Odor Fences Have No Effect on Wild Boar Movement and Home Range Size
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Wild Boar Telemetry
2.3. Deterrent Application
2.4. Statistic Evaluation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacigalupo, S.A.; Chang, Y.; Dixon, L.K.; Gubbins, S.; Kucharski, A.J.; Drewe, J.A. The Importance of Fine-Scale Predictors of Wild Boar Habitat Use in an Isolated Population. Ecol. Evol. 2022, 12, e9031. [Google Scholar] [CrossRef] [PubMed]
- Milda, D.; Ramesh, T.; Kalle, R.; Gayathri, V.; Thanikodi, M.; Ashish, K. Factors Driving Human–Wild Pig Interactions: Implications for Wildlife Conflict Management in Southern Parts of India. Biol. Invasions 2023, 25, 221–235. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild Boar Populations up, Numbers of Hunters down? A Review of Trends and Implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Drimaj, J.; Kamler, J.; Hošek, M.; Plhal, R.; Mikulka, O.; Zeman, J.; Drápela, K. Reproductive Potential of Free-Living Wild Boar in Central Europe. Eur. J. Wildl. Res. 2020, 66, 75. [Google Scholar] [CrossRef]
- Gethöffer, F.; Sodeikat, G.; Pohlmeyer, K. Reproductive Parameters of Wild Boar (Sus scrofa) in Three Different Parts of Germany. Eur. J. Wildl. Res. 2007, 53, 287–297. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of Wild Boar (Sus scrofa) in Its Introduced and Native Range: A Review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Tack, J. Wild Boar (Sus scrofa) Populations in Europe; A Scientific Review of Population Trends and Implications for Management; European Landowners’ Organization: Brussels, Belgium, 2018; pp. 29–30. [Google Scholar]
- Vetter, S.G.; Ruf, T.; Bieber, C.; Arnold, W. What Is a Mild Winter? Regional Differences in within-Species Responses to Climate Change. PLoS ONE 2015, 10, e0132178. [Google Scholar] [CrossRef]
- Touzot, L.; Schermer, É.; Venner, S.; Delzon, S.; Rousset, C.; Baubet, É.; Gaillard, J.M.; Gamelon, M. How Does Increasing Mast Seeding Frequency Affect Population Dynamics of Seed Consumers? Wild Boar as a Case Study. Ecol. Appl. 2020, 30, e02134. [Google Scholar] [CrossRef]
- Oja, R.; Kaasik, A.; Valdmann, H. Winter Severity or Supplementary Feeding—Which Matters More for Wild Boar? Acta Theriol. 2014, 59, 553–559. [Google Scholar] [CrossRef]
- Pandey, P.; Shaner, P.J.L.; Sharma, H.P. The Wild Boar as a Driver of Human-Wildlife Conflict in the Protected Park Lands of Nepal. Eur. J. Wildl. Res. 2016, 62, 103–108. [Google Scholar] [CrossRef]
- Davoli, M.; Ghoddousi, A.; Sabatini, F.M.; Fabbri, E.; Caniglia, R.; Kuemmerle, T. Changing Patterns of Conflict between Humans, Carnivores and Crop-Raiding Prey as Large Carnivores Recolonize Human-Dominated Landscapes. Biol. Conserv. 2022, 269, 109553. [Google Scholar] [CrossRef]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African Swine Fever Status in Europe. Viruses 2019, 11, 3101. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, A.; Bosch, J.; Sánchez-Vizcaíno, J.M.; Ito, S.; Muñoz, C.; Iglesias, I.; Avilés, M.M. African Swine Fever Survey in a European Context. Pathogens 2022, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- You, S.; Liu, T.; Zhang, M.; Zhao, X.; Dong, Y.; Wu, B.; Wang, Y.; Li, J.; Wei, X.; Shi, B. African Swine Fever Outbreaks in China Led to Gross Domestic Product and Economic Losses. Nat. Food 2021, 2, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Depner, K.; Gortazar, C.; Guberti, V.; Masiulis, M.; More, S.; Olßsevskis, E.; Thulke, H.H.; Viltrop, A.; Wozniakowski, G.; Abrahantes, J.C.; et al. Epidemiological Analyses of African Swine Fever in the Baltic States and Poland (Update September 2016–September 2017). EFSA J. 2017, 15, e05068. [Google Scholar] [CrossRef]
- Mason-D’Croz, D.; Bogard, J.R.; Herrero, M.; Robinson, S.; Sulser, T.B.; Wiebe, K.; Willenbockel, D.; Godfray, H.C.J. Modelling the Global Economic Consequences of a Major African Swine Fever Outbreak in China. Nat. Food 2020, 1, 221–228. [Google Scholar] [CrossRef]
- Mpemba, H.; Yang, F.; MacLeod, K.J.; Wen, D.; Liu, Y.; Jiang, G. Influences of Predator Cues on the Incidence of Ungulates, Mesopredators and Top Predators in the Greater Khingan Mountains, Northeastern China. Pak. J. Zool. 2023, 55, 269–280. [Google Scholar] [CrossRef]
- Villalobos, A.; Schlyter, F.; Dekker, T.; Larsson Herrera, S.; Birgersson, G.; Löf, M. Predator Odor Can Reduce Acorn Removal by Granivorous Rodents in Mixed Oak Forest Stands. For. Ecol. Manag. 2023, 548, 121411. [Google Scholar] [CrossRef]
- Wang, Z.N.; Wang, H.; Shen, Y.Z.; Li, F.K.; Xiao, J.X.; Yang, Y.; Lv, S.J. Behavioural and Physiological Responses of Small Tail Han Sheep to Predators. Animal 2023, 17, 100884. [Google Scholar] [CrossRef]
- Verschut, T.A.; Carlsson, M.A.; Hambäck, P.A. Scaling the Interactive Effects of Attractive and Repellent Odours for Insect Search Behaviour. Sci. Rep. 2019, 9, 15309. [Google Scholar] [CrossRef]
- Schlageter, A.; Haag-Wackernagel, D. Evaluation of an Odor Repellent for Protecting Crops from Wild Boar Damage. J. Pest Sci. 2012, 85, 209–215. [Google Scholar] [CrossRef]
- Bíl, M.; Andrášik, R.; Bartonička, T.; Křivánková, Z.; Sedoník, J. An Evaluation of Odor Repellent Effectiveness in Prevention of Wildlife-Vehicle Collisions. J. Environ. Manag. 2018, 205, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Walter, W.D.; Lavelle, M.J.; Fischer, J.W.; Johnson, T.L.; Hygnstrom, S.E.; VerCauteren, K.C. Management of Damage by Elk (Cervus elaphus) in North America: A Review. Wildl. Res. 2010, 37, 630–646. [Google Scholar] [CrossRef]
- Bíl, M.; Kušta, T.; Andrášik, R.; Cícha, V.; Brodská, H.; Jezek, M.; Keken, Z. No Clear Effect of Odour Repellents on Roe Deer Behaviour in the Vicinity of Roads. Wildl. Biol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Zamojska, J.; Bandyk, A.; Olejarski, P. Results of the Monitoring of the Effectiveness of Repellents against Wild Boar in the Fields. Prog. Plant Prot. 2014, 54, 159–162. [Google Scholar] [CrossRef]
- Elmeros, M.; Winbladh, J.K.; Andersen, P.N.; Madsen, A.B.; Christensen, J.T. Effectiveness of Odour Repellents on Red Deer (Cervus elaphus) and Roe Deer (Capreolus capreolus): A Field Test. Eur. J. Wildl. Res. 2011, 57, 1223–1226. [Google Scholar] [CrossRef]
- Santilli, F.; Mori, L.; Galardi, L. Evaluation of Three Repellents for the Prevention of Damage to Olive Seedlings by Deer. Eur. J. Wildl. Res. 2004, 50, 85–89. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Crump, D.R.; Sullivan, D.S. Use of Predator Odors as Repellents to Reduce Feeding Damage by Herbivores—IV. Northern Pocket Gophers (Thomomys talpoides). J. Chem. Ecol. 1988, 14, 379–389. [Google Scholar] [CrossRef]
- Kušta, T.; Keken, Z.; Ježek, M.; Kůta, Z. Effectiveness and Costs of Odor Repellents in Wildlife-Vehicle Collisions: A Case Study in Central Bohemia, Czech Republic. Transp. Res. Part D Transp. Environ. 2015, 38, 1–5. [Google Scholar] [CrossRef]
- Ascensão, F.; Barrientos, R.; D’Amico, M. Wildlife Collisions Put a Dent in Road Safety. Science 2021, 374, 1208. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Miranda, M.A.; Bicout, D.; Bøtner, A.; Butterworth, A.; Calistri, P.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Michel, V.; et al. African Swine Fever in Wild Boar. EFSA J. 2018, 16, e05344. [Google Scholar] [PubMed]
- Smith, G.C.; Brough, T.; Podgórski, T.; Ježek, M.; Šatrán, P.; Vaclavek, P.; Delahay, R. Defining and Testing a Wildlife Intervention Framework for Exotic Disease Control. Ecol. Solut. Evid. 2022, 3, e12192. [Google Scholar] [CrossRef]
- Olejarz, A.; Faltusová, M.; Börger, L.; Güldenpfennig, J.; Jarský, V.; Ježek, M.; Mortlock, E.; Silovský, V.; Podgórski, T. Worse Sleep and Increased Energy Expenditure yet No Movement Changes in Sub-Urban Wild Boar Experiencing an Influx of Human Visitors (Anthropulse) during the COVID-19 Pandemic. Sci. Total Environ. 2023, 879, 163106. [Google Scholar] [CrossRef] [PubMed]
- Bíl, M.; Sedoník, J.; Andrášik, R.; Kušta, T.; Keken, Z. Olfactory Repellents Decrease the Number of Ungulate-Vehicle Collisions on Roads: Results of a Two-Year Carcass Study. J. Environ. Manag. 2024, 365, 121561. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System (Version 3.36). Open Source Geospatial Foundation Project. 2024. Available online: https://qgis.org/ (accessed on 1 September 2024).
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.2.2.); R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https:///www.R-project.org/ (accessed on 1 September 2024).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Jori, F.; Massei, G.; Licoppe, A.; Ruiz-Fons, F.; Linden, A.; Václavek, P.; Chenais, E.; Rosell, C. Management of wild boar populations in the European Union before and during the ASF crisis. In Understanding and Combatting African Swine Fever: A European perspective; Iacolina, L., Penrith, M.-L., Bellini, S., Chenais, E., Jori, F., Montoya, M., Ståhl, K., Gavier-Widén, D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; ISBN 9789086869107. [Google Scholar]
- Iacolina, L.; Scandura, M.; Bongi, P.; Apollonio, M. Nonkin Associations in Wild Boar Social Units. J. Mammal. 2009, 90, 666–674. [Google Scholar] [CrossRef]
- Massei, G.; Genov, P.V.; Staines, B.W.; Gorman, M.L. Factors Influencing Home Range and Activity of Wild Boar (Sus scrofa) in a Mediterranean Coastal Area. J. Zool. 1997, 242, 411–423. [Google Scholar] [CrossRef]
- Cavazza, S.; Brogi, R.; Apollonio, M. Sex-Specific Seasonal Variations of Wild Boar Distance Traveled and Home Range Size. Curr. Zool. 2023, 70, 284–290. [Google Scholar] [CrossRef]
- Russo, L.; Massei, G.; Genov, P.V. Daily Home Range and Activity of Wild Boar in a Mediterranean Area Free from Hunting. Ethol. Ecol. Evol. 1997, 9, 287–294. [Google Scholar] [CrossRef]
- Benten, A.; Hothorn, T.; Vor, T.; Ammer, C. Wildlife Warning Reflectors Do Not Mitigate Wildlife–Vehicle Collisions on Roads. Accid. Anal. Prev. 2018, 120, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Schlageter, A.; Haag-Wackernagel, D. Effectiveness of Solar Blinkers as a Means of Crop Protection from Wild Boar Damage. Crop Prot. 2011, 30, 1216–1222. [Google Scholar] [CrossRef]
- Eguchi, Y.; Tanida, H.; Tanaka, T.; Yoshimoto, T. Color Discrimination in Wild Boars. J. Ethol. 1997, 15, 1–7. [Google Scholar] [CrossRef]
- Denzin, N.; Helmstädt, F.; Probst, C.; Conraths, F.J. Testing Different Deterrents as Candidates for Short-Term Reduction in Wild Boar Contacts—A Pilot Study. Animals 2020, 10, 2156. [Google Scholar] [CrossRef] [PubMed]
- Desmecht, D.; Gerbier, G.; Gortázar Schmidt, C.; Grigaliuniene, V.; Helyes, G.; Kantere, M.; Korytarova, D.; Linden, A.; Miteva, A.; Neghirla, I.; et al. Epidemiological Analysis of African Swine Fever in the European Union (September 2019 to August 2020). EFSA J. 2021, 19, e06572. [Google Scholar] [CrossRef]
- Palencia, P.; Blome, S.; Brook, R.K.; Ferroglio, E.; Jo, Y.S.; Linden, A.; Montoro, V.; Penrith, M.L.; Plhal, R.; Vicente, J.; et al. Tools and Opportunities for African Swine Fever Control in Wild Boar and Feral Pigs: A Review. Eur. J. Wildl. Res. 2023, 69, 1–22. [Google Scholar] [CrossRef]
- Mysterud, A.; Rolandsen, C.M. Fencing for Wildlife Disease Control. J. Appl. Ecol. 2019, 56, 519–525. [Google Scholar] [CrossRef]
- Vercauteren, K.C.; Lavelle, M.J.; Hygnstrom, S. Fences and Deer-Damage Management: A Review of Designs and Efficacy. Wildl. Soc. Bull. 2006, 34, 191–200. [Google Scholar] [CrossRef]
- Cukor, J.; Linda, R.; Mahlerová, K.; Vacek, Z.; Faltusová, M.; Marada, P.; Havránek, F.; Hart, V. Different Patterns of Human Activities in Nature during COVID-19 Pandemic and African Swine Fever Outbreak Confirm Direct Impact on Wildlife Disruption. Sci. Rep. 2021, 11, 20791. [Google Scholar] [CrossRef]
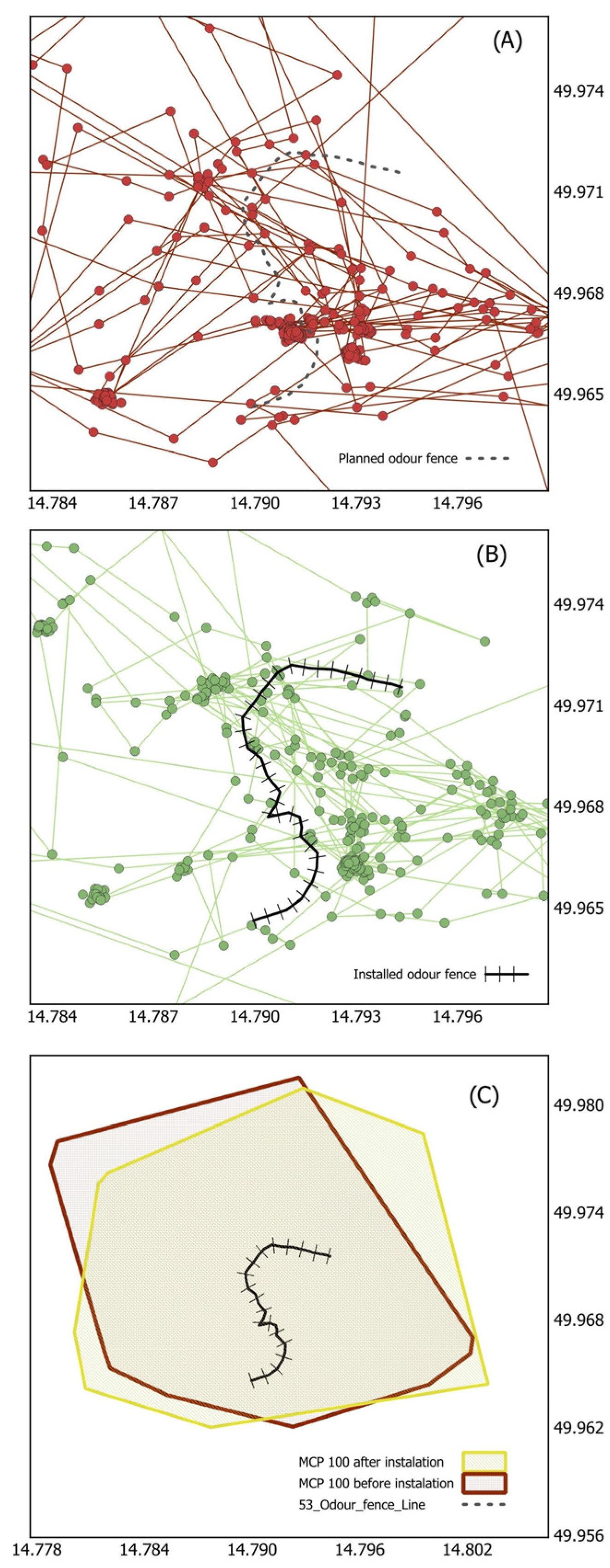

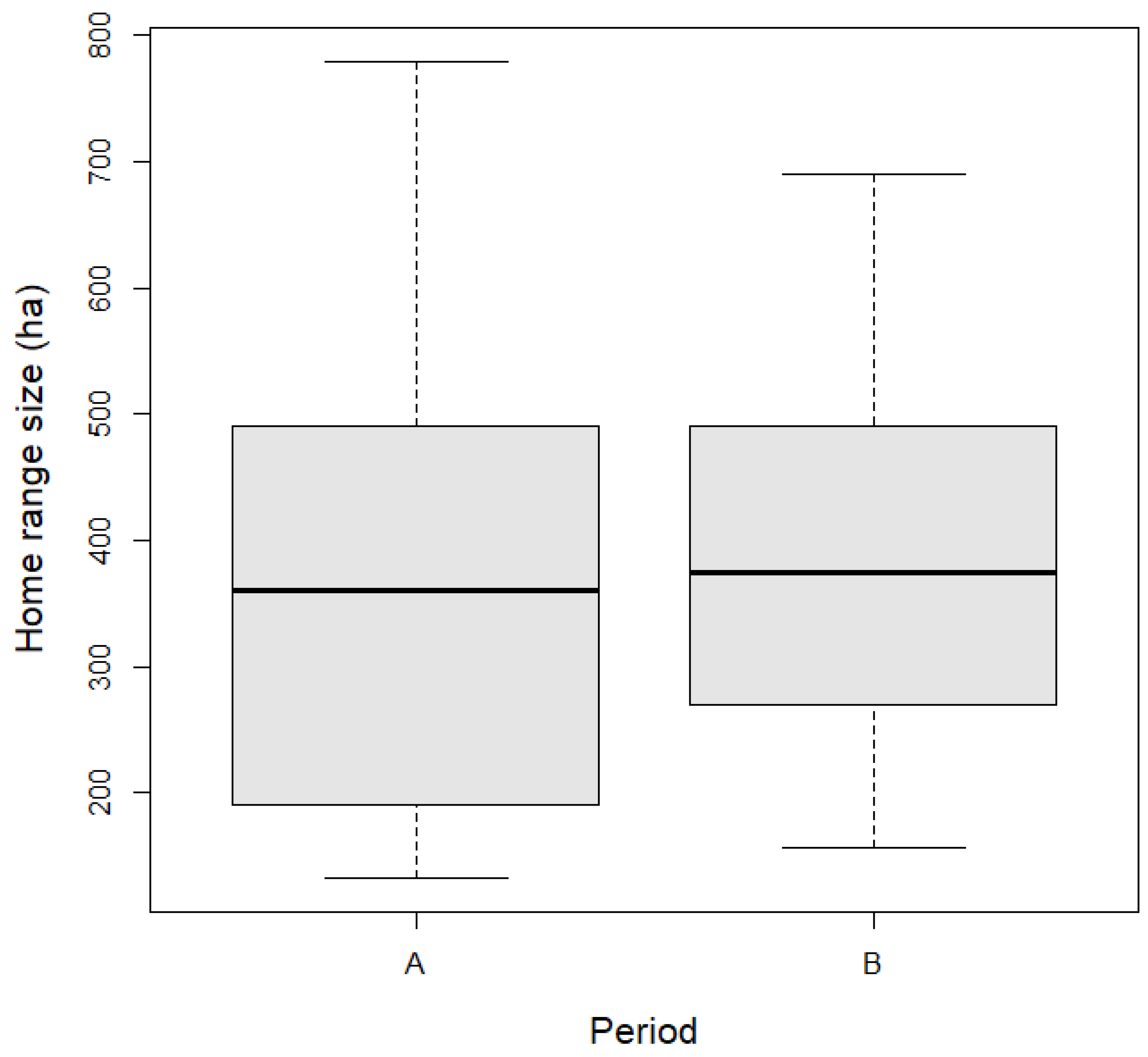
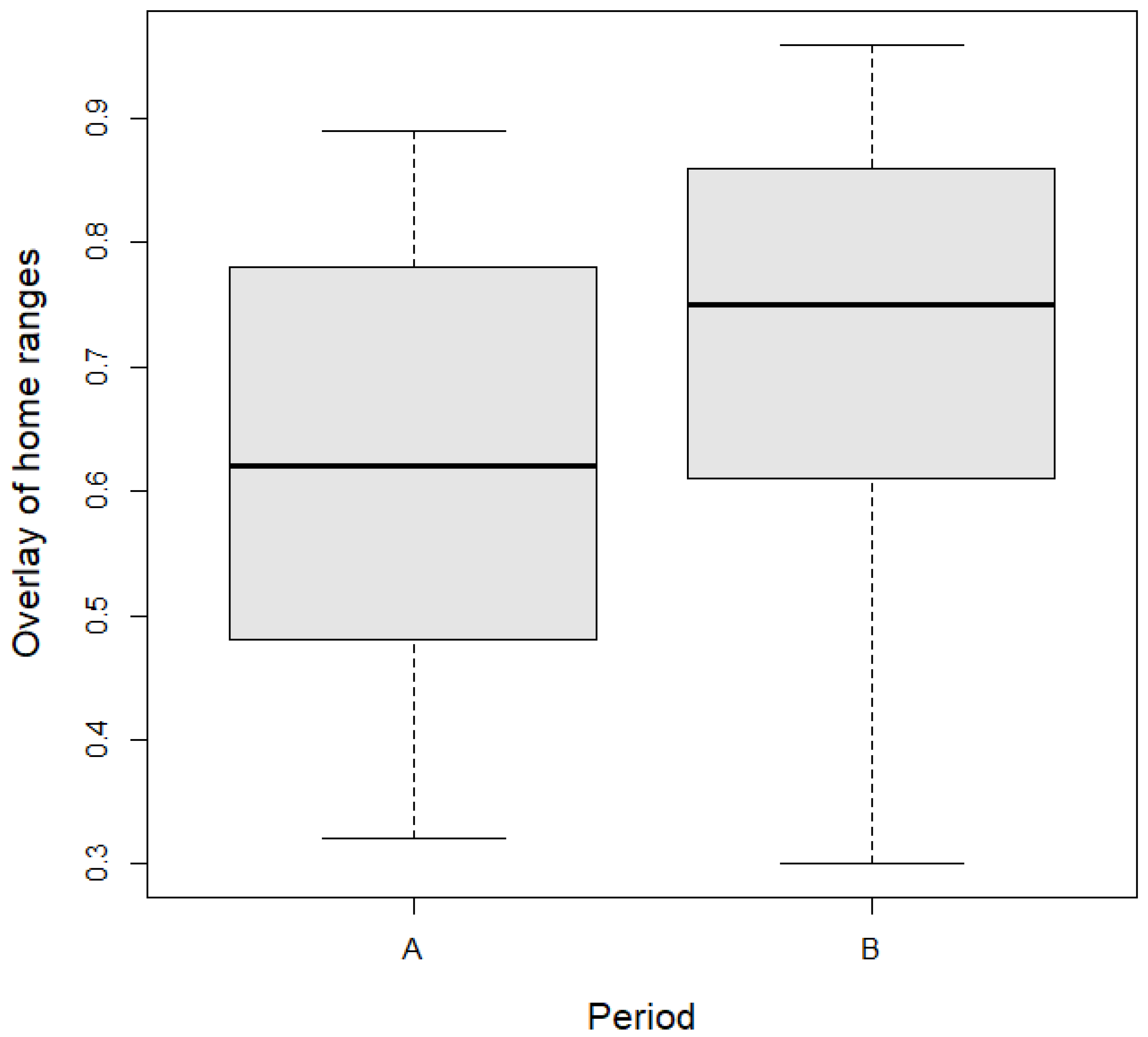
| Variable | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 23.6748 | 4.4175 | 5.359 | 0.0526 |
| Period (B) | −0.6111 | 2.8010 | −0.218 | 0.8287 |
| Sex (M) | −1.0040 | 3.3941 | −0.296 | 0.7693 |
| Age (S) | −2.8906 | 3.0233 | −0.956 | 0.3464 |
| Variable | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 388.5824 | 57.0172 | 6.815 | 0.0083 |
| Period (B) | 0.2222 | 55.0948 | 0.004 | 0.9968 |
| Sex (M) | −72.0198 | 66.5263 | −1.083 | 0.2872 |
| Age (S) | 35.0190 | 59.4157 | 0.589 | 0.5599 |
| Variable | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 0.6528 | 0.0641 | 10.191 | 0.0033 |
| Period (B) | 0.0861 | 0.0601 | 1.433 | 0.1619 |
| Sex (M) | −0.0048 | 0.0726 | 0.066 | 0.9478 |
| Age (S) | −0.0442 | 0.0648 | −0.682 | 0.5002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faltusová, M.; Ježek, M.; Ševčík, R.; Silovský, V.; Cukor, J. Odor Fences Have No Effect on Wild Boar Movement and Home Range Size. Animals 2024, 14, 2556. https://doi.org/10.3390/ani14172556
Faltusová M, Ježek M, Ševčík R, Silovský V, Cukor J. Odor Fences Have No Effect on Wild Boar Movement and Home Range Size. Animals. 2024; 14(17):2556. https://doi.org/10.3390/ani14172556
Chicago/Turabian StyleFaltusová, Monika, Miloš Ježek, Richard Ševčík, Václav Silovský, and Jan Cukor. 2024. "Odor Fences Have No Effect on Wild Boar Movement and Home Range Size" Animals 14, no. 17: 2556. https://doi.org/10.3390/ani14172556





