Detection of Gastrointestinal Pathogens with Zoonotic Potential in Horses Used in Free-Riding Activities during a Countrywide Study in Greece
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
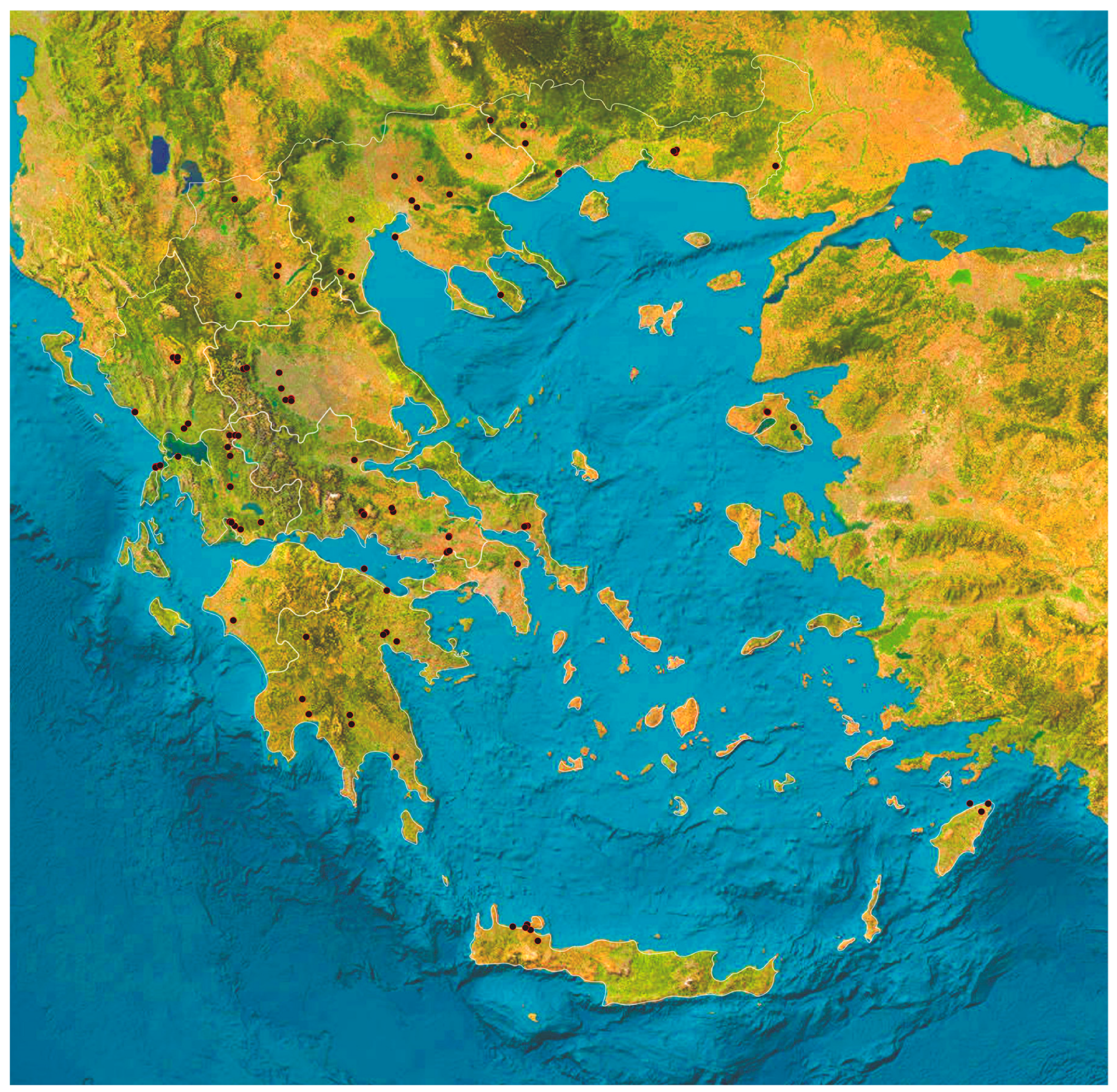
2.1. Animals and Samplings
2.2. Laboratory Examinations
2.3. Data Management and Analysis
3. Results
3.1. Detection of Potentially Zoonotic Pathogens in Faecal Samples
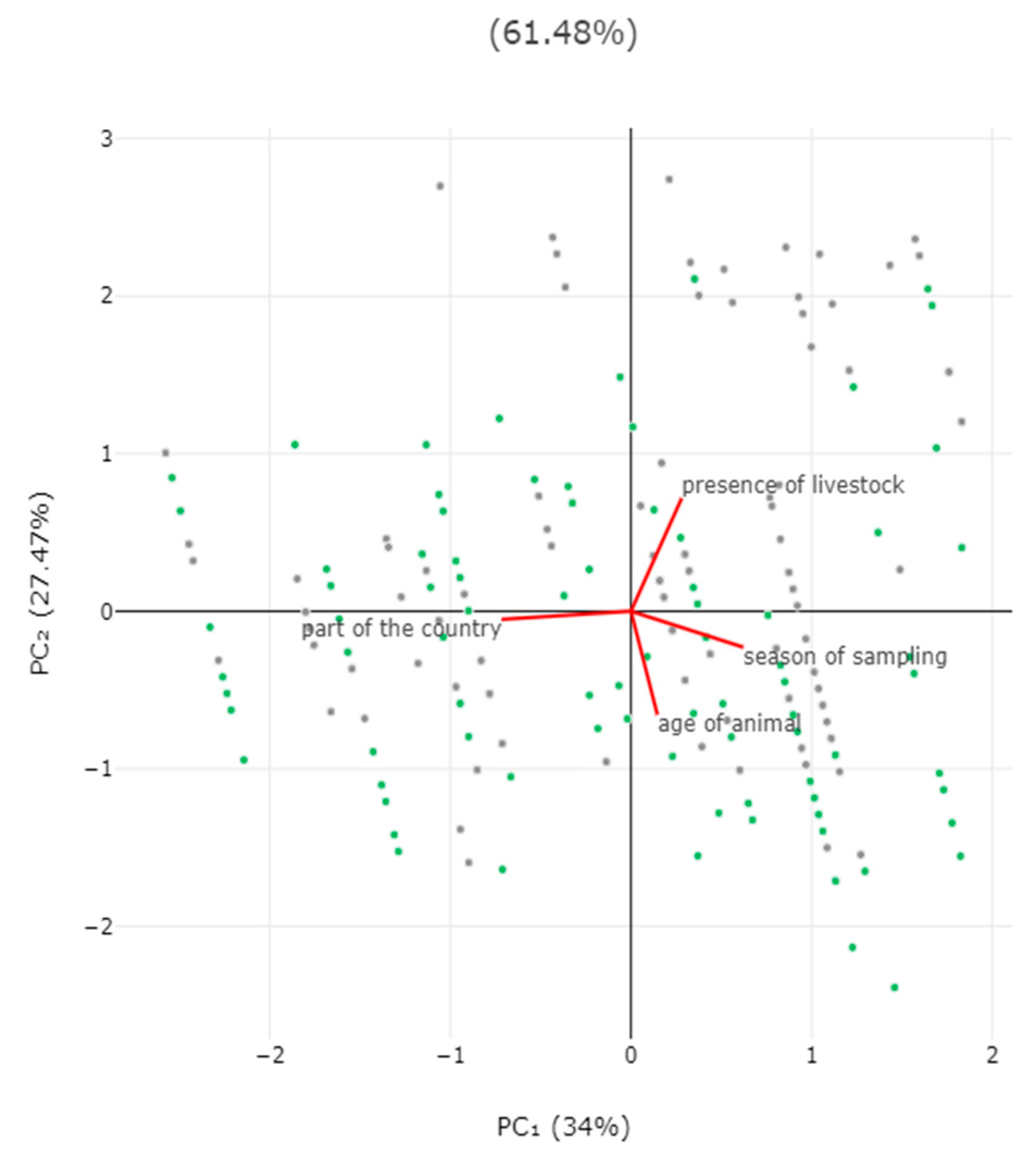
3.2. Predictors
4. Discussion
4.1. Frequency of Detection and Identity of Potentially Zoonotic Pathogens
4.2. The Use of FilmArray® GI Panel Technology in Samples from Horses
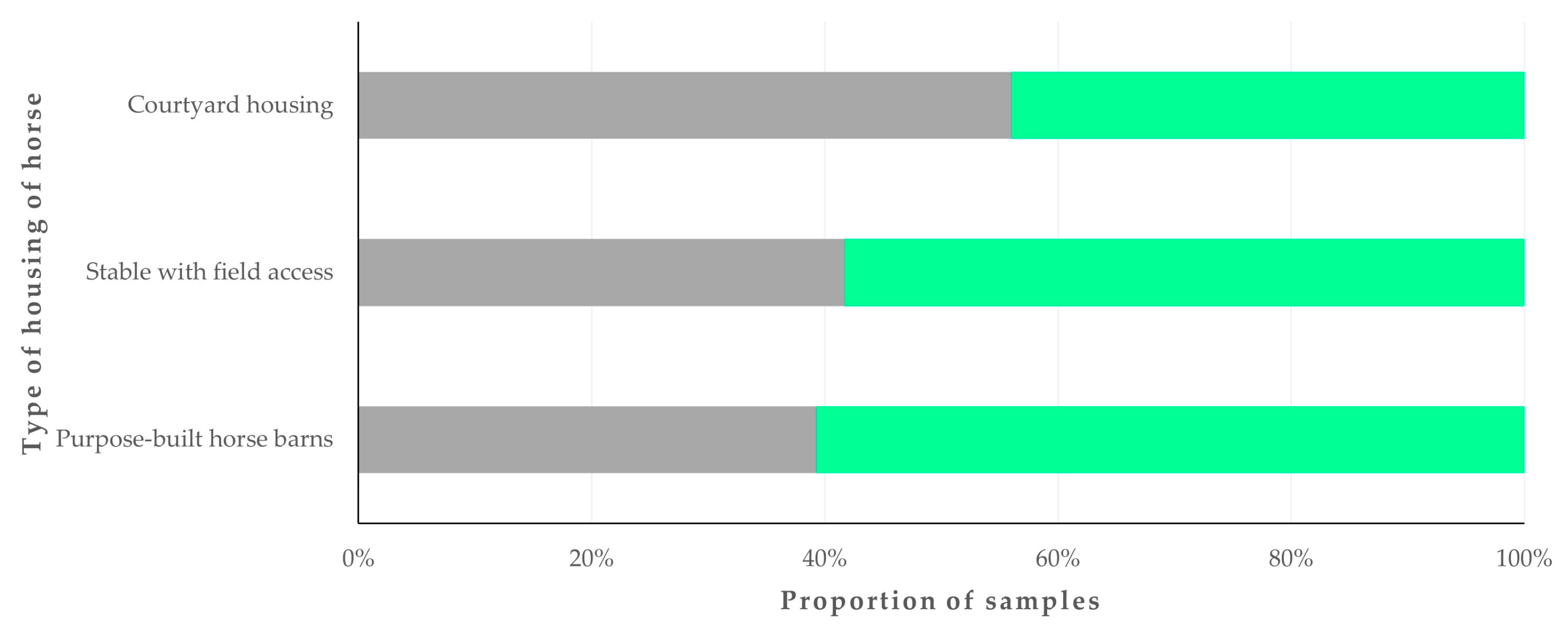
4.3. Predictors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dwyer, R. Equine zoonoses: Consequences of horse-human interactions. In Zoonoses-Infections Affecting Humans and Animals; Sing, A., Ed.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Sack, A.; Oladunni, F.S.; Gonchigoo, B.; Chambers, T.M.; Gray, G.C. Zoonotic diseases from horses: A systematic review. Vector Borne Zoonotic Dis. 2020, 20, 484–495. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union one health 2021 zoonoses report. EFSA J. 2022, 20, e07666. [Google Scholar]
- Ruple-Czerniak, A.A.; Aceto, H.W.; Bender, J.B.; Paradis, M.R.; Shaw, S.P.; Van Metre, D.C.; Weese, J.S.; Wilson, D.A.; Wilson, J.; Morley, P.S. Syndromic surveillance for evaluating the occurrence of healthcare-associated infections in equine hospitals. Equine Vet. J. 2014, 46, 435–440. [Google Scholar] [CrossRef]
- Weese, J.S. Infection control and biosecurity in equine disease control. Equine Vet. J. 2014, 46, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.B.; Dagley, K.; Tainsh, J. Insights into the economic consequences of the 2007 equine influenza outbreak in Australia. Aust. Vet. J. 2011, 89, 151–158. [Google Scholar] [CrossRef]
- Uzal, F.A.; Arroyo, L.G.; Navarro, M.A.; Gomez, D.E.; Asín, J.; Henderson, E. Bacterial and viral enterocolitis in horses: A review. J. Vet. Diagn. Investig. 2022, 34, 354–375. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, J.H.; Kroeze, E.V. Infectious Diseases of the Horse: Diagnosis, Pathology, Management, and Public Health, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Uzal, F.A.; Diab, S.S. Gastritis, enteritis, and colitis in horses. Vet. Clin. N. Am. Equine Pract. 2015, 31, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.S.; Arrowood, M.; Kokoskin, E.P.; Paltridge, G.P.; Pillai, D.R.; Procop, G.W.; Ryan, N.; Shimizu, R.Y.; Visvesvara, G. Practical guidance for clinical microbiology laboratories: Laboratory diagnosis of parasites from the gastrointestinal tract. Clin. Microbiol. Rev. 2018, 31, e00025-17. [Google Scholar] [CrossRef]
- Båverud, V. Clostridium difficile infections in animals with special reference to the horse: A review. Vet. Q. 2002, 24, 203–219. [Google Scholar] [CrossRef]
- Weese, J.S.; Staempfli, H.R.; Prescott, J.F. Survival of Clostridium difficile and its toxins in equine feces: Implications for diagnostic test selection and interpretation. J. Vet. Diagn. Investig. 2000, 12, 332–336. [Google Scholar] [CrossRef]
- Ward, M.P.; Alinovi, C.A.; Couëtil, L.L.; Wu, C.C. Evaluation of a PCR to detect Salmonella in faecal samples of horses admitted to a veterinary teaching hospital. J. Vet. Diagn. Investig. 2005, 17, 118–123. [Google Scholar] [CrossRef]
- Burgess, B.A. Salmonella in horses. Vet. Clin. N. Am. Equine Pract. 2023, 39, 25–35. [Google Scholar] [CrossRef]
- De Souza, P.N.B.; Bomfim, T.C.B.; Huber, F.; Abboud, L.C.S.; Gomes, R.S. Natural infection by Cryptosporidium sp., Giardia sp. and Eimeria leuckarti in three groups of equines with different handlings in Rio de Janeiro, Brazil. Vet. Parasitol. 2009, 160, 327–333. [Google Scholar] [CrossRef]
- Kauter, A.; Brombach, J.; Luebke-Becker, A.; Kannapin, D.; Bang, C.; Franzenburg, S.; Stoeckle, S.D.; Mellmann, A.; Scherff, N.; Koeck, R. Antibiotic prophylaxis and hospitalization of horses subjected to median laparotomy: Gut microbiota trajectories and abundance increase of Escherichia. Front. Microbiol. 2023, 14, 1228845. [Google Scholar] [CrossRef] [PubMed]
- Dimola, S.; Caruso, M.L. Helicobacter pylori in animals affecting the human habitat through the food chain. Anticanc. Res. 1999, 19, 3889–3894. [Google Scholar]
- Lambe, H.; Sykes, B.W. Prevalence of salmonella faecal shedding in at-risk hospitalised cases in an equine hospital in New Zealand: A pilot study. Equine Vet. Educ. 2022, 34, E554–E557. [Google Scholar] [CrossRef]
- Buss, S.N.; Leber, A.; Chapin, K.; Fey, P.D.; Bankowski, M.J.; Jones, M.K.; Rogatcheva, M.; Kanack, K.J.; Bourzac, K.M. Multicenter evaluation of the BioFire FilmArray Gastrointestinal Panel for etiologic diagnosis of infectious gastroenteritis. J. Clin. Microbiol. 2015, 53, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, P.; Croucher, D.; Hewitt, J. Preliminary evaluation of BioFire FilmArray® Gastrointestinal Panel for the detection of noroviruses and other enteric viruses from wastewater and shellfish. Environ. Sci. Pollut. Res. Int. 2018, 25, 27657–27661. [Google Scholar] [CrossRef] [PubMed]
- Khare, Ρ.; Espy, M.J.; Cebelinski, E.; Boxrud, D.; Sloan, L.M.; Cunningham, S.A.; Pritt, B.S.; Patel, R.; Binnicker, M.J. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J. Clin. Microbiol. 2014, 52, 3667–3673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, H.; Zhao, W.; Zhang, H.; Wang, W.; Ling, X.; Xiao, Y.; Guo, J.; Huang, Z.; Xu, Y.; et al. Evaluation of the BioFire FilmArray Gastrointestinal Panel and real-time Polymerase Chain Reaction assays for the detection of major diarrheagenic pathogens by a multicenter diarrheal disease surveillance program in China. Foodborne Pathog. Dis. 2019, 16, 788–798. [Google Scholar] [CrossRef]
- US Food and Drug Administration. 510(k) Substantial Equivalence Determination Decision Summary; Number: K140407, Clearance for the FilmArray® Gastrointestinal (GI) Panel Microorganism Multiplex Nucleic Acid-Based Assay; U.S. Food and Drug Administration: Washington, DC, USA, 2014. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/k140407.pdf (accessed on 20 November 2019).
- Rodriguez, E.; Martin-Carrillo, N.; Valladares, B.; Foronda, P. Study of zoonotic enteric pathogens of Atelerix algirus in Tenerife, Canary Islands, Spain. Front. Vet. Sci. 2020, 7, 579602. [Google Scholar]
- Tsilipounidaki, K.; Florou, Z.; Lianou, D.T.; Michael, C.K.; Katsarou, E.I.; Skoulakis, A.; Fthenakis, G.C.; Petinaki, E. Detection of zoonotic gastrointestinal pathogens in dairy sheep and goats by using FilmArray® multiplex-PCR technology. Microorganisms 2022, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.; Panda, S.; Mengels, G.; Martinez, X.; Azpizoz, F.; Dore, J.; Guarner, F.; Manichanh, C. Processing faecal samples: A step forward for standards in microbial community analysis. BMC Microbiol. 2014, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- BioFire. FilmArray Gastrointestinal Panel. Available online: https://www.biomerieux.com/us/en/our-offer/clinical-products/biofire-gastrointestinal-panel.html (accessed on 15 July 2023).
- Hu, Q.; Luo, J.; Cheng, F.; Wang, P.; Gong, P.; Lv, X.; Wang, X.; Yang, M.; Wei, P. Spatial profiles of the bacterial microbiota throughout the gastrointestinal tract of dairy goats. Appl. Microbiol. Biotechnol. 2024, 108, 356. [Google Scholar] [CrossRef]
- Merkies, K.; Franzin, O. Enhanced understanding of horse–human interactions to optimize welfare. Animals 2021, 11, 1347. [Google Scholar] [CrossRef]
- Claassen, S.; du Toit, E.; Kaba, M.; Moodley, C.; Zar, H.J.; Nicol, M.P. A comparison of the efficiency of five different commercial DNA extraction kits for extraction of DNA from faecal samples. J. Microbiol. Methods 2013, 94, 103–110. [Google Scholar] [CrossRef]
- Stewart, H.L.; Pitta, D.; Indugu, N.; Vecchiarelli, B.; Engiles, J.B.; Southwood, L.L. Characterization of the fecal microbiota of healthy Horses. Am. J. Vet. Res. 2018, 79, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Engering, A.; Hogerwerf, L.; Slingenbergh, J. Pathogen–host–environment interplay and disease emergence. Emerg. Microbes Infect. 2013, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lönker, N.S.; Fechner, K.; Abd El Wahed, A. Horses as a crucial part of one health. Vet. Sci. 2020, 7, 28. [Google Scholar] [CrossRef]
- Pusterla, N. Equine coronaviruses. Vet. Clin. N. Am. Equine Pract. 2023, 39, 55–71. [Google Scholar] [CrossRef]
- Merkies, K.; Hayman, B.; Ijichi, C.L. Examining the human–horse bond from the human perspective. Anthrozoös 2024, 37, 231–244. [Google Scholar] [CrossRef]
- Moriarty, E.M.; Downing, M.; Bellamy, J.; Gilpin, B.J. Concentrations of faecal coliforms, Escherichia coli, enterococci and Campylobacter spp. in equine faeces. N. Z. Vet. J. 2015, 63, 104–109. [Google Scholar] [CrossRef]
- Theelen, M.J.P.; Luiken, R.E.C.; Wagenaar, J.A.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Rossen, J.W.A.; Zomer, A.L. The equine faecal microbiota of healthy horses and ponies in The Netherlands: Impact of host and environmental factors. Animals 2021, 11, 1762. [Google Scholar] [CrossRef] [PubMed]
- Paruch, L.; Paruch, A.M. Molecular identification of infectious enteropathogens in faeces of healthy horses. Microbiol. Insights 2022, 15, 11786361221089005. [Google Scholar] [CrossRef] [PubMed]
- Maunula, L.; Miettinen, I.T.; von Bonsdorff, C.H. Norovirus outbreaks from drinking water. Emerg. Infect. Dis. 2005, 11, 1716–1721. [Google Scholar] [CrossRef]
- Morse, E.V.; Kersting, K.W.; Smith, L.E., Jr.; Myhrom, E.P.; Greenwood, D.E. Salmonellosis: Possible transmission from horse to human to dog of infection. Am. J. Public Health 1978, 68, 497–499. [Google Scholar] [CrossRef]
- Soza-Ossandón, P.; Rivera, D.; Tardone, R.; Riquelme-Neira, R.; García, P.; Hamilton-West, C.; Adell, A.D.; González-Rocha, G.; Moreno-Switt, A.I. Widespread environmental presence of multidrug-resistant Salmonella in an equine veterinary hospital that received local and international horses. Front. Vet. Sci. 2020, 7, 346. [Google Scholar] [CrossRef]
- Bolzoni, L.; Conter, M.; Lamperti, L.; Scaltriti, E.; Morganti, M.; Poeta, A.; Vecchi, M.; Paglioli, S.; Rampini, A.; Ramoni, P.; et al. Salmonella in horses at slaughter and public health effects in Italy. Int. J. Food Microbiol. 2024, 408, 110429. [Google Scholar] [CrossRef]
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence factors of enteric pathogenic Escherichia coli: A review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Karakaya, E.; Aydin, F.; Kayman, T.; Abay, S. Escherichia coli in different animal feces: Phylotypes and virulence genes. World J. Microbiol. Biotechnol. 2023, 39, 14. [Google Scholar] [CrossRef]
- Hurvell, B. Zoonotic Yersinia enterocolitica infection: Host range, clinical manifestations, and transmission between animals and man. In Yersinia enterocolitica; Bottone, E.J., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 145–159. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union one health 2020 zoonoses report. EFSA J. 2021, 19, 6971. [Google Scholar]
- Søgaard, K.K.; Hinic, V.; Goldenberger, D.; Gensch, A.; Schweitzer, M.; Bättig, V.; Siegemund, M.; Bassetti, S.; Bingisser, R.; Tamm, M.; et al. Evaluation of the clinical relevance of the Biofire© FilmArray pneumonia panel among hospitalized patients. Infection 2024, 52, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.; Williams, B.A.; Smidt, H.; Verstegen, M.W.A.; Mosenthin, R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 2006, 7, 35–51. [Google Scholar]
- Chen, S.; Luo, S.; Yan, C. Gut microbiota implications for health and welfare in farm animals: A review. Animals 2021, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Kempf, F.; La Ragione, R.; Chirullo, B.; Schouler, C.; Velge, P. Super shedding in enteric pathogens: A review. Microorganisms 2022, 10, 2101. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.S.; Figueiredo, E.; McAllister, T.; Stanford, K. Farm to fork impacts of super-shedders and high-event periods on food safety. Trends Food Sci. Technol. 2022, 127, 129–142. [Google Scholar] [CrossRef]
- Rossi, F.; Santonicola, S.; Amadoro, C.; Marino, L.; Colavita, G. Food and drinking water as sources of pathogenic protozoans: An update. Appl. Sci. 2024, 14, 5339. [Google Scholar] [CrossRef]
- Cabral, J.P.S. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Elsohaby, I.; Villa, L. Zoonotic diseases: Understanding the risks and mitigating the threats. BMC Vet. Res. 2023, 19, 186. [Google Scholar] [CrossRef]
- Guerra, V.; Tiago, I.; Aires, A.; Coelho, C.; Nunes, J.; Martins, L.O.; Veríssimo, A. The gastrointestinal microbiome of browsing goats (Capra hircus). PLoS ONE 2022, 17, e0276262. [Google Scholar] [CrossRef]
- Park, J.; Kim, E.B. Differences in microbiome and virome between cattle and horses in the same farm. Asian-Australas. J. Anim. Sci. 2020, 33, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Gebhart, C.J.; Lavoie, J.P.; Drolet, R. Lawsonia intracellularis. In Equine Infectious Diseases; Sellon, D.C., Long, M.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 316–321.e2. [Google Scholar]
- Fernandes, K.A.; Gee, E.K.; Rogers, C.W.; Kittelmann, S.; Biggs, P.J.; Bermingham, E.N.; Bolwell, C.F.; Thomas, D.G. Seasonal variation in the faecal microbiota of mature adult horses maintained on pasture in New Zealand. Animals 2021, 11, 2300. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, S.; Dagurova, O.; Radnagurueva, A.; Kozlova, A.; Izotova, A.; Krylova, A.; Noskov, S.; Begmatov, S.; Patutina, E.; Barkhutova, D.D. Fecal microbiota and diet composition of Buryatian horses grazing warm- and cold-season grass pastures. Microorganisms 2023, 11, 1947. [Google Scholar] [CrossRef] [PubMed]

| Pathogens | Frequency of Detection (n) (Prevalence (95% CI)) |
|---|---|
| Campylobacter spp. (jejuni, coli, upsaliensis) | 0 (0.0%, 0.0–1.7%) |
| Clostridium difficile (toxin A/B) | 1 (0.5%, 0.1–2.5%) |
| Plesiomonas shigelloides | 0 (0.0%, 0.0–1.7%) |
| Salmonella spp. | 1 (0.5%, 0.1–2.5%) |
| Vibrio spp. (parahaemolyticus, vulnificus, cholerae) | 1 (0.5%, 0.1–2.5%) |
| Vibrio cholerae | 0 (0.0%, 0.0–1.7%) |
| Yersinia enterocolitica | 7 (3.1%, 1.5–6.3%) |
| Enteroaggregative Escherichia coli | 0 (0.0%, 0.0–1.7%) |
| Enteropathogenic E. coli | 43 (19.2%, 14.6–24.9%) |
| Enterotoxigenic E. coli lt/st | 16 (7.1%, 4.4–11.3%) |
| Shiga-like toxin-producing E. coli stx1/stx2 | 31 (13.8%, 9.9–19.0%) |
| E. coli O157 | 11 (4.9%, 2.8–8.6%) |
| Shigella/enteroinvasive E. coli | 2 (0.9%, 0.2–3.5%) |
| Cryptosporidium spp. | 0 (0.0%, 0.0–1.7%) |
| Cyclospora cayetanensis | 0 (0.0%, 0.0–1.7%) |
| Entamoeba histolytica | 0 (0.0%, 0.0–1.7%) |
| Giardia duodenalis | 7 (3.1%, 1.5–6.3%) |
| Adenovirus F40/41 | 0 (0.0%, 0.0–1.7%) |
| Astrovirus | 0 (0.0%, 0.0–1.7%) |
| Norovirus GI/GII | 2 (0.9%, 0.2–3.5%) |
| Rotavirus A | 0 (0.0%, 0.0–1.7%) |
| Sapovirus (I, II, IV, and V) | 0 (0.0%, 0.0–1.7%) |
| Combinations of Pathogens | Frequency of Detection (n) |
|---|---|
| Enteropathogenic E. coli and G. duodenalis | 3 |
| Enteropathogenic E. coli and Salmonella | 1 |
| Enterotoxigenic E. coli lt/st—Shiga-like toxin-producing E. coli stx1/stx2—E. coli O157 and Norovirus GI/GII | 1 |
| Shiga-like toxin-producing E. coli stx1/stx2—E. coli O157 and Norovirus GI/GII | 1 |
| Variables | Odds Risk/Odds Ratio 1 | p-Value |
|---|---|---|
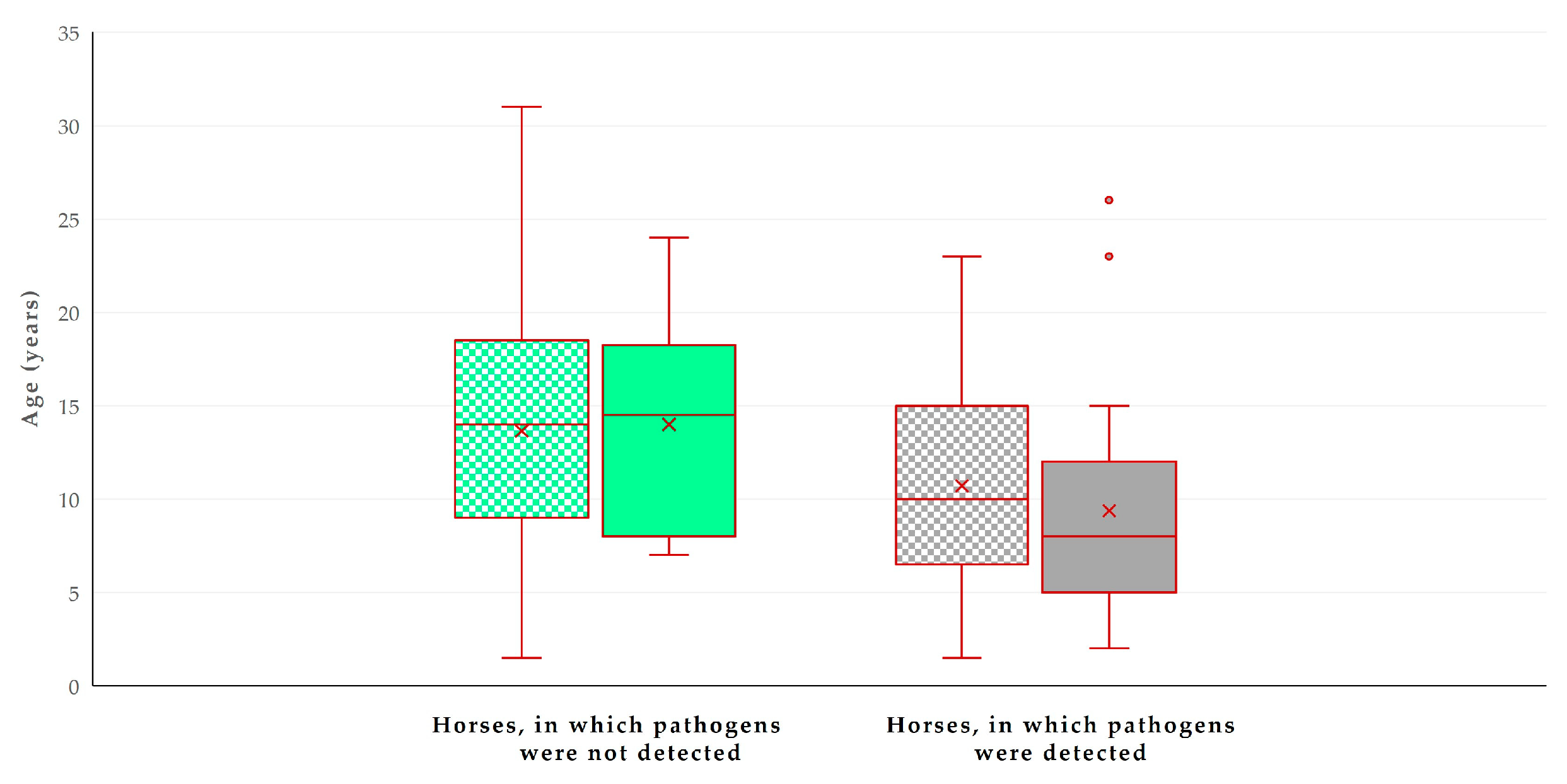
| Age of horse | 0.0001 | |
| Per unit (year) decrease | 1.021 ± 1.005 | <0.0001 |
| Presence of livestock at the same premisesas horses | 0.014 | |
| Yes (63.2% 2) | 2.654 (1.289–5.462) | 0.008 |
| No (39.2%) | reference |
| Variables | Odds Ratios 1 | p-Value |
|---|---|---|
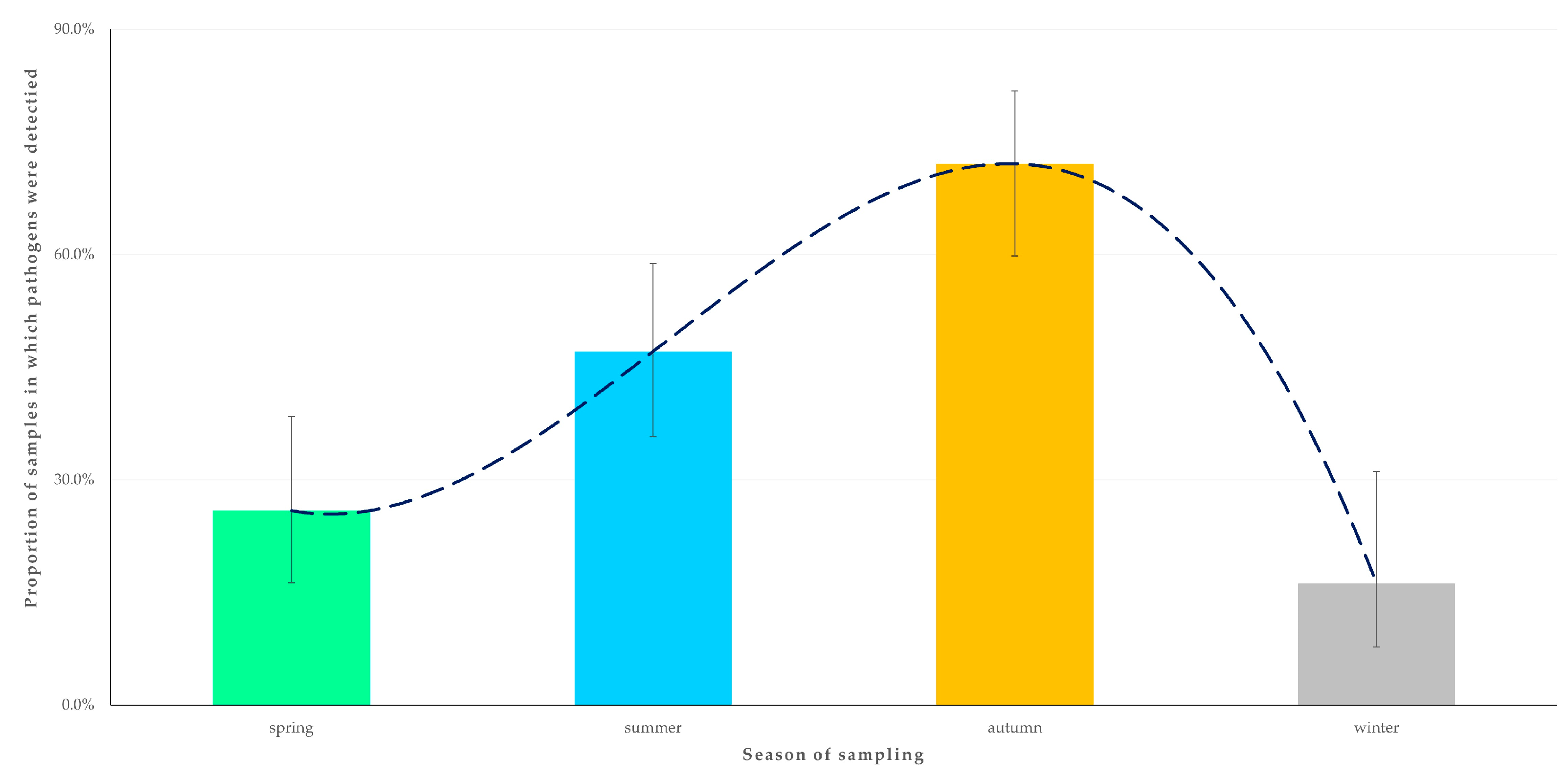
| Season when sampling of horse took place | 0.049 | |
| Spring (0.0% 2) | 1.171 (0.023–59.938) | 0.937 |
| Summer (0.0%) | reference | |
| Autumn (8.2%) | 13.336 (0.722–246.398) | 0.08 |
| Winter (2.7%) | 5.630 (0.224–141.727) | 0.29 |
| Variables | Odds Ratios/Odds Risk 1 | p-Value |
|---|---|---|
| Presence of livestock at the same premises | 0.004 | |
| Yes (57.1% 2) | 7.177 (1.537–33.511) | 0.012 |
| No (15.7%) | reference |
| Variables | Odds Ratios/Odds Risk 1 | p-Value |
|---|---|---|
| Age of horse | 0.0006 | |
| Per year decrease | 1.017 ± 1.005 | 0.0004 |
| Presence of livestock at the same premises | 0.032 | |
| Yes (24.7% 2) | 2.277 (1.123–4.615) | 0.023 |
| No (12.6%) | reference | |
| Season when sampling took place | 0.033 | |
| Spring (17.2%) | 1.333 (0.417–4.266) | 0.63 |
| Summer (35.3%) | 3.491 (1.203–10.134) | 0.022 |
| Autumn (68.9%) | 14.147 (4.770–41.961) | <0.0001 |
| Winter (13.5%) | reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyrnenopoulou, P.; Tsilipounidaki, K.; Florou, Z.; Gkountinoudis, C.-G.; Tyropoli, K.; Starras, A.; Peleki, C.; Marneris, D.; Arseniou, N.; Lianou, D.T.; et al. Detection of Gastrointestinal Pathogens with Zoonotic Potential in Horses Used in Free-Riding Activities during a Countrywide Study in Greece. Animals 2024, 14, 2566. https://doi.org/10.3390/ani14172566
Tyrnenopoulou P, Tsilipounidaki K, Florou Z, Gkountinoudis C-G, Tyropoli K, Starras A, Peleki C, Marneris D, Arseniou N, Lianou DT, et al. Detection of Gastrointestinal Pathogens with Zoonotic Potential in Horses Used in Free-Riding Activities during a Countrywide Study in Greece. Animals. 2024; 14(17):2566. https://doi.org/10.3390/ani14172566
Chicago/Turabian StyleTyrnenopoulou, Panagiota, Katerina Tsilipounidaki, Zoi Florou, Christos-Georgios Gkountinoudis, Konstantina Tyropoli, Alexandros Starras, Christina Peleki, Danai Marneris, Nikoletta Arseniou, Daphne T. Lianou, and et al. 2024. "Detection of Gastrointestinal Pathogens with Zoonotic Potential in Horses Used in Free-Riding Activities during a Countrywide Study in Greece" Animals 14, no. 17: 2566. https://doi.org/10.3390/ani14172566






