Effects of Social Group Housing on the Behavioral and Physiological Responses of Captive Sub-Adult Giant Pandas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feeding Management
2.2. Behavior Observation and Data Collection
2.3. Determination of Heart Rate Variability (HRV)
2.4. Measurement of Urinary Cortisol
2.5. 16S rDNA Amplicon Sequencing
2.6. Data Statistics and Analysis
3. Result
3.1. Behavioral Differences of Giant Pandas under Different Feeding Modes
3.2. Difference of Heart Rate Variability under Different Feeding Modes
3.3. Difference of Urinary Cortisol in Giant Pandas under Different Feeding Modes
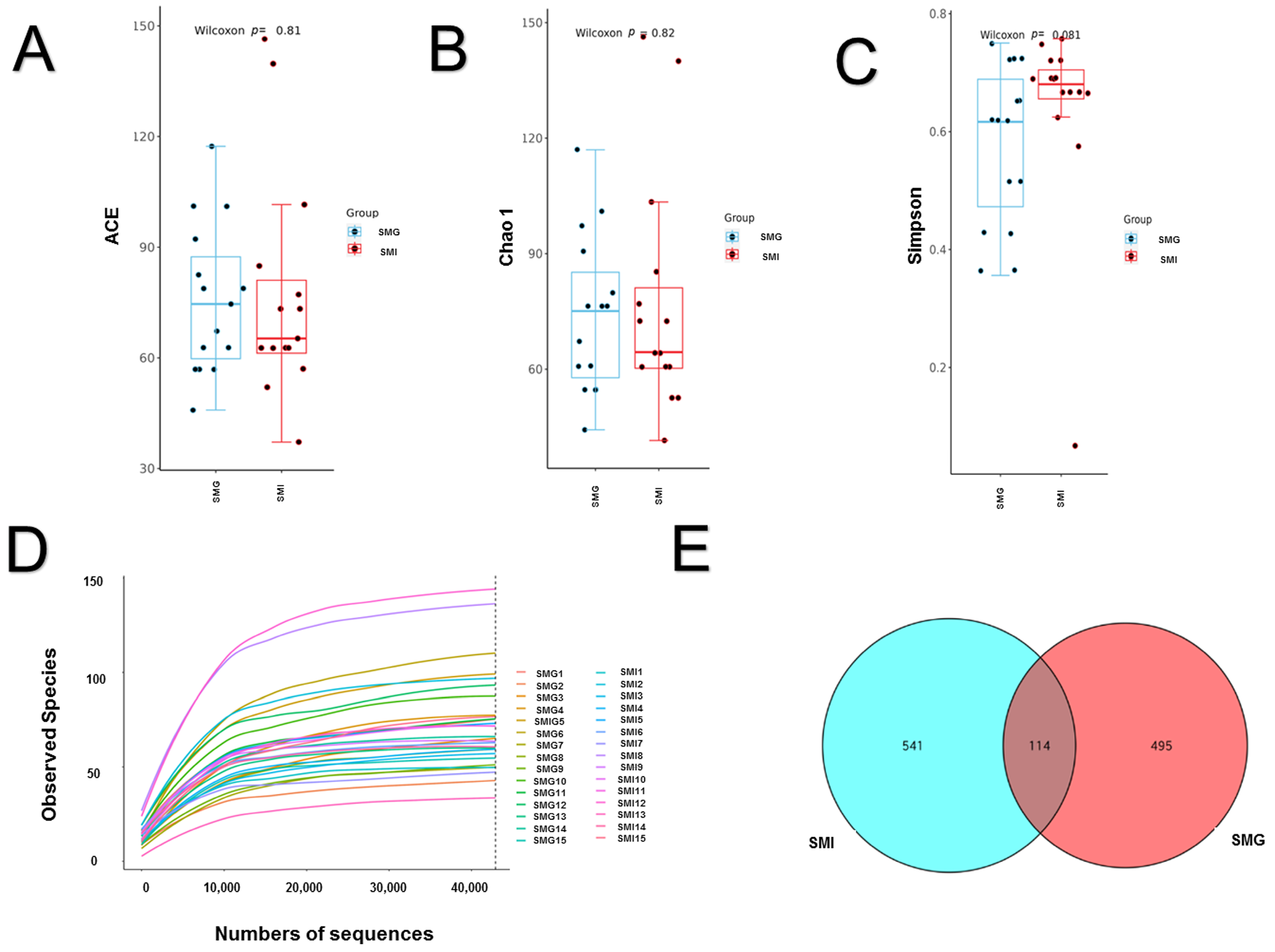
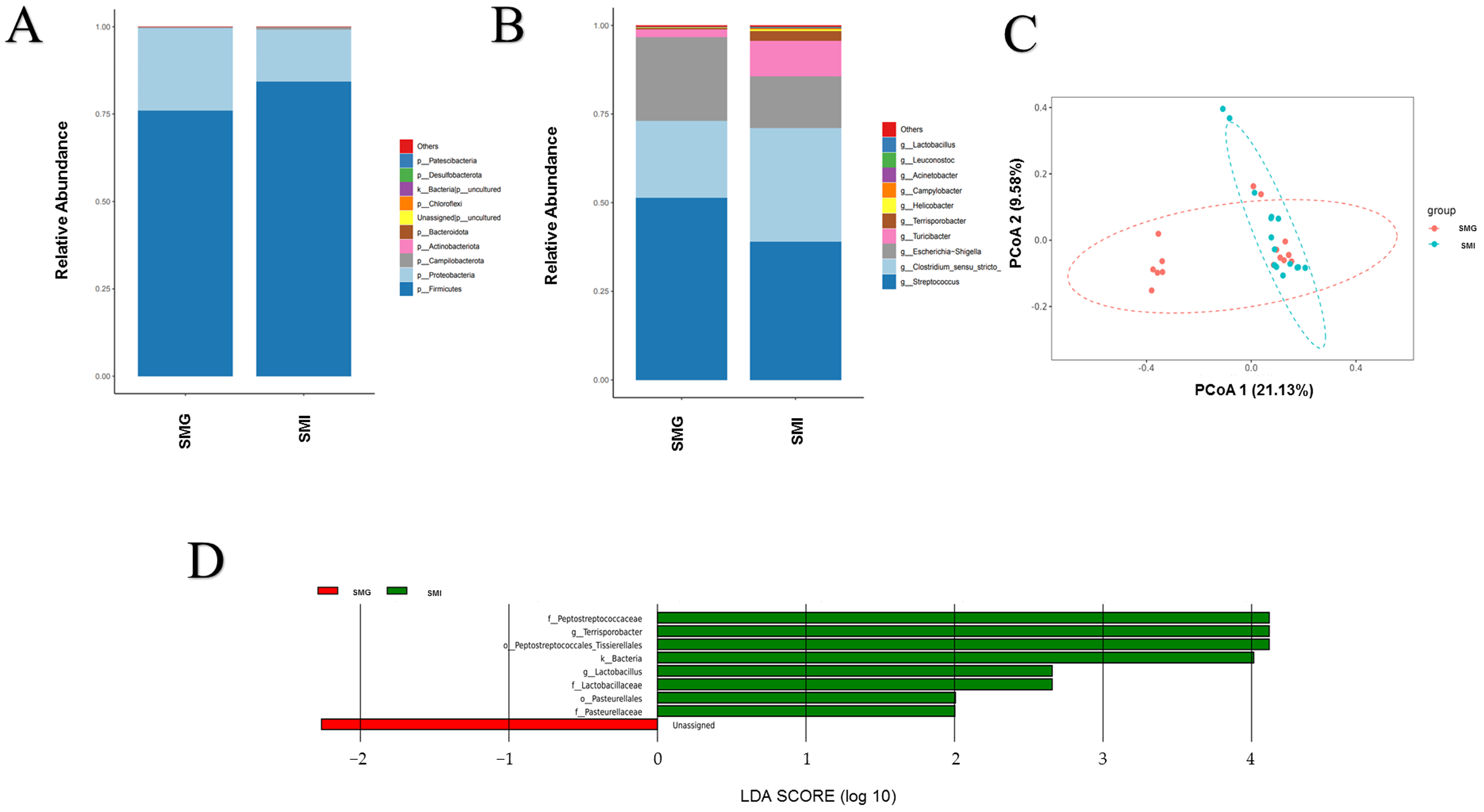
3.4. Difference of Fecal Microorganisms in Giant Pandas under Different Feeding Modes
4. Discussion
4.1. Influence of Social Environment on Giant Panda Behavior
4.2. The Effects of Group Breeding on HRV, Cortisol, and Fecal Microbiota in Giant Pandas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dehnhard, M.; Hildebrandt, T.B.; Knauf, T.; Jewgenow, K.; Kolter, L.; Göritz, F. Comparative endocrine investigations in three bear species based on urinary steroid metabolites and volatiles. Theriogenology 2006, 66, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.F.; Zhan, X.J.; Wu, H.; Zhang, S.N.; Meng, T.; Bruford, M.W.; Wei, F.W. Conservation implications of drastic reductions in the smallest and most isolated populations of giant pandas. Conserv. Biol. 2010, 24, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Pan, W.S.; Xie, Z.; Wildt, D. The giant panda as a social, biological and conservation phenomenon. In Giant Pandas: Biology, Veterinary Medicine and Management; Wildt, D.E., Zhang, A., Zhang, H., Janssen, D.L., Ellis, S., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–16. [Google Scholar]
- Wang, Y.; Wei, W.; Yuan, F.Y.; Cao, D.D.; Zhang, Z.J. The Science Underlying Giant Panda Conservation Translocations. Animals 2023, 13, 3332. [Google Scholar] [CrossRef] [PubMed]
- SFA. The Fourth National Giant Panda Survey; State Forestry Administration of China: Beijing, China, 2015.
- Hong, M.S.; Wei, W.; Zhou, H.; Tang, J.F.; Han, H.; Zhang, Z.J. Creative conservation in China: Releasing captive giant pandas into the wild. Environ. Sci. Pollut. Res. 2019, 30, 31548–31549. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Wang, D.J.; Wei, F.W. Panda downlisted but not out of the woods. Conserv. Lett. 2018, 11, e12355. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhang, X.H.; Ayala, J.; Hou, R. Effects of different nursing methods on the behavioral response of adult captive giant pandas (Ailuropoda melanoleuca). Animals 2021, 11, 626. [Google Scholar] [CrossRef]
- Liu, D.Z.; Wang, Z.P.; Tian, H.; Yu, C.Q.; Zhang, G.Q.; Wei, R.P.; Zhang, H.M. Behavior of giant pandas (Ailuropoda melanoleuca) in captive conditions: Gender differences and enclosure effects. Zoo Biol. 2003, 22, 77–82. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Ellis, S.; Forthman, D.L.; Shepherdson, D.J. Commentary: Improving well-being for captive giant pandas: Theoretical and practical issues. Zoo Biol. Publ. Affil. Am. Zoo Aquar. Assoc. 2003, 22, 347–354. [Google Scholar] [CrossRef]
- Mason, G.J. Stereotypies and suffering. Behav. Process. 1991, 25, 103–115. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends. Ecol. Evol. 2010, 12, 713–721. [Google Scholar] [CrossRef]
- Swaisgood, R.R. Current status and future directions of applied behavioral research for animal welfare and conservation. Appl. Anim. Behav. Sci. 2007, 102, 139–162. [Google Scholar] [CrossRef]
- Schneider, L.; Kemper, N.; Spindler, B. Stereotypic behavior in fattening bulls. Animals 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Swaisgood, R.R.; Lindburg, D.; White, A.M.; Zhang, H.; Zhou, X. Chemical communication in giant pandas: Experimentation and application. In Giant Pandas: Biology and Conservation; Lindburg, D., Baragona, K., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 106–120. [Google Scholar]
- Charlton, B.D.; Keating, J.L.; Li, R.G.; Huang, Y.; Swaisgood, R.R. Female giant panda (Ailuropoda melanoleuca) chirps advertise the caller’s fertile phase. Proc. Biol. Sci. 2010, 277, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.B.; Hu, J.C.; Pan, W.S.; Zhu, J. The Giant Pandas of Wolong; University of Chicago Press: Chicago, IL, USA, 1985. [Google Scholar]
- Zhu, X.J.; Lindburg, D.G.; Pan, W.S.; Forney, K.A.; Wang, D.J. The reproductive strategy of giant pandas (Ailuropoda melanoleuca): Infant growth and development and mother-infant relationships. J. Zool. 2010, 253, 141–155. [Google Scholar] [CrossRef]
- Available online: https://animaldiversity.org/accounts/Ursidae/ (accessed on 23 August 2024).
- Carlstead, K.; Seidensticker, J.; Baldwin, R. Environmental enrichment for zoo bears. Zoo Biol. 1991, 10, 3–16. [Google Scholar] [CrossRef]
- Fernandez, E.J. Appetitive search behaviors and stereotypies in polar bears (Ursus maritimus). Behav. Process. 2021, 182, 104299. [Google Scholar] [CrossRef]
- Forthman, D.L.; Bakeman, R. Environmental and social influences on enclosure use and activity patterns of captive sloth bears (Ursus ursinus). Zoo Biol. 1992, 11, 405–415. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Guo, L.R.; Gu, B.; Liu, H.; Hou, A.Y.; Liu, X.F.; Sun, L.X.; Liu, D.Z. Stereotypic behavior and fecal cortisol level in captive giant pandas in relation to environmental enrichment. Zoo Biol. 2006, 25, 445–459. [Google Scholar] [CrossRef]
- Liu, H.; Duan, H.; Wang, C. Effects of ambient environmental factors on the stereotypic behaviors of giant pandas (Ailuropoda melanoleuca). PLoS ONE 2017, 12, e0170167. [Google Scholar] [CrossRef]
- Zhang, J.D.; Hull, V.; Huang, J.Y.; Zhou, S.Q.; Xu, W.H. Activity patterns of the giant panda (Ailuropoda melanoleuca). J. Mammal. 2015, 96, 1116–1127. [Google Scholar] [CrossRef]
- Billman, G.E. Heart rate variability–a historical perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhang, X.H.; Wang, X.Y.; Liu, Y.L.; An, J.H.; Wang, D.H.; Cai, Z.G.; Hou, R. Intestinal acetic acid regulates the synthesis of sex pheromones in captive giant pandas. Front. Microbiol. 2023, 14, 1234676. [Google Scholar] [CrossRef] [PubMed]
- Maunder, M.; Byers, O. The IUCN technical guidelines on the management of ex situ populations for conservation: Reflecting major changes in the application of ex situ conservation. Oryx 2005, 39, 95–98. [Google Scholar] [CrossRef]
- Pitsko, L.E. Wild Tigers in Captivity: A Study of the Effects of the Captive Environment on Tiger Behavior. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2003. [Google Scholar]
- Altman, J.D.; Gross, K.L.; Lowry, S.R. Nutritional and behavioral effects of gorge and fast feeding in captive lions. J. Appl. Anim. Welf. Sci. 2005, 8, 47–57. [Google Scholar] [CrossRef]
- McPhee, M.E. Generations in captivity increases behavioral variance: Considerations for captive breeding and reintroduction programs. Biol. Conserv. 2004, 115, 71–77. [Google Scholar] [CrossRef]
- Meng, X.X.; Cody, N.; Gong, B.C.; Xiang, L.L. Stable fighting strategies to maintain social ranks in captive male Alpine musk deer (Moschus sifanicus). Anim. Sci. J. 2012, 83, 617–622. [Google Scholar] [CrossRef]
- Swaney, W.; Kendal, J.; Capon, H.; Brown, C.; Laland, K.N. Familiarity facilitates social learning of foraging behaviour in the guppy. Anim. Behav. 2001, 62, 591–598. [Google Scholar] [CrossRef]
- Zhan, M.Y.; Wang, A.S.; Yao, Y.; Zhou, Y.M.; Zhang, S.; Fu, X.H.; Zhou, J.; Pei, E.L.; Wang, L. An amateur gut microbial configuration formed in giant panda for striving to digest cellulose in bamboo: Systematic evidence from intestinal digestive enzymes, functional genes and microbial structures. Front. Microbiol. 2022, 13, 926515. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Martin-Wintle, M.S.; Owen, M.A.; Zhou, X.; Zhang, H.M. Developmental stability of foraging behavior: Evaluating suitability of captive giant pandas for translocation. Anim. Conserv. 2018, 21, 474–482. [Google Scholar] [CrossRef]
- Rushen, J.; Lawrence, A.B.; Terlouw, E.M.C. Stereotypic animal behaviour: Fundamentals and applications to welfare. Livestock Prod. Sci. 1994, 40, 359–360. [Google Scholar]
- Finch, K.; Sach, F.; Fitzpatrick, M.; Masters, N.; Rowden, L.J. Longitudinal improvements in zoo-housed elephant welfare: A case study at zsl whipsnade zoo. Animals 2020, 10, 2029. [Google Scholar] [CrossRef]
- Lasky, M.; Campbell, J.; Osborne, J.A.; Ivory, E.L.; Lasky, J.; Kendall, C.J. Increasing browse and social complexity can improve zoo elephant welfare. Zoo Biol. 2021, 40, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.J.; Latham, N.R. Can’t stop, won’t stop: Is stereotypy a reliable animal welfare indicator. Anim. Welf. 2004, 13, 57–69. [Google Scholar] [CrossRef]
- Mellor, D.; Patterson-Kane, E.; Stafford, K.J. The Sciences of Animal Welfare; UFAW Animal Welfare: Oxford, UK, 2009; pp. 72–93. [Google Scholar]
- Murphy, E.; Nordquist, R.E.; van der Staay, F.J. A review of behavioural methods to study emotion and mood in pigs, Sus scrofa. Appl. Anim. Behav. Sci. 2014, 159, 9–28. [Google Scholar] [CrossRef]
- Fagen, R. Animal Play Behavior; Oxford University Press: New York, NY, USA, 1981. [Google Scholar]
- Wilson, M.L. An Investigation into the Factors That Affect Play Fighting Behavior in Giant Pandas; Georgia Institute of Technology: Atlanta, GA, USA, 2005. [Google Scholar]
- Kleiman, D.G. Mammalian sociobiology and zoo breeding pro-grams. Zoo Biol. 1994, 13, 423–432. [Google Scholar] [CrossRef]
- Snyder, R.J.; Zhang, A.J.; Zhang, Z.H.; Li, G.H.; Tian, Y.Z.; Huang, X.M.; Luo, L.; Bloomsmith, M.A.; Forthman, D.L.; Maple, T.L. Behavioral and developmental consequences of early rearing experience for captive giant pandas (Ailuropoda melanoleuca). J. Comp. Psychol. 2003, 117, 235. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Schmalbach, I.; Herhaus, B.; Pässler, S.; Runst, S.; Berth, H.; Wolff-Stephan, S.; Petrowski, K. Cortisol reactivity in patients with anorexia nervosa after stress induction. Transl. Psychiatry 2020, 10, 275. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Costanzo, E.S. Psychoneuroimmunology and health psychology: An integrative model. Brain Behav. Immun. 2003, 17, 225–232. [Google Scholar] [CrossRef]
- O’Connor, D.B.; Thayer, J.F.; Vedhara, K. Stress and health: A review of psychobiological processes. Annu. Rev. Psychol. 2021, 72, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.H.M.A.; Coelho, F.M.; Oliveira, V.M.C.; Yamaki, F.L.; Pereira, G.G.; Soares, E.C.; Fedullo, J.D.; Pereira, R.C.; Ito, F.H. Electrocardiographic parameters of captive lions (Panthera leo) and tigers (Panthera tigris) immobilized with ketamine plus xylazine. J. Zoo Wildl. Med. 2008, 39, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Jung, K.C.; Choe, J.H.; Kim, B.C. Effects of muscle cortisol concentration on muscle fiber characteristics, pork quality, and sensory quality of cooked pork. Meat Sci. 2012, 94, 490–498. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Schaefer, A.L.; Cook, N.J.; Scott, S.L. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welf. 2005, 14, 319–325. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Dzviti, M.; Mapfumo, L.; Muchenje, V. Relationship between saliva and blood cortisol in handled cows. Asian-Australas J. Anim. Sci. 2019, 32, 734–741. [Google Scholar] [CrossRef]
- Tatemoto, P.; Broom, D.M.; Zanella, A.J. Changes in Stereotypies: Effects over Time and over Generations. Animals 2022, 12, 2504. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhou, Y.Y.; Fu, H.; Xiong, X.W.; Fang, S.M.; Jiang, H.; Wu, J.Y.; Yang, H.; Gao, J.; Huang, L.S. Expanded catalog of microbial genes and metagenome-assembled genomes from the pig gut microbiome. Nat. Commun. 2021, 12, 1106. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: From fiction to function. Am. J. Physiol. Gastrointest. Liver. Physiol. 2019, 317, G602–G608. [Google Scholar] [CrossRef]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; Lozupone, C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Maintenance of gut microbiome stability for optimum intestinal health in pigs–a review. J. Anim. Sci. Biotechnol. 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Cornwallis, C.K.; Botero, C.A.; Rubenstein, D.R.; Downing, P.A.; West, S.A.; Griffin, A.S. Cooperation facilitates the colonization of harsh environments. Nat. Ecol. Evol. 2017, 1, 57. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef] [PubMed]


| Group | Name | Studbook | Birth | Sex | Sir | Dam | Time of Capture of Behavioral Data |
|---|---|---|---|---|---|---|---|
| SMI | Qi Hang | 1132 | 2018 | Male | Xiong Bing | Qing He | 2023.7.24–2023.7.29 (24 h) |
| SMI | Qi Cheng | 1131 | 2018 | Female | Xiong Bing | Qing He | 2023.8.15–2023.8.21 (24 h) |
| SMI | Fu Wa | 948 | 2015 | Female | Qiao Qiao | Ke Lin | 2023.9.12–2023.9.18 (24 h) |
| SMG | Guo Guo | 1252 | 2020 | Female | Xiong Bing | Qing He | 2023.7.24–2023.7.29 (24 h) |
| SMG | Qiang Qiang | 1251 | 2020 | Male | Xiong Bing | Qing He | 2023.8.15–2023.8.21 (24 h) |
| SMG | Fu Shuang | 1250 | 2020 | Female | Gong Zai | Fu Lu | 2023.9.12–2023.9.18 (24 h) |
| Behavior Category | Behavior | Definition |
|---|---|---|
| feeding behavior | take food drink water | Eat bamboo, bamboo shoots, steamed bread, etc., drink water. |
| resting behavior | rest | Maintain a stationary state in various postures, with or without closed eyes. |
| playing behavior | play with objects | Play with objects such as bamboo sticks, twigs, toys or water. |
| self-play | Including backward rolling and swinging limbs, forward rolling, inverted spinning around objects, and climbing. Climbing walls and railings, etc. | |
| interactive play | Two or more giant pandas play with each other. | |
| stereotyped behavior | bite the rail | Repeatedly gnawing on the railing of the enclosure, rubbing against it, or even sucking on it. |
| step by step | Constantly moving back and forth in the enclosure. | |
| turn around | A highly specialized pacing behavior that accurately follows the same route every time (circle). |
| Index | SMI | SMG | p-Value |
|---|---|---|---|
| SDANN | 56.541 ± 23 | 29.505 ± 12 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Fu, Q.; Wang, X.-Y.; Zhang, X.-H.; Liu, Y.-L.; Hou, R.; Zhang, M.-Y. Effects of Social Group Housing on the Behavioral and Physiological Responses of Captive Sub-Adult Giant Pandas. Animals 2024, 14, 2545. https://doi.org/10.3390/ani14172545
Yuan B, Fu Q, Wang X-Y, Zhang X-H, Liu Y-L, Hou R, Zhang M-Y. Effects of Social Group Housing on the Behavioral and Physiological Responses of Captive Sub-Adult Giant Pandas. Animals. 2024; 14(17):2545. https://doi.org/10.3390/ani14172545
Chicago/Turabian StyleYuan, Bo, Qin Fu, Xue-Ying Wang, Xiao-Hui Zhang, Yu-Liang Liu, Rong Hou, and Ming-Yue Zhang. 2024. "Effects of Social Group Housing on the Behavioral and Physiological Responses of Captive Sub-Adult Giant Pandas" Animals 14, no. 17: 2545. https://doi.org/10.3390/ani14172545
APA StyleYuan, B., Fu, Q., Wang, X.-Y., Zhang, X.-H., Liu, Y.-L., Hou, R., & Zhang, M.-Y. (2024). Effects of Social Group Housing on the Behavioral and Physiological Responses of Captive Sub-Adult Giant Pandas. Animals, 14(17), 2545. https://doi.org/10.3390/ani14172545






