] [CrossRef]
- Robles, V.; Martínez-Pastor, F. Flow Cytometric Methods for Sperm Assessment. In Spermatogenesis; Springer: Berlin/Heidelberg, Germany, 2013; pp. 175–186. [Google Scholar]
- Evenson, D.P. Sperm Chromatin Structure Assay (SCSA®) for Fertility Assessment. Curr. Protoc. 2022, 2, e508. [Google Scholar] [CrossRef] [PubMed]
- Albal, M.S.; Silvestri, G.; Kiazim, L.G.; Vining, L.M.; Zak, L.J.; Walling, G.A.; Haigh, A.M.; Harvey, S.C.; Harvey, K.E.; Griffin, D.K. Supplementation of Porcine in Vitro Maturation Medium with FGF2, LIF, and IGF1 Enhances Cytoplasmic Maturation in Prepubertal Gilts Oocytes and Improves Embryo Quality. Zygote 2022, 30, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Dai, X.; Demott, R.P.; Redfern, K.; Mirando, M.A. Movement Characteristics of Boar Sperm Obtained from the Oviduct or Hyperactivated in Vitro. J. Androl. 1992, 13, 75–80. [Google Scholar] [CrossRef]
- Ho, H.-C.; Suarez, S.S. Hyperactivation of Mammalian Spermatozoa: Function and Regulation. Reproduction 2001, 122, 519–526. [Google Scholar] [CrossRef]
- Yanagimachi, R. Mammalian Fertilization. Physiol. Reprod. 1994, 1, 273–279. [Google Scholar]
- Suarez, S.S. Control of Hyperactivation in Sperm. Hum. Reprod. Update 2008, 14, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Day, B.N. Effects of Follicular Fluid at Fertilization in Vitro on Sperm Penetration in Pig Oocytes. Reproduction 1993, 99, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Nagai, T. Regulation of in Vitro Penetration of Frozen–Thawed Boar Spermatozoa by Caffeine and Adenosine. Mol. Reprod. Dev. Inc. Gamete Res. 2001, 58, 424–431. [Google Scholar] [CrossRef]
- García-Roselló, E.; Matás, C.; Cánovas, S.; Moreira, P.N.; Gadea, J.; Coy, P. Influence of Sperm Pretreatment on the Efficiency of Intracytoplasmic Sperm Injection in Pigs. J. Androl. 2006, 27, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Avery, B.; Greve, T. Impact of PercollR on Bovine Spermatozoa Used for in Vitro Insemination. Theriogenology 1995, 44, 871–878. [Google Scholar] [CrossRef]
- Coetzee, K.; Franken, D.R.; Kruger, T.F.; Lombard, C.J. Effect of Multiple Centrifugations on the Evaluation of the Acrosome Reaction in Human Spermatozoa. Andrologia 1992, 24, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Silva, G.; López-Torres, A.S.; Maldonado-Rosas, I.; Mata-Martínez, E.; Larrea, F.; Torres-Flores, V.; Treviño, C.L.; Chirinos, M. Effects of Semen Processing on Sperm Function: Differences between Swim-up and Density Gradient Centrifugation. World J. Mens. Health 2021, 39, 740. [Google Scholar] [CrossRef]
- Zini, A.; Finelli, A.; Phang, D.; Jarvi, K. Influence of Semen Processing Technique on Human Sperm DNA Integrity. Urology 2000, 56, 1081–1084. [Google Scholar] [CrossRef]
- Malvezzi, H.; Sharma, R.; Agarwal, A.; Abuzenadah, A.M.; Abu-Elmagd, M. Sperm Quality after Density Gradient Centrifugation with Three Commercially Available Media: A Controlled Trial. Reprod. Biol. Endocrinol. 2014, 12, 121. [Google Scholar] [CrossRef]
- Takeshima, T.; Yumura, Y.; Kuroda, S.; Kawahara, T.; Uemura, H.; Iwasaki, A. Effect of Density Gradient Centrifugation on Reactive Oxygen Species in Human Semen. Syst. Biol. Reprod. Med. 2017, 63, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-M.; Wang, W.-H.; Abeydeera, L.R.; Petersen, A.L.; Kim, J.-H.; Murphy, C.; Day, B.N.; Prather, R.S. Pronuclear Location before the First Cell Division Determines Ploidy of Polyspermic Pig Embryos. Biol. Reprod. 1999, 61, 1340–1346. [Google Scholar] [CrossRef] [PubMed]

| Group | Head Abn. Mean% ± SEM | Tail Abn. Mean% ± SEM | CD Mean% ± SEM |
|---|---|---|---|
| Control | 7.6 ± 0.7 | 10.5 ± 1.2 a | 11.5 ± 1.6 a |
| MCS | 6.7 ± 0.5 | 4.9 ± 0.6 b | 3.6 ± 0.6 b |
| DGS | 7.4 ± 0.5 | 8.6 ± 1.0 c | 8.8 ± 1.2 c |
| p value * | 0.402 | <0.001 | <0.001 |
| H * | 1.82 | 16.744 | 19.383 |
| Group | DFI Mean% ± SEM | HDS Mean% ± SEM |
|---|---|---|
| Control | 2.4 ± 0.3 a | 2.3 ± 0.4 a |
| MCS | 0.7 ± 0.1 b | 0.7 ± 0.1 b |
| DGS | 1.1 ± 0.2 b | 1.7 ± 0.3 a |
| p value * | <0.001 | <0.001 |
| H * | 27.76 | 27.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Albal, M.; Aquilina, M.C.; Kiazim, L.G.; Zak, L.J.; Griffin, D.K.; Ellis, P.J. Effect of Two Different Sperm Selection Methods on Boar Sperm Parameters and In Vitro Fertilisation Outcomes. Animals 2024, 14, 2544. https://doi.org/10.3390/ani14172544
Serrano-Albal M, Aquilina MC, Kiazim LG, Zak LJ, Griffin DK, Ellis PJ. Effect of Two Different Sperm Selection Methods on Boar Sperm Parameters and In Vitro Fertilisation Outcomes. Animals. 2024; 14(17):2544. https://doi.org/10.3390/ani14172544
Chicago/Turabian StyleSerrano-Albal, Maria, Marie Claire Aquilina, Lucas G. Kiazim, Louisa J. Zak, Darren K. Griffin, and Peter J. Ellis. 2024. "Effect of Two Different Sperm Selection Methods on Boar Sperm Parameters and In Vitro Fertilisation Outcomes" Animals 14, no. 17: 2544. https://doi.org/10.3390/ani14172544
APA StyleSerrano-Albal, M., Aquilina, M. C., Kiazim, L. G., Zak, L. J., Griffin, D. K., & Ellis, P. J. (2024). Effect of Two Different Sperm Selection Methods on Boar Sperm Parameters and In Vitro Fertilisation Outcomes. Animals, 14(17), 2544. https://doi.org/10.3390/ani14172544









