Trophic Ecology of the Pyjama Shark Poroderma africanum (Gmelin, 1789) Elucidated by Stable Isotopes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Stable Isotope Analysis (SIA)
2.3. Statistical Analysis
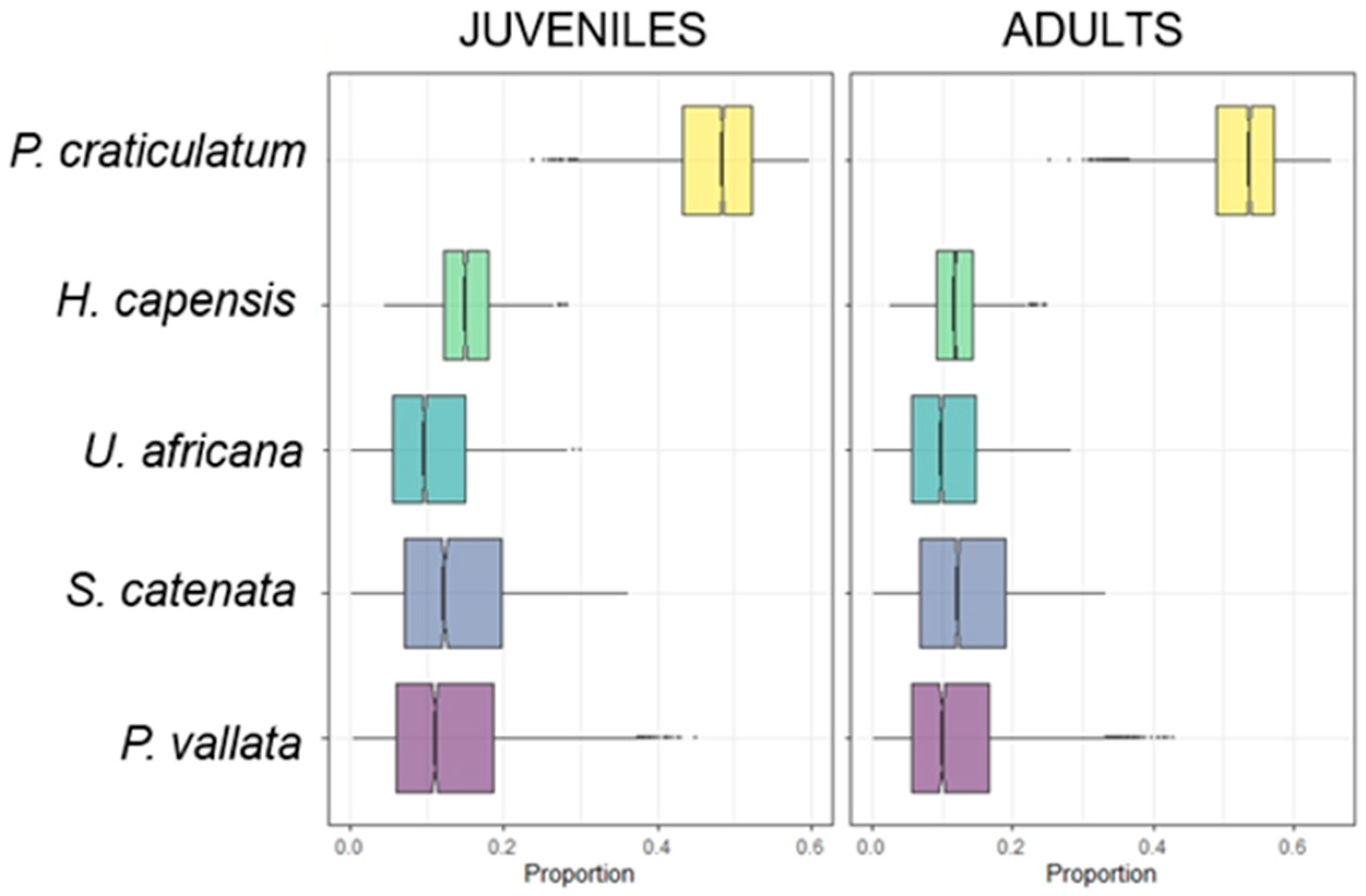
2.4. Mixing Models
| Sources | Mean δ13C (‰) | Mean δ15N (‰) | SD δ13C (‰) | SD δ15N (‰) |
|---|---|---|---|---|
| Perinereis nuntia vallata [32] | −12.7 | 11.8 | 0.3 | 0.1 |
| Sesarma catenata [32] | −17.0 | 10.1 | 1 | 0.2 |
| Upogebia africana [32] | −15.0 | 9.3 | 1 | 0.4 |
| Hyporhamphus capensis [33] | −22.3 | 13.6 | 0.01 | 0.01 |
| Phalium craticulatum [34] | −14.8 | 15.0 | 0.01 | 0.01 |
3. Results
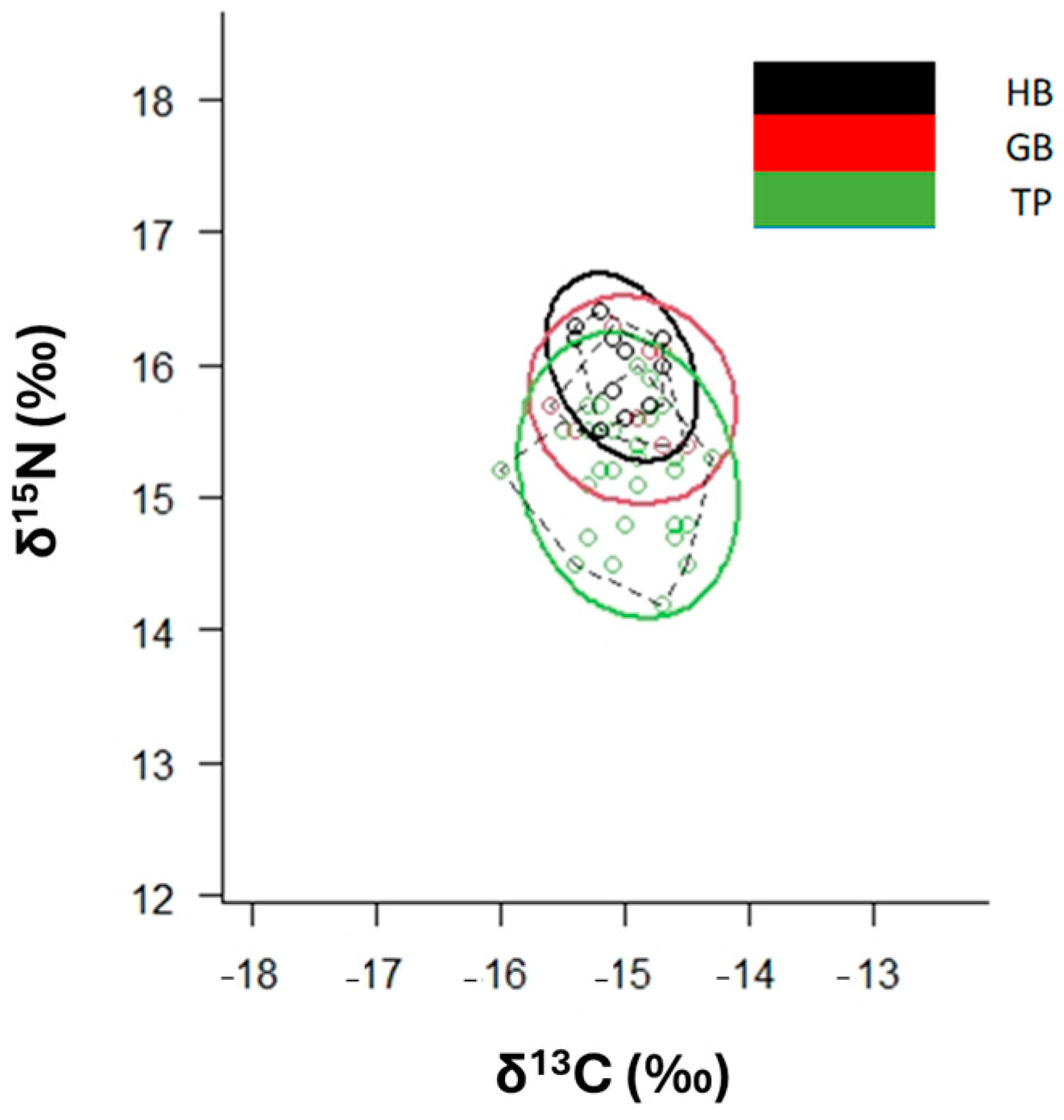
3.1. Overall Isotopic Composition
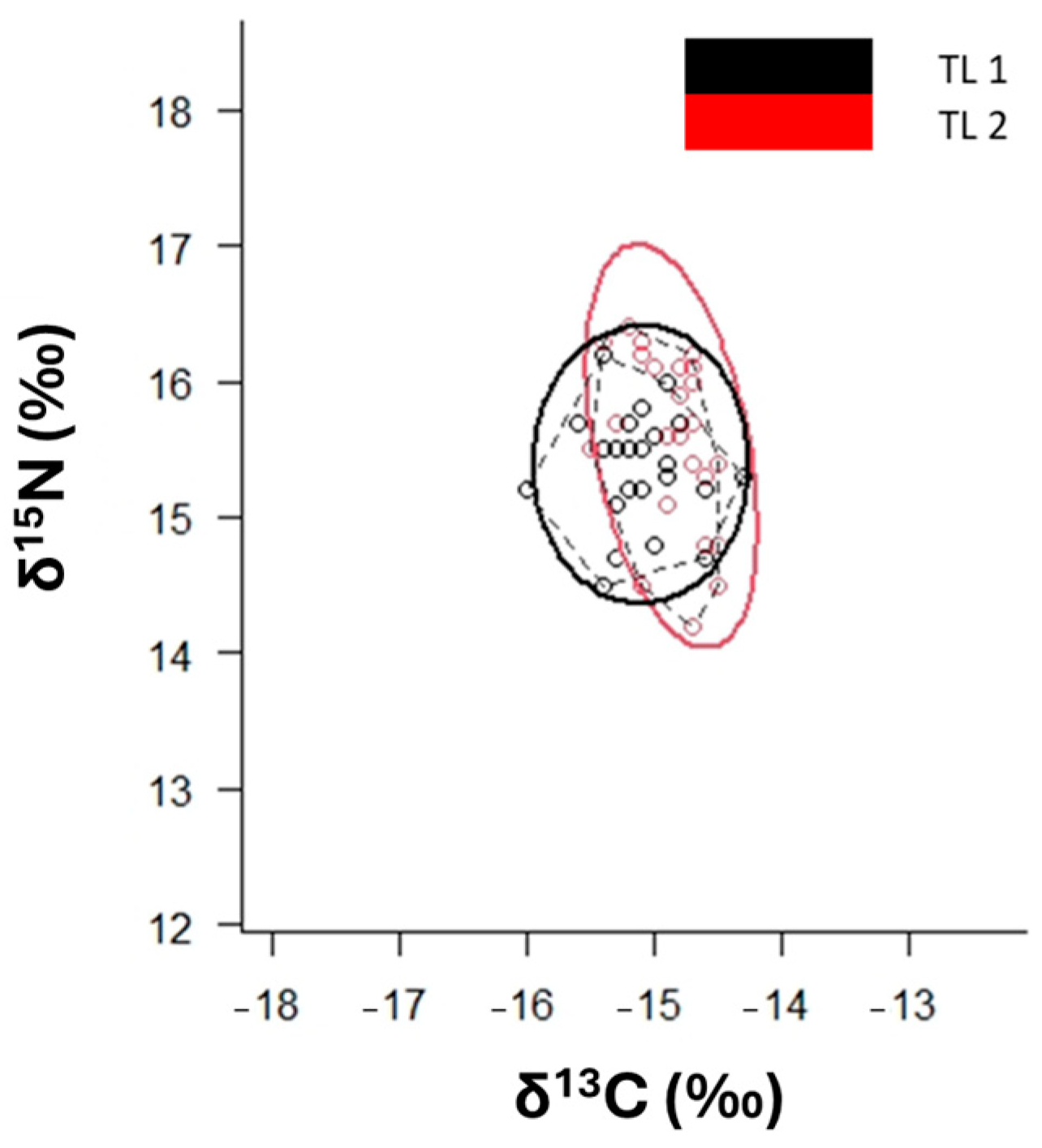
3.2. Mixing Models and Niche Width
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compagno, L.J.V. Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes 1990, 28, 33–75. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Bascompte, J.; Melián, C.J. Simple trophic modules for complex food webs. Ecology 2005, 86, 2868–2873. [Google Scholar] [CrossRef]
- Ellis, J.K.; Musick, J.A. Ontogenetic changes in the diet of the sandbar shark, Carcharhinus plumbeus, in lower Chesapeake Bay and Virginia (USA) coastal waters. Environ. Biol. Fishes 2007, 80, 51–67. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Braccini, M.; Newman, S.J.; Harvey, E.S. Global patterns in the bycatch of sharks and rays. Mar. Pol. 2015, 54, 86–97. [Google Scholar] [CrossRef]
- Estes, J.; Crooks, K.; Holt, R.D. Predators, Ecological Role of Encyclopedia of Biodiversity, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Heupel, M.R.; Knip, D.M.; Simpfendorfer, C.A.; Dulvy, N.K. Sizing up the ecological role of sharks as predators. Mar. Ecol. Prog. Ser. 2014, 495, 291–298. [Google Scholar] [CrossRef]
- Matich, P.; Heithaus, M.R. Multi-tissue stable isotope analysis and acoustic telemetry reveal seasonal variability in the trophic interactions of juvenile bull sharks in a coastal estuary. J. Anim. Ecol. 2014, 83, 199–213. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Skomal, G.B.; Fisk, A.T. Stable isotopes from multiple tissues reveal diet switching in sharks. Mar. Ecol. Prog. Ser. 2005, 302, 199–206. [Google Scholar] [CrossRef]
- Shiffman, D.S.; Gallagher, A.J.; Boyle, M.D.; Hammerschlag-Peyer, C.M.; Hammerschlag, N. Stable isotope analysis as a tool for elasmobranch conservation research: A primer for non-specialists. Mar. Freshw. Res. 2012, 63, 635–643. [Google Scholar] [CrossRef]
- Domi, N.; Bouquegneau, J.M.; Krishna, D. Feeding ecology of five commercial shark species of the Celtic Sea through stable isotope and trace metal analysis. Mar. Environ. Res. 2005, 60, 551–569. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Drouillard, K.G.; Fisk, A.T. Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Can. J. Fish. Aquat. Sci. 2006, 63, 345–353. [Google Scholar] [CrossRef]
- Hussey, N.E.; Chapman, D.D.; Donnelly, E.; Abercrombie, D.L.; Fisk, A.T. Fin-icky samples: An assessment of shark fin as a source material for stable isotope analysis. Limnol. Oceanogr. Methods 2011, 9, 524–532. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006; Volume 521. [Google Scholar] [CrossRef]
- Van der Heever, G.M.; Van der Lingen, C.D.; Leslie, R.W.; Gibbons, M.J. Spatial and ontogenetic variability in the diet and trophic ecology of two co-occurring catsharks (Scyliorhinidae) off South Africa. Afr. J. Mar. Sci. 2020, 42, 423–438. [Google Scholar] [CrossRef]
- Hobson, K.A. Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 1999, 120, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Compagno, L.J.V.; Ebert, D.A.; Smale, M.J. Guide to the Sharks and Rays of Southern Africa; Struik Publishers: Cape Town, South Africa, 1989. [Google Scholar]
- McQuaid, C.D. The establishment and maintenance of vertical size gradients in populations of Littorina africana knysnaensis (Philippi) on an exposed rocky shore. J. Exp. Mar. Biol. Ecol. 1981, 54, 77–89. [Google Scholar] [CrossRef]
- McQuaid, C.D.; Branch, G.M. Trophic structure of rocky intertidal communities: Response to wave action and implications for energy flow. Mar. Ecol. Prog. Ser. Oldendorf 1985, 22, 153–161. [Google Scholar] [CrossRef]
- Emanuel, B.P.; Bustamante, R.H.; Branch, G.M.; Eekhout, S.; Odendaal, F.J. A zoogeographic and functional approach to the selection of marine reserves on the west coast of South Africa. S. Afr. J. Mar. Sci. 1992, 12, 341–354. [Google Scholar] [CrossRef]
- Watling, R.J.; Watling, H.R. Metal Surveys in South African Estuaries. III. Hartenbos, Little Brak and Great Brak Rivers (Mossel Bay). Water SA 1982, 8, 108–113. [Google Scholar]
- Fanelli, E.; Da Ros, Z.; Martino, I.; Donato, F.; Azzurro, E.; Bargione, G.; Lucchetti, A. Crowding in the middle of marine food web: A focus on Raja asterias and other Mediterranean batoids. Mar. Environ. Res. 2023, 183, 105830. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities. Approach Stat. Anal. Interpret. 2001, 2, 1–168. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, K. PERMANOVA+ for Primer: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electr. 2001, 4, 9. [Google Scholar]
- Parnell, A. SIMMR: A Stable Isotope Mixing Model. R Package Version 0.4.5. 2021. Available online: https://CRAN.R-project.org/package=simmr (accessed on 30 June 2024).
- Parnell, A.C.; Phillips, D.L.; Bearhop, S.; Semmens, B.X.; Ward, E.J.; Moore, J.W.; Jackson, A.L.; Grey, J.; Kelly, D.J.; Inger, R. Bayesian stable isotope mixing models. Environmetrics 2013, 24, 387–399. [Google Scholar] [CrossRef]
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Canad. J. L Zool. 2014, 92, 823–835. [Google Scholar] [CrossRef]
- Tilley, A.; López-Angarita, J.; Turner, J.R. Diet reconstruction and resource partitioning of a Caribbean marine mesopredator using stable isotope Bayesian modelling. PLoS ONE 2013, 8, e79560. [Google Scholar] [CrossRef]
- Richoux, N.B.; Froneman, P.W. Assessment of spatial variation in carbon utilization by benthic and pelagic invertebrates in a temperate South African estuary using stable isotope signatures. Estuar. Coast. Shelf Sci. 2007, 71, 545–558. [Google Scholar] [CrossRef]
- Bergamino, L.; Dalu, T.; Whitfield, A.K.; Carassou, L.; Richoux, N.B. Stable isotope evidence of food web connectivity by a top predatory fish (Argyrosomus japonicus: Sciaenidae: Teleostei) in the Kowie Estuary, South Africa. Afr. J. Mar. Sci. 2014, 36, 207–213. [Google Scholar] [CrossRef]
- De Lecea, A.M.; Fennessy, S.T.; Smit, A.J. Processes controlling the benthic food web of a mesotrophic bight (KwaZulu-Natal, South Africa) revealed by stable isotope analysis. Mar. Ecol. Prog. Ser. 2013, 484, 97–114. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. An. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Kim, S.L.; Casper, D.R.; Galván-Magaña, F.; Ochoa-Díaz, R.; Hernández-Aguilar, S.B.; Koch, P.L. Carbon and nitrogen discrimination factors for elasmobranch soft tissues based on a long-term controlled feeding study. Environ. Biol. Fishes 2012, 95, 37–52. [Google Scholar] [CrossRef]
- Caracausi, L.; Da Ros, Z.; Masia Lillo, P.; Ligas, A.; Fanelli, E. Trophic ecology of Scyliorhinus canicula in the Mediterranean Sea: Literature review and insights from a Tyrrhenian case-study. 2024; Submitted. [Google Scholar]
- Di Lorenzo, M.; Vizzini, S.; Signa, G.; Andolina, C.; Palo, G.B.; Gristina, M.; Mazzoldi, C.; Colloca, F. Ontogenetic trophic segregation between two threatened smooth-hound sharks in the Central Mediterranean Sea. Sci. Rep. 2020, 10, 11011. [Google Scholar] [CrossRef]
- Jennings, S.; Reñones, O.; Morales-Nin, B.; Polunin, N.V.; Moranta, J.; Coll, J. Spatial variation in the δ15N and δ13C stable isotope composition of plants, invertebrates and fishes on Mediterranean reefs: Implications for the study of trophic pathways. Mar. Ecol. Prog. Ser. 1997, 146, 109–116. [Google Scholar] [CrossRef]
- Fanelli, E.; Menicucci, S.; Malavolti, S.; Da Ros, Z.; Biagiotti, I.; Canduci, G.; De Felice, A.; Leonori, I. The pelagic food web of the Western Adriatic Sea: A focus on small pelagics. Sci. Rep. 2023, 13, 14554. [Google Scholar] [CrossRef] [PubMed]
- Rabehagasoa, N.; Lorrain, A.; Bach, P.; Potier, M.; Jaquemet, S.; Richard, P.; Ménard, F. Isotopic niches of the blue shark Prionace glauca and the silky shark Carcharhinus falciformis in the southwestern Indian Ocean. Endang. Species Res. 2012, 17, 83–92. [Google Scholar] [CrossRef]
- Dainty, A.M. Biology and Ecology of Four Catshark Species in the Southwestern Cape, South Africa. MSc Thesis, University of Cape Town, Cape Town, South Africa, 2002. [Google Scholar]
- Tanaka, H.; Ohshimo, S.; Takagi, N.; Ichimaru, T. Investigation of the geographical origin and migration of anchovy Engraulis japonicus in Tachibana Bay, Japan: A stable isotope approach. Fish. Res. 2010, 102, 217–220. [Google Scholar] [CrossRef]
- Trueman, C.N.; MacKenzie, K.M.; Palmer, M.R. Identifying migrations in marine fishes through stable-isotope analysis. J. Fish Biol. 2012, 81, 826–847. [Google Scholar] [CrossRef]
- Graham, B.S.; Koch, P.L.; Newsome, S.D.; McMahon, K.W.; Aurioles, D. Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. In Isoscapes; Springer: Dordrecht, The Netherlands, 2010; pp. 299–318. [Google Scholar] [CrossRef]
- Lee, K.Y.; Graham, L.; Spooner, D.E.; Xenopoulos, M.A. Tracing anthropogenic inputs in stream foods webs with stable carbon and nitrogen isotope systematics along an agricultural gradient. PLoS ONE 2018, 13, e0200312. [Google Scholar] [CrossRef]
- Davias, L.A.; Kornis, M.S.; Breitburg, D.L. Environmental factors influencing δ13C and δ15N in three Chesapeake Bay fishes. ICES J. Mar. Sci. 2014, 71, 689–702. [Google Scholar] [CrossRef]
- Elmhagen, B.; Ludwig, G.; Rushton, S.P.; Helle, P.; Lindén, H. Top predators, mesopredators and their prey: Interference ecosystems along bioclimatic productivity gradients. J. Anim. Ecol. 2010, 79, 785–794. [Google Scholar] [CrossRef] [PubMed]

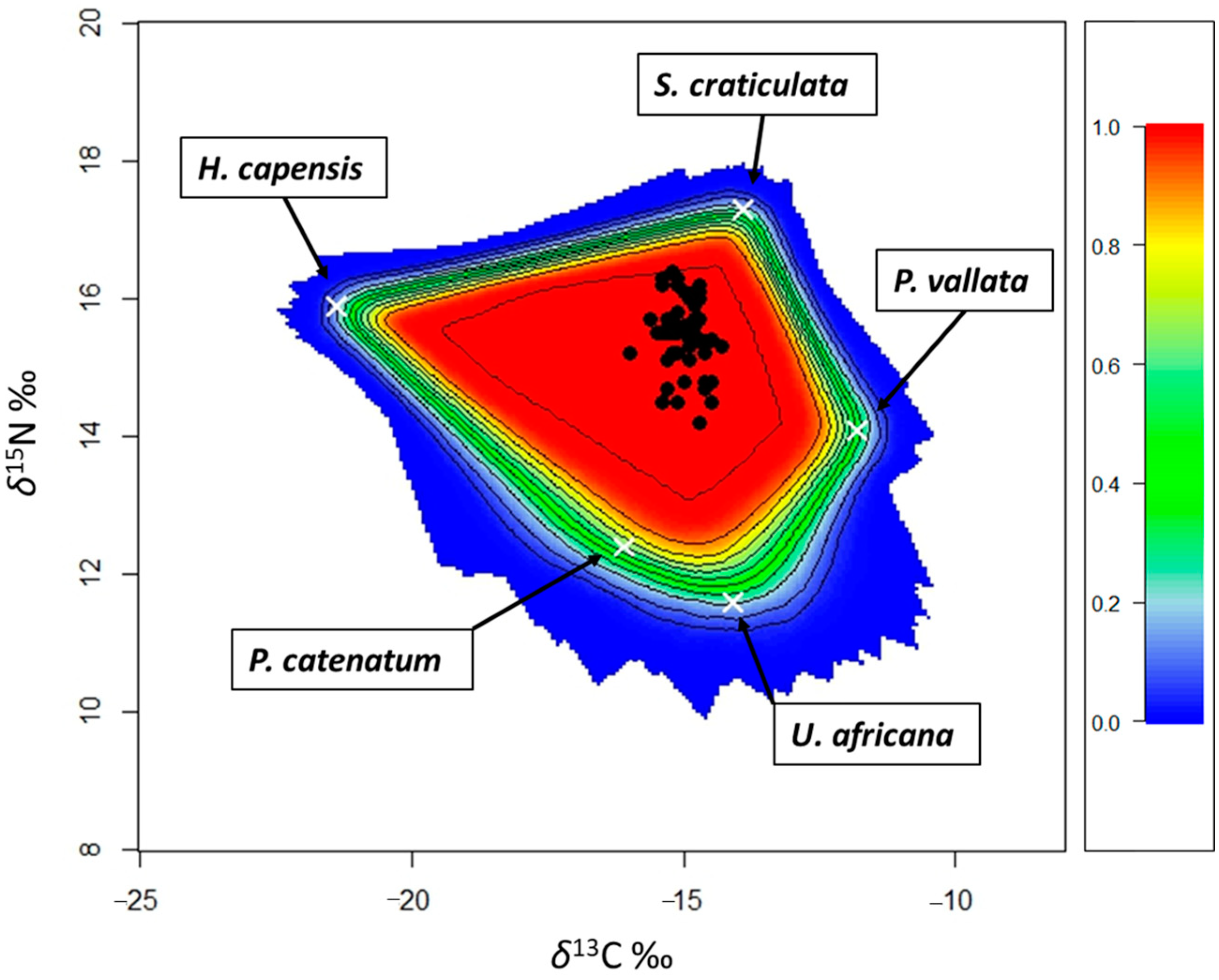
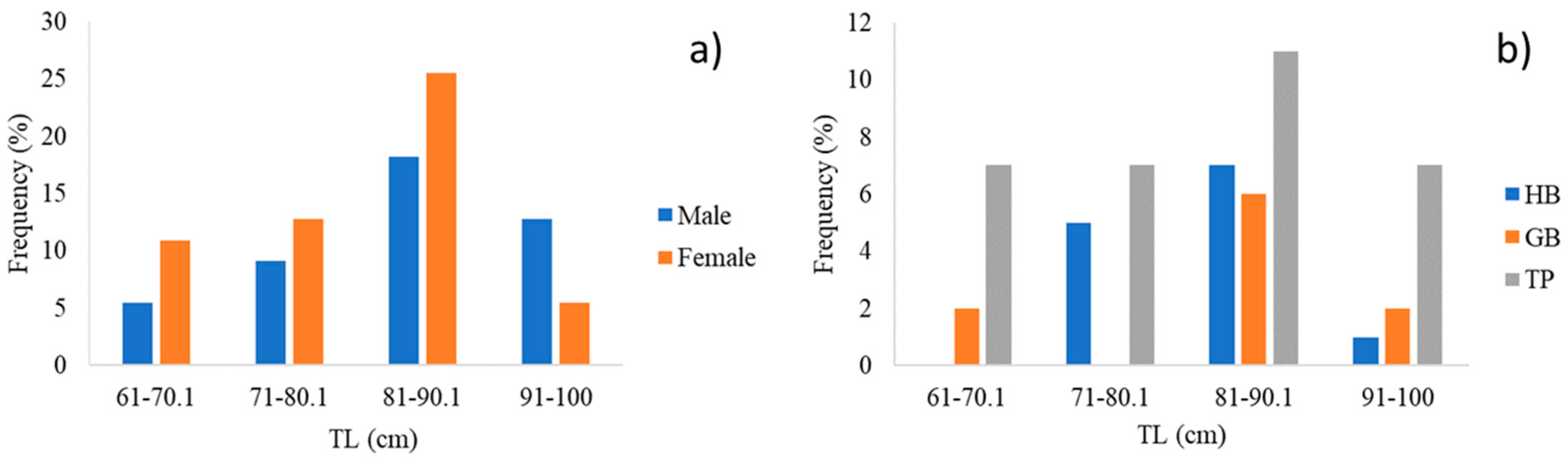
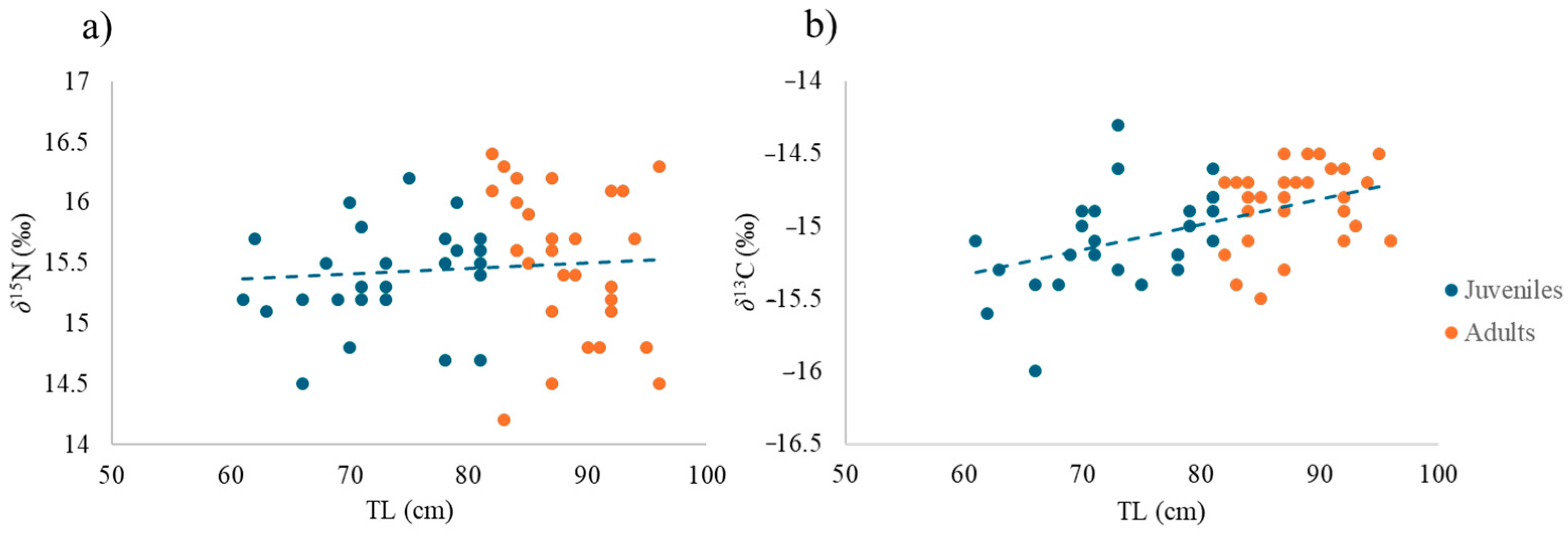
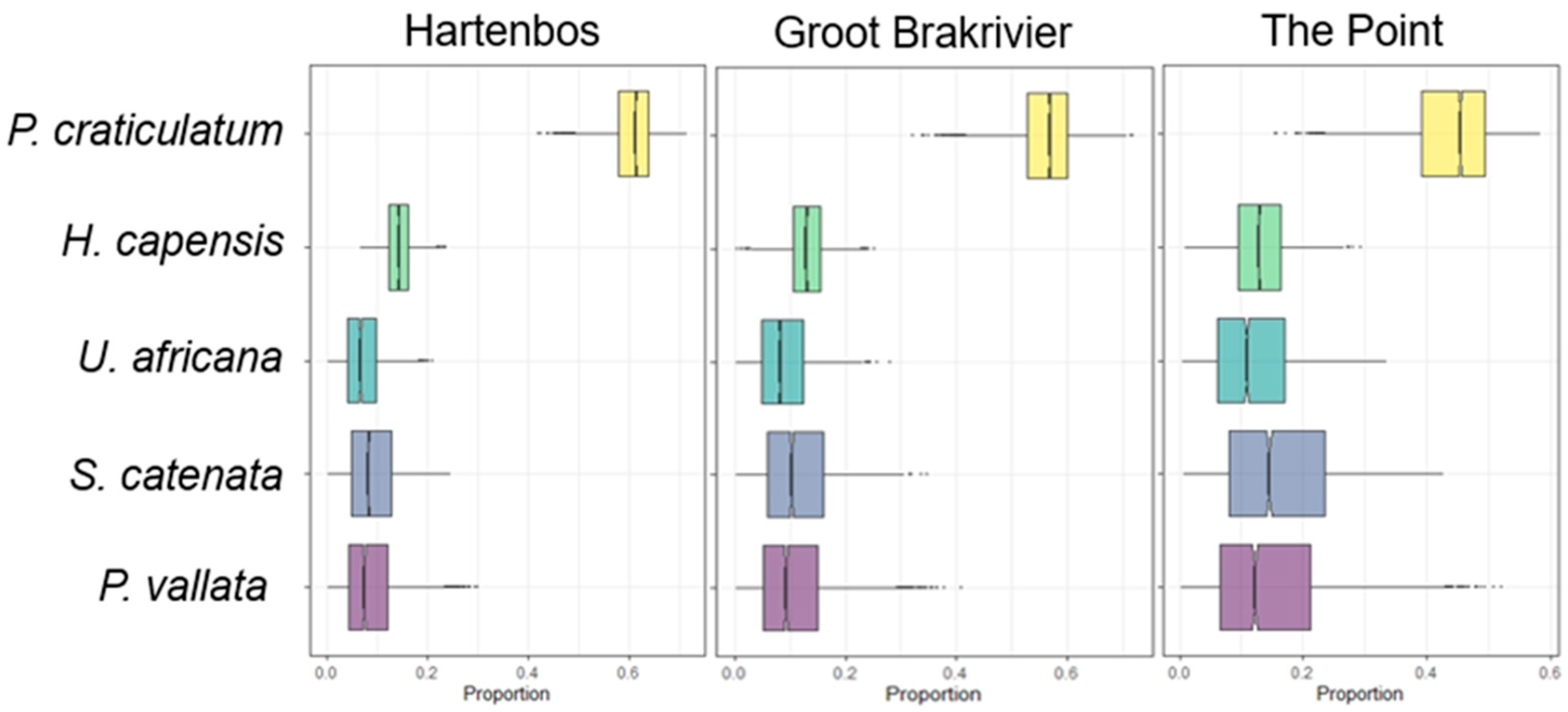
| Model | Groups | Communities |
|---|---|---|
| 1 | TP, HB and GB | 1 |
| 2 | TP, HB and GB | 2 (M and F) |
| 3 | AD and JUV | 1 |
| Mean δ13C (‰) | SD δ13C (‰) | Mean δ15N (‰) | SD δ15N (‰) | |
|---|---|---|---|---|
| F | −15 | 0.3 | 15.5 | 0.4 |
| M | −14.9 | 0.3 | 15.4 | 0.6 |
| JUV | −15.1 | 0.3 | 15.4 | 0.4 |
| AD | −14.9 | 0.3 | 15.5 | 0.6 |
| HB | −15.0 | 0.2 | 16.0 | 0.3 |
| GB | −14.9 | 0.3 | 15.7 | 0.3 |
| TP | −15.0 | 0.4 | 15.2 | 0.4 |
| (a) | δ15N | δ13C | |||||
| Source | df | MS | Pseudo-F | P(MC) | MS | Pseudo-F | P(MC) |
| TL | 1 | 0.22 | 1.60 | n.s. | 0.71 | 7.12 | * |
| Sex | 1 | 0.00 | 0.03 | n.s. | 0.00 | 0.04 | n.s. |
| Site | 2 | 3.18 | 23.38 | * | 0.04 | 0.35 | n.s. |
| TL×Sex | 1 | 0.00 | 0.00 | n.s. | 0.01 | 0.11 | n.s. |
| TL×Site | 2 | 0.19 | 1.39 | n.s. | 0.11 | 1.15 | n.s. |
| Sex×Site | 2 | 0.40 | 2.95 | n.s. | 0.14 | 1.46 | n.s. |
| TL×Sex×Site | 2 | 0.19 | 1.38 | n.s. | 0.06 | 0.57 | n.s. |
| Residuals | 43 | 0.14 | 0.10 | ||||
| Total | 54 | ||||||
| (b) | |||||||
| Groups | t | P(MC) | |||||
| HB, GB | 0.94 | n.s. | |||||
| HB, TP | 4.94 | * | |||||
| GB, TP | 4.16 | * | |||||
| Groups | t | P(MC) | |||||
| F, M | 2.19 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caracausi, L.; Da Ros, Z.; Premici, A.; Gennari, E.; Fanelli, E. Trophic Ecology of the Pyjama Shark Poroderma africanum (Gmelin, 1789) Elucidated by Stable Isotopes. Animals 2024, 14, 2559. https://doi.org/10.3390/ani14172559
Caracausi L, Da Ros Z, Premici A, Gennari E, Fanelli E. Trophic Ecology of the Pyjama Shark Poroderma africanum (Gmelin, 1789) Elucidated by Stable Isotopes. Animals. 2024; 14(17):2559. https://doi.org/10.3390/ani14172559
Chicago/Turabian StyleCaracausi, Luca, Zaira Da Ros, Alice Premici, Enrico Gennari, and Emanuela Fanelli. 2024. "Trophic Ecology of the Pyjama Shark Poroderma africanum (Gmelin, 1789) Elucidated by Stable Isotopes" Animals 14, no. 17: 2559. https://doi.org/10.3390/ani14172559







