Protective Effects of Tea Tree Oil on Inflammatory Injury of Porcine Intestinal Epithelial Cells Induced by Lipopolysaccharide In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line Culture
2.2. Experimental Design
2.3. Cell Viability
2.4. Cytokine Secretion
2.5. Cell Apoptosis
2.6. Gene Expression
2.7. Statistical Analysis
3. Results
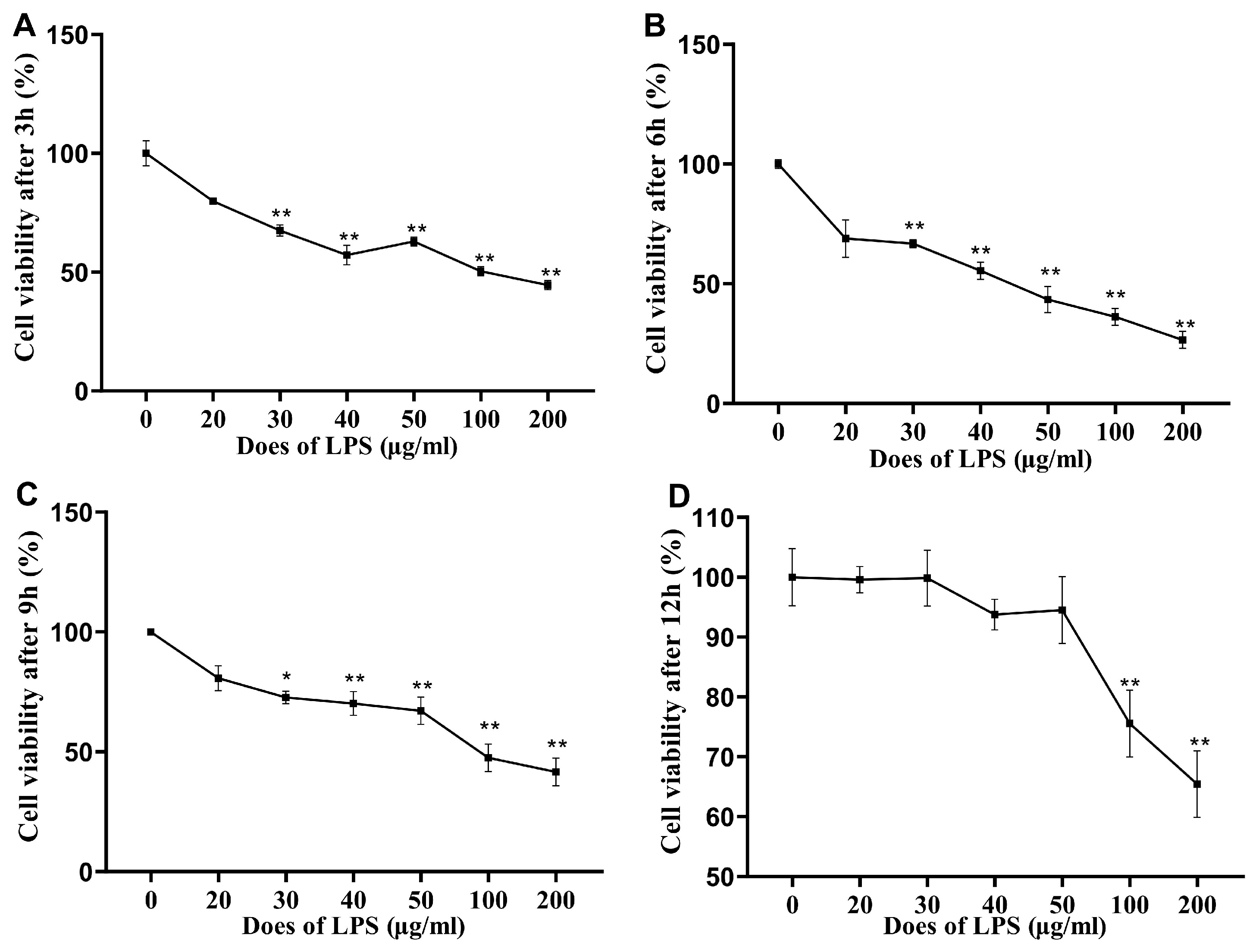
3.1. Cell Viability after LPS Stimulation
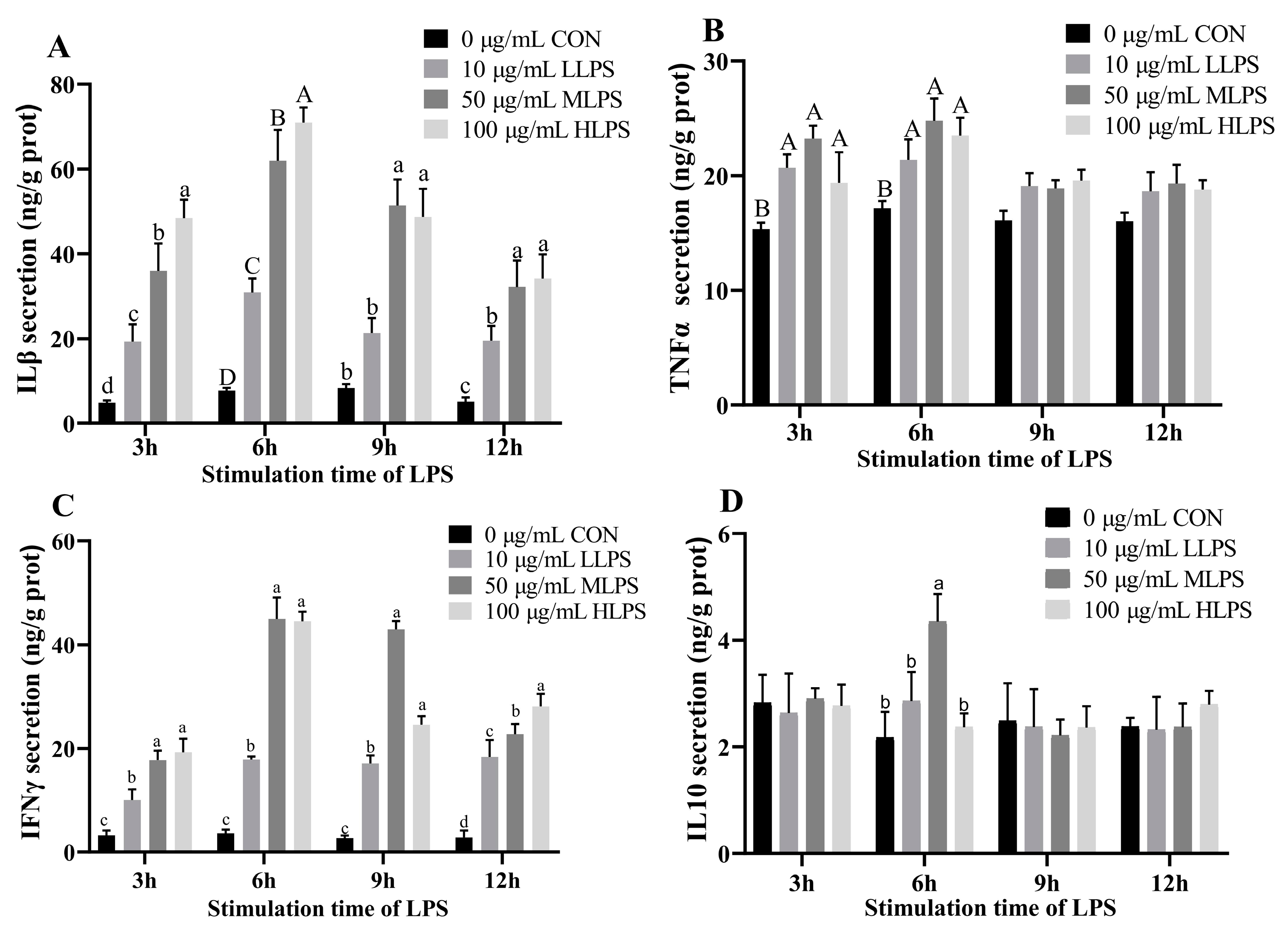
3.2. Cytokine Secretion after LPS Stimulation
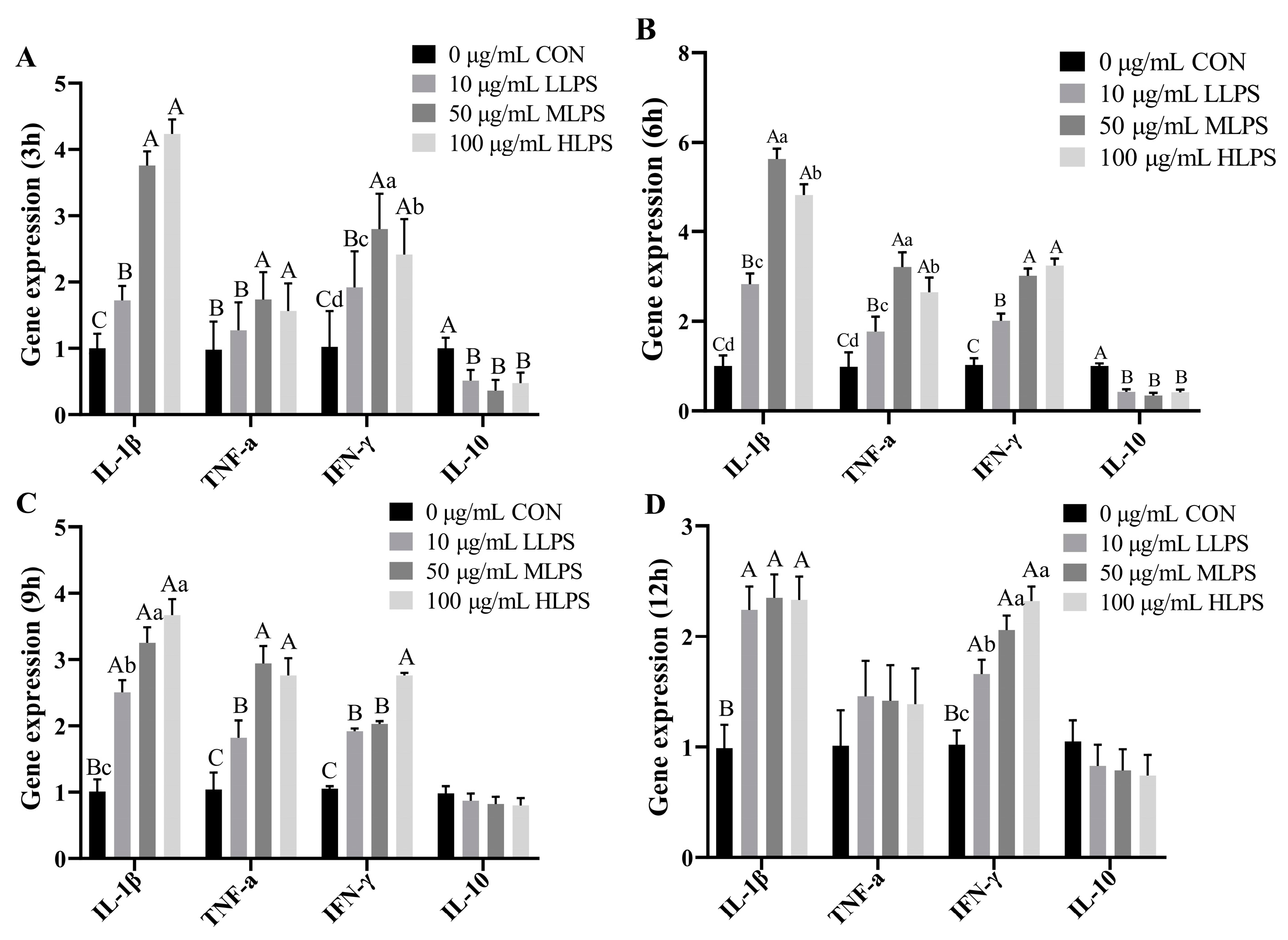
3.3. Gene Expression of Cytokines after LPS Stimulation
3.4. Viability of IPI-2I Cells after Tea Tree Oil Treatment
3.5. Cell Apoptosis
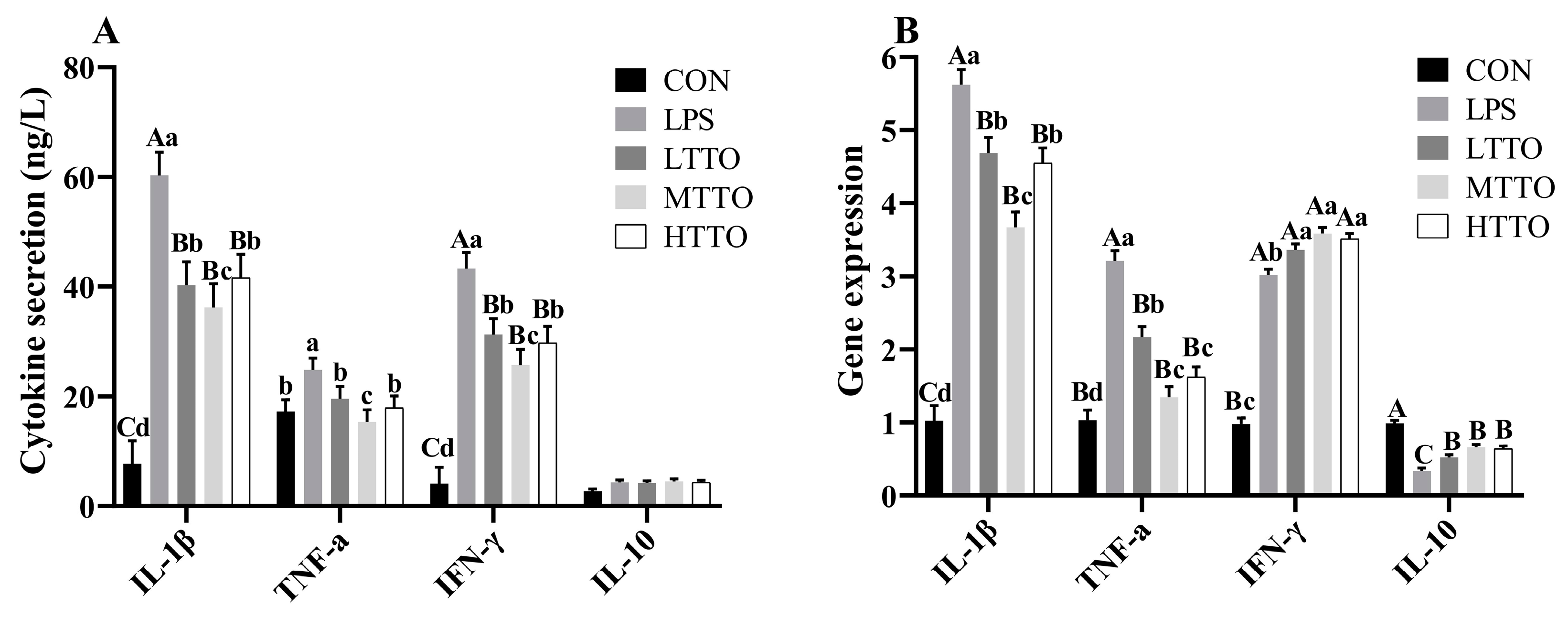
3.6. Cytokine Secretion and Gene Expression
3.7. Gene Expression of NF-κB and TLR4
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Chen, L.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. L-theanine improves intestinal barrier functions by increasing tight junction protein expression and attenuating inflammatory reaction in weaned piglets. J. Funct. Foods 2023, 100, 105400. [Google Scholar]
- Li, E.; Li, C.; Horn, N.; Ajuwon, K.M. Quercetin attenuates deoxynivalenol-induced intestinal barrier dysfunction by activation of Nrf2 signaling pathway in IPEC-J2 cells and weaned piglets. Curr. Res. Toxicol. 2023, 5, 100122. [Google Scholar] [PubMed]
- Yuan, B.; Liu, M.; Luo, S.; Qu, Q.; Zhu, M.; Wang, Z.; Zhang, X.; Xie, G.; Li, B.; Wang, W. ETEC regulates GPR109A expression in intestinal epithelial cells mediated by inflammatory factors secreted by macrophages. Res. Vet. Sci. 2023, 154, 15–21. [Google Scholar]
- Arce, C. Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2 and IPI-2I) in response to LPS from Salmonella typhimurium. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 161–174. [Google Scholar]
- Hammer, K. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2004, 53, 1081–1085. [Google Scholar]
- Dong, L. Dietary tea tree oil supplementation improves the intestinal mucosal immunity of weanling piglets. Anim. Feed Sci. Technol. 2019, 255, 114209. [Google Scholar]
- Livak, K.J. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Xie, M. MiR-339 attenuates LPS-induced intestinal epithelial cells inflammatory responses and apoptosis by targeting TLR4. Genes Genom 2020, 42, 1097–1105. [Google Scholar]
- Chang, Y. Glucagon-like peptide 2 attenuates intestinal mucosal barrier injury through the MLCK/pMLC signaling pathway in a piglet model. J. Cell. Physiol. 2021, 236, 3015–3032. [Google Scholar]
- Zhao, X. Capsaicin attenuates lipopolysaccharide-induced inflammation and barrier dysfunction in intestinal porcine epithelial cell line-J2. Front. Physiol. 2021, 12, 715469. [Google Scholar]
- Wang, F. Effects of porcine epidemic diarrhea virus infection on Toll-like receptor expression and cytokine levels in porcine intestinal epithelial cells. Pol. J. Vet. Sci. 2020, 23, 119–126. [Google Scholar]
- Iacovelli, F.; Romeo, A.; Lattanzio, P.; Ammendola, S.; Battistoni, A.; La Frazia, S.; Vindigni, G.; Unida, V.; Biocca, S.; Gaziano, R.; et al. Deciphering the broad antimicrobial activity of melaleuca alternifolia tea tree oil by combining experimental and computational investigations. Int. J. Mol. Sci. 2023, 24, 12432. [Google Scholar] [CrossRef]
- Yong, Y. Tea tree oil terpinen-4-ol protects gut barrier integrity by upregulation of tight junction proteins via the ERK1/2-signaling pathway. Front. Nutr. 2021, 8, 1315. [Google Scholar]
- Greay, S. Induction of necrosis and cell cycle arrest in murine cancer cell lines by Melaleuca alternifolia (tea tree) oil and terpinen-4-ol. Cancer Chemother. Pharmacol. 2010, 65, 877–888. [Google Scholar]
- Söderberg, T.A. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology 1996, 107, 99–109. [Google Scholar]
- Angulo, S. Probiotic sonicates selectively induce mucosal immune cells apoptosis through ceramide generation via neutral sphingomyelinase. PLoS ONE 2011, 6, e16953. [Google Scholar]
- Taher, I.; El-Masry, E.; Abouelkheir, M.; Taha, A.E. Anti-inflammatory effect of metformin against an experimental model of LPS-induced cytokine storm. Exp. Ther. Med. 2023, 26, 415. [Google Scholar]
- Nogueira, M. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778. [Google Scholar] [PubMed]
- Zhang, G. Effects of tea tree oil supplementation on growth performance, antioxidant capacity, immune status and microbial community in weaned pigs. Arch. Anim. Nutr. 2021, 75, 121–136. [Google Scholar]
- Golab, M. Mechanisms involved in the anti-inflammatory action of inhaled tea tree oil in mice. Exp. Biol. Med. 2007, 232, 420–426. [Google Scholar]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.; JLeyva-Gómez, G. Therapeutic applications of terpenes on inflammatory diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar]
- Cui, Y.; Zhang, F.; Xu, W.; Li, Z.; Zou, J.; Gao, P.; Hu, J. Effects of Si-Miao-Yong-An decoction on myocardial I/R rats by regulating gut microbiota to inhibit LPS-induced TLR4/NF-κB signaling pathway. BMC Complement. Med. Ther. 2023, 23, 180. [Google Scholar]
- Ren, H.; Li, K.; Min, Y.; Qiu, B.; Huang, X.; Luo, J.; Qi, L.; Kang, M.; Xia, P.; Qiao, H.; et al. Rehmannia glutinosa polysaccharides: Optimization of the decolorization process and antioxidant and anti-inflammatory effects in LPS-stimulated porcine intestinal epithelial cells. Antioxidants 2023, 12, 914. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequence (5′-3′) | Fragment, bp |
|---|---|---|
| GAPDH | 5′ GTCGGAGTGAACGGATTTGGC 3′ 5′ GGAGGTCAATGAAGGGGTCA 3′ | 106 |
| IL-1β | 5′ GTGGCAGGACCTACACTCTTC 3′ 5′ TTCCTTCAGAATGCCGTCCTC 3′ | 115 |
| IL10 | 5′ GTGGCAGCCAGCATTAAGTC 3′ 5′ AACTCTTCACTGGGCCGAAG 3′ | 103 |
| IFN-γ | 5′ GGCCATTCAAAGGAGCATGGA 3′ 5′ TCACTGATGGCTTTGCGCT 3′ | 144 |
| TNF-α | 5′ GCCCTTCCACCAACGTTTTC 3′ 5′ CAAGGGCTCTTGATGGCAGA 3′ | 97 |
| NF-κB p65 | 5′ AGATCTTCCTGCTGTGCGAC 3′ 5′ GTCGGCTTGTGAAAAGGAGC 3′ | 98 |
| TLR4 | 5′ GTGGCCTCCAAGGAACAAGA 3′ 5′ CTGGTGTTCACACGCACAAG 3′ | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Yuan, Q.; Qiu, G.; Zhang, Y.; Wang, H.; Yu, L. Protective Effects of Tea Tree Oil on Inflammatory Injury of Porcine Intestinal Epithelial Cells Induced by Lipopolysaccharide In Vitro. Animals 2024, 14, 2577. https://doi.org/10.3390/ani14172577
Dong L, Yuan Q, Qiu G, Zhang Y, Wang H, Yu L. Protective Effects of Tea Tree Oil on Inflammatory Injury of Porcine Intestinal Epithelial Cells Induced by Lipopolysaccharide In Vitro. Animals. 2024; 14(17):2577. https://doi.org/10.3390/ani14172577
Chicago/Turabian StyleDong, Li, Qingqing Yuan, Guangzhi Qiu, Yongsheng Zhang, Hongrong Wang, and Lihuai Yu. 2024. "Protective Effects of Tea Tree Oil on Inflammatory Injury of Porcine Intestinal Epithelial Cells Induced by Lipopolysaccharide In Vitro" Animals 14, no. 17: 2577. https://doi.org/10.3390/ani14172577
APA StyleDong, L., Yuan, Q., Qiu, G., Zhang, Y., Wang, H., & Yu, L. (2024). Protective Effects of Tea Tree Oil on Inflammatory Injury of Porcine Intestinal Epithelial Cells Induced by Lipopolysaccharide In Vitro. Animals, 14(17), 2577. https://doi.org/10.3390/ani14172577







