An Automated Sprinkler Cooling System Effectively Alleviates Heat Stress in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
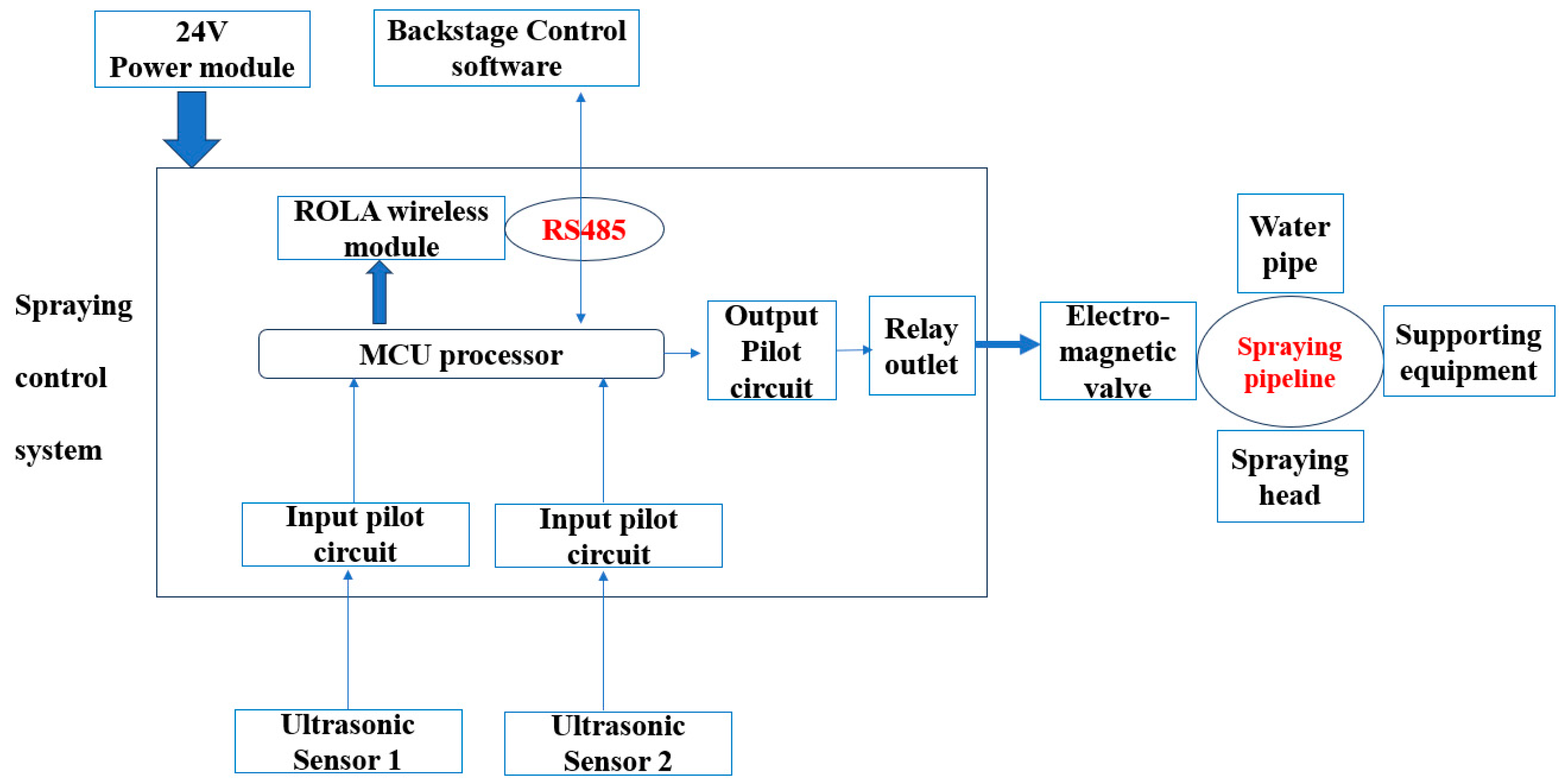
2. Materials and Methods
2.1. Experiment Animals and Management
2.2. Feed Intake and Composition Analysis
2.3. Milk Production and Composition
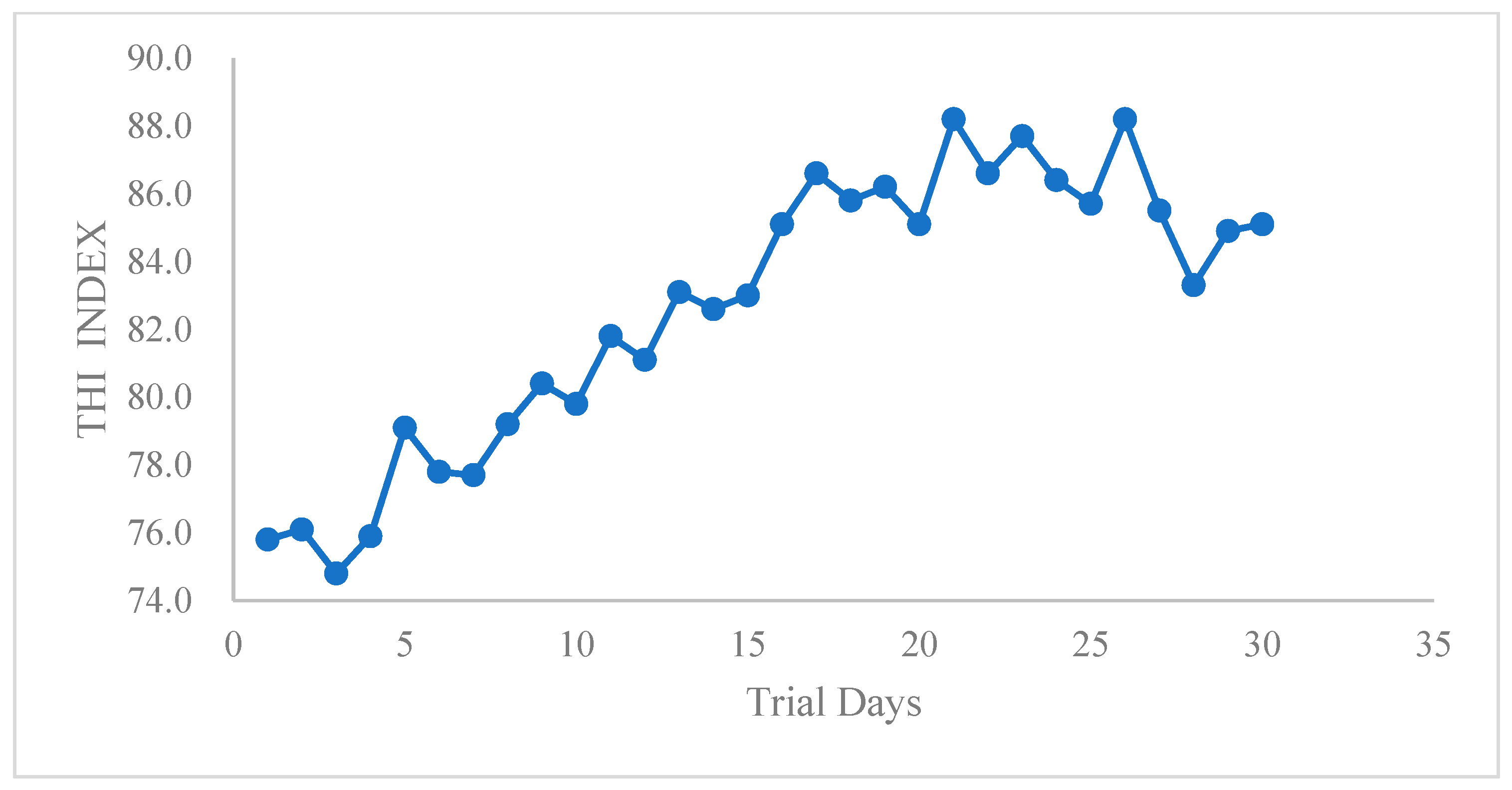
2.4. Body Temperature Measurement
2.5. Rumen Content Collection and Fermentation Parameter Analysis
2.6. Rumen Microbial Communities Measurement
2.7. Statistical Analysis
3. Results
3.1. Effects of Automatic Spraying on Body Temperature, Milk Yield and Content, and Pregnancy Rate under Heat Stress Conditions
3.2. Effects of Automatic Spraying on Rumen Fermentable Parameters under the Heat Stress Condition
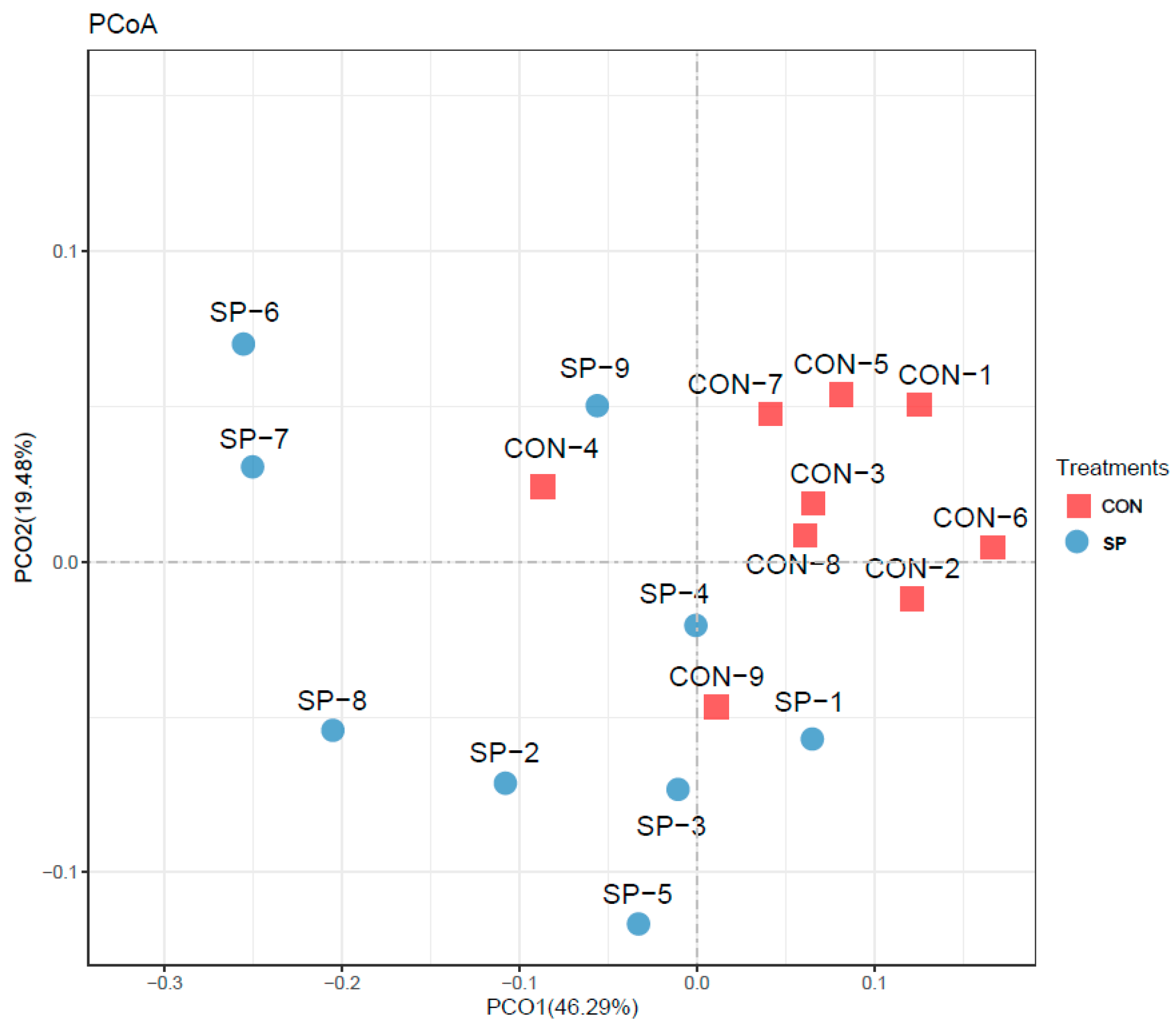
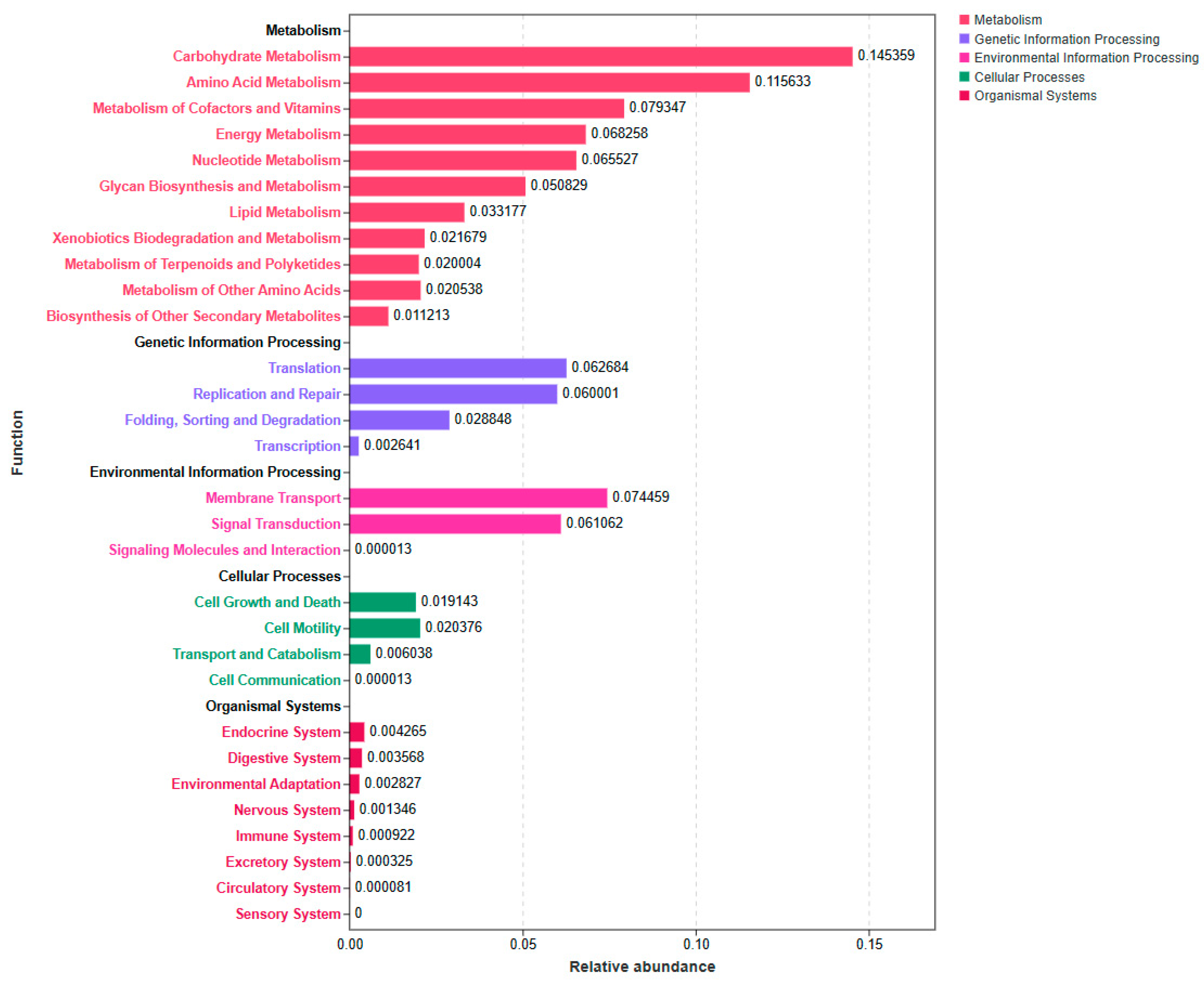
3.3. Effects of Automatic Spraying on Rumen Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tokarska, K.B.; Stolpe, M.B.; Sippel, S.; Fischer, E.M.; Smith, C.J.; Lehner, F.; Knutti, R. Past warming trend constrains future warming in CMIP6 models. Sci. Adv. 2020, 6, eaaz9549. [Google Scholar] [CrossRef] [PubMed]
- Ebi, K.L.; Capon, A.; Berry, P.; Broderick, C.; de Dear, R.; Havenith, G.; Honda, Y.; Kovats, R.S.; Ma, W.; Malik, A.; et al. Hot weather and heat extremes: Health risks. Lancet 2021, 398, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Nonaka, I.; Higuchi, K.; Takusari, N.; Kurihara, M.; Takenaka, A.; Mitsumori, M.; Kajikawa, H.; Aminov, R.I. Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe 2007, 13, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.J. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Koester, L.R.; Hayman, K.; Anderson, C.J.; Tibbs-Cortes, B.W.; Daniels, K.M.; Seggerman, F.M.; Gorden, P.J.; Lyte, M.; Schmitz-Esser, S. Influence of a sodium-saccharin sweetener on the rumen content and rumen epithelium microbiota in dairy cattle during heat stress. J. Anim. Sci. 2023, 101, 403. [Google Scholar] [CrossRef]
- Pinto, S.; Hoffmann, G.; Ammon, C.; Amon, T. Critical THI thresholds based on the physiological parameters of lactating dairy cows. J. Therm. Biol. 2020, 88, 102523. [Google Scholar] [CrossRef]
- Dahl, G.E.; Tao, S.; Laporta, J. Heat stress impacts immune status in cows across the life cycle. Front. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Pohler, K.G.; Mulliniks, J.T.; Ríus, A.G. Lowering rumen-degradable and rumen-undegradable protein improved amino acid metabolism and energy utilization in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2018, 101, 386–395. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Rhoads, M.L. Effects of periconceptional heat stress on primiparous and multiparous daughters of Holstein dairy cows. Theriogenology 2020, 150, 458–463. [Google Scholar] [CrossRef]
- Li, G.; Chen, S.; Chen, J.; Peng, D.; Gu, X. Predicting rectal temperature and respiration rate responses in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2020, 103, 5466–5484. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian-Aust. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Tresoldi, G.; Schütz, K.E.; Tucker, C.B. Cooling cows with sprinklers: Effects of soaker flow rate and timing on behavioral and physiological responses to heat load and production. J. Dairy Sci. 2019, 102, 528–538. [Google Scholar] [CrossRef]
- Igono, M.O.; Johnson, H.D.; Steevens, B.J.; Krause, G.F.; Shanklin, M.D. Physiological, productive, and economic benefits of shade, spray, and fan system versus shade for Holstein cows during summer heat. J. Dairy Sci. 1987, 70, 1069–1079. [Google Scholar] [CrossRef]
- Gallardo, M.R.; Valtorta, S.E.; Leva, P.E.; Gaggiotti, M.C.; Conti, G.A.; Gregoret, R.F. Diet and cooling interactions on physiological responses of grazing dairy cows, milk production and composition. Int. J. Biometeorol. 2005, 50, 90–95. [Google Scholar] [CrossRef]
- Honig, H.; Miron, J.; Lehrer, H.; Jackoby, S.; Zachut, M.; Zinou, A.; Portnick, Y.; Moallem, U. Performance and welfare of high-yielding dairy cows subjected to 5 or 8 cooling sessions daily under hot and humid climate. J. Dairy Sci. 2012, 95, 3736–3742. [Google Scholar] [CrossRef]
- Chen, J.M.; Schütz, K.E.; Tucker, C.B. Cooling cows efficiently with water spray: Behavioral, physiological, and production responses to sprinklers at the feed bunk. J. Dairy Sci. 2016, 99, 4607–4618. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T.; et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Kelly, W.J.; Janssen, P.H.; Attwood, G.T. Rumen microbial (meta)genomics and its application to ruminant production. Animal 2013, 7 (Suppl. 1), 184–201. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: Implications for engineering improved ruminal fermentations. Front. Microbiol. 2015, 6, 296. [Google Scholar] [CrossRef]
- Paz, H.A.; Hales, K.E.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C.; Berry, E.D.; Flythe, M.D.; Spangler, M.L.; Fernando, S.C. Rumen bacterial community structure impacts feed efficiency in beef cattle. J. Anim. Sci. 2018, 96, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Liu, J.X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Zhou, M.; Ghoshal, B.; Stothard, P.; Guan, L.L. Distinctive roles between rumen epimural and content bacterial communities on beef cattle feed efficiency: A combined analysis. Curr. Res. Microb. Sci. 2021, 2, 100085. [Google Scholar] [CrossRef]
- Ragaller, V.; Lebzien, P.; Bigalke, W.; Südekum, K.H.; Hüthera, L.; Flachowsky, G. Effects of folic acid supplementation to rations differing in the concentrate to roughage ratio on ruminal fermentation, nutrient flow at the duodenum, and on serum and milk variables of dairy cows. Arch. Anim. Nutr. 2010, 64, 484–503. [Google Scholar] [CrossRef] [PubMed]
- Seck, M.; Linton, J.A.V.; Allen, M.S.; Castagnino, D.S.; Chouinard, P.Y.; Girard, C.L. Apparent ruminal synthesis of B vitamins in lactating dairy cows fed diets with different forage-to-concentrate ratios. J. Dairy Sci. 2017, 100, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of vitamin B2 (riboflavin) by microorganisms: An overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef]
- Beckett, L.; Gleason, C.B.; Bedford, A.; Liebe, D.; Yohe, T.T.; Hall, M.B.; Daniels, K.M.; White, R.R. Rumen volatile fatty acid molar proportions, rumen epithelial gene expression, and blood metabolite concentration responses to ruminally degradable starch and fiber supplies. J. Dairy Sci. 2021, 104, 8857–8869. [Google Scholar] [CrossRef]
- Zhao, S.; Min, L.; Zheng, N.; Wang, J. Effect of heat stress on bacterial composition and metabolism in the rumen of lactating dairy cows. Animals 2019, 9, 925. [Google Scholar] [CrossRef]
- Kim, S.H.; Ramos, S.C.; Valencia, R.A.; Cho, Y.I.; Lee, S.S. Heat stress: Effects on rumen microbes and host physiology, and strategies to alleviate the negative impacts on lactating dairy cows. Front. Microbiol. 2022, 13, 804562. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Pang, F.; Zheng, Z.; Teng, Z.; Miao, T.; Fu, T.; Rushdi, H.E.; Yang, L.; Gao, T.; et al. Novel insights into heat tolerance using metabolomic and high-throughput sequencing analysis in dairy cows rumen fluid. Animal 2022, 16, 100478. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Y.; Liu, W.; Du, D.; Jiang, W.; Wang, Z.; Li, N.; Hu, Z. Altered rumen microbiome and correlations of the metabolome in heat-stressed dairy cows at different growth stages. Microbiol. Spectr. 2023, 11, e0331223. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ding, Y.; Wang, Y.; Zhou, G.; Guo, H.; Chen, Y.; Yang, Y. Temperature and humidity index (THI)-induced rumen bacterial community changes in goats. Appl. Microbiol. Biotechnol. 2019, 103, 3193–3203. [Google Scholar] [CrossRef]
- Correia, S.G.; Carvalho, B.F.; Schwan, R.F.; de Figueiredo Vilela, L.; Moreno Meneses, J.A.; Gionbelli, M.P.; Luiza da Silva Ávila, C. Heat stress influence the microbiota and organic acids concentration in beef cattle rumen. J. Therm. Biol. 2021, 97, 102897. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Bai, H.; Zhang, J.; Mao, S.; Jin, W. Live yeast supplementation altered the bacterial community’s composition and function in rumen and hindgut and alleviated the detrimental effects of heat stress on dairy cows. J. Anim. Sci. 2023, 101, 410. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001.
- Tucker, C.B.; Rogers, A.R.; Schütz, K. Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Appl. Anim. Behav. Sci. 2008, 2–4, 141–154. [Google Scholar] [CrossRef]
- Saini, G.; Kumar, S.; Pandey, A.K.; Yadav, V.; Sharma, S. Intensity of estrus expression-valuable obvious determinant of fertility in Bos indicus cows. Anim. Biotechnol. 2023, 34, 3867–3876. [Google Scholar] [CrossRef]
- NY/T 815-2004; Beef Cattle Feeding Standards. Institute of Animal Husbandry, CAOA: Harbin, China, 2004.
- Shen, J.S.; Chai, Z.; Song, L.J.; Liu, J.X.; Wu, Y.M. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 2012, 95, 5978–5984. [Google Scholar] [CrossRef]
- Xue, F.; Sun, F.; Jiang, L.; Hua, D.; Wang, Y.; Nan, X.; Zhao, Y.; Xiong, B. Effects of partial replacement of dietary forage using kelp powder (thallus laminariae) on ruminal fermentation and lactation performances of dairy cows. Animals 2019, 9, 852. [Google Scholar] [CrossRef]
- Wang, H.; Hao, W.; Yang, L.; Li, T.; Zhao, C.; Yan, P.; Wei, S. Procyanidin B2 alleviates heat-induced oxidative stress through the Nrf2 pathway in bovine mammary epithelial cells. Int. J. Mol. Sci. 2022, 23, 7769. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Cheng, J.B.; Shi, B.L.; Yang, H.J.; Zheng, N.; Wang, J.Q. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J. Zhejiang Univ. Sci. B 2015, 16, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, L.; Xue, F.; Mao, S.; Xiong, B.; Ma, Z.; Jiang, L. Effects of bamboo leaf extract on the production performance, rumen fermentation parameters, and rumen bacterial communities of heat-stressed dairy cows. Anim. Biosci. 2021, 34, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, Z.; Tan, Y.; Chang, S.; Zheng, H.; Wang, H.; Yan, T.; Guru, T.; Hou, F. Selenium yeast dietary supplement affects rumen bacterial population dynamics and fermentation parameters of tibetan sheep (Ovis aries) in alpine meadow. Front. Microbiol. 2021, 12, 663945. [Google Scholar] [CrossRef] [PubMed]
- Dado-Senn, B.; Skibiel, A.L.; Fabris, T.F.; Zhang, Y.; Dahl, G.E.; Peñagaricano, F.; Laporta, J. RNA-Seq reveals novel genes and pathways involved in bovine mammary involution during the dry period and under environmental heat stress. Sci. Rep. 2018, 8, 11096. [Google Scholar] [CrossRef]
- Zhao, C.; Bao, L.; Qiu, M.; Wu, K.; Zhao, Y.; Feng, L.; Xiang, K.; Zhang, N.; Hu, X.; Fu, Y. Commensal cow Roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice. Cell Rep. 2022, 41, 111681. [Google Scholar] [CrossRef]
- Martens, H.; Leonhard-Marek, S.; Röntgen, M.; Stumpff, F. Magnesium homeostasis in cattle: Absorption and excretion. Nutr. Res. Rev. 2018, 31, 114–130. [Google Scholar] [CrossRef]
- Xue, Y.; Lin, L.; Hu, F.; Zhu, W.; Mao, S. Disruption of ruminal homeostasis by malnutrition involved in systemic ruminal microbiota-host interactions in a pregnant sheep model. Microbiome 2020, 8, 138. [Google Scholar] [CrossRef]
- Uyeno, Y.; Sekiguchi, Y.; Tajima, K.; Takenaka, A.; Kurihara, M.; Kamagata, Y. An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe 2010, 16, 27–33. [Google Scholar] [CrossRef]
| Items | Content |
|---|---|
| Ingredients (%) | |
| Corn | 17.7 |
| Corn silage | 24.5 |
| Soybean meal | 12.3 |
| Cottonseed meal | 3.3 |
| Pressure corn piece | 8.2 |
| Leymus chinensis | 10.2 |
| Distiller’s dried grains with soluble (DDGS) | 3.1 |
| Alfalfa hay | 14.3 |
| Beet pulp | 4.8 |
| Premix (1) | 1.0 |
| NaCl | 0.6 |
| Total | 100 |
| Chemical composition | |
| NE (2) (MJ/kg) | 7.13 |
| EE (%) | 4.56 |
| CP (%) | 17.36 |
| ADF (%) | 18.52 |
| NDF (%) | 31.34 |
| Ca (%) | 0.68 |
| P (%) | 0.41 |
| Items | SP (n = 9) | CON (n = 9) | SE | p-Value |
|---|---|---|---|---|
| Body temperature | 38.6 | 38.9 | 0.10 | 0.046 |
| DMI | 23.3 | 21.4 | 0.68 | 0.043 |
| Milk yield | 31.3 | 29.4 | 0.56 | 0.046 |
| Milk fat | 3.76 | 3.63 | 0.11 | 0.056 |
| Milk protein | 3.37 | 3.34 | 0.03 | 0.412 |
| SCC | 13.77 | 19.39 | 2.46 | 0.033 |
| Items | SP (n = 9) | CON (n = 9) | SE | p-Value |
|---|---|---|---|---|
| Rumen pH | 6.08 | 6.14 | 0.08 | 0.351 |
| NH3-N | 17.73 | 15.43 | 1.53 | 0.168 |
| Acetate | 67.67 | 59.29 | 3.97 | 0.047 |
| Propionate | 23.79 | 20.44 | 1.65 | 0.054 |
| Isobutyrate | 1.17 | 0.65 | 0.16 | 0.004 |
| Butyrate | 16.52 | 14.25 | 1.08 | 0.049 |
| Valerate | 1.95 | 2.07 | 0.37 | 0.744 |
| Isovalerate | 2.40 | 2.44 | 0.34 | 0.915 |
| TVFA | 113.51 | 99.15 | 6.88 | 0.035 |
| A:P | 2.88 | 2.92 | 0.09 | 0.622 |
| Items | SP (n = 9) | CON (n = 9) | SE | p-Value |
|---|---|---|---|---|
| Shannon | 7.85 | 7.66 | 0.10 | 0.182 |
| Simpson | 0.98 | 0.98 | 0.00 | 0.342 |
| Ace | 2356.5 | 2171.4 | 52.0 | 0.018 |
| Chao1 | 2256.3 | 2116.6 | 51.2 | 0.062 |
| observed_species | 1934.9 | 1772.5 | 44.8 | 0.017 |
| Items | SP (n = 9) | CON (n = 9) | SE | p-Value |
|---|---|---|---|---|
| p__Actinobacteria | 6.47 | 5.51 | 0.76 | 0.229 |
| p__Fibrobacteres | 1.48 | 1.20 | 0.10 | 0.086 |
| p__Firmicutes | 15.44 | 15.35 | 0.11 | 0.940 |
| p__Bacteroidetes | 13.57 | 13.17 | 0.20 | 0.078 |
| p__Tenericutes | 8.96 | 8.55 | 0.23 | 0.101 |
| p__Cyanobacteria | 2.88 | 2.15 | 0.74 | 0.335 |
| p__Patescibacteria | 5.50 | 4.88 | 0.53 | 0.266 |
| p__Proteobacteria | 5.94 | 5.67 | 0.45 | 0.564 |
| p__Spirochaetes | 8.00 | 7.36 | 0.40 | 0.133 |
| Others | 5.82 | 5.20 | 0.42 | 0.122 |
| Items | SP (n = 9) | CON (n = 9) | SE | p-Value |
|---|---|---|---|---|
| g__Prevotella | 18.66 | 16.75 | 1.239 | 0.064 |
| g__Ruminococcaceae | 15.64 | 20.20 | 0.189 | 0.005 |
| g__Succiniclasticum | 11.42 | 9.76 | 0.321 | 0.029 |
| g__Lachnospiraceae | 5.38 | 5.41 | 0.282 | 0.623 |
| g__Eubacterium | 4.49 | 4.07 | 0.206 | 0.141 |
| g__Ruminococcus | 4.37 | 4.95 | 0.256 | 0.015 |
| g__Shuttleworthia | 1.95 | 1.37 | 0.182 | 0.272 |
| g__Prevotellaceae | 1.28 | 1.25 | 0.237 | 0.371 |
| g__Acetitomaculum | 0.992 | 0.741 | 0.121 | 0.063 |
| g__Lachnoclostridium | 0.713 | 0.751 | 0.012 | 0.137 |
| g__Butyrivibrio | 0.534 | 0.418 | 0.034 | 0.035 |
| g__Ruminiclostridium | 0.258 | 0.284 | 0.041 | 0.108 |
| g__Pseudobutyrivibrio | 0.253 | 0.182 | 0.014 | 0.009 |
| g__Selenomonas | 0.074 | 0.041 | 0.021 | 0.167 |
| g__Lactobacillus | 0.069 | 0.061 | 0.010 | 0.089 |
| g__Bifidobacterium | 0.036 | 0.021 | 0.013 | 0.048 |
| g__Escherichia-Shigella | 0.024 | 0.033 | 0.008 | 0.271 |
| g__Bacteroides | 0.022 | 0.024 | 0.006 | 0.345 |
| g__Succinivibrio | 0.014 | 0.071 | 0.012 | 0.033 |
| g__Streptococcus | 0.019 | 0.014 | 0.003 | 0.029 |
| g__Butyricicoccus | 0.011 | 0.011 | 0.006 | 0.132 |
| others | 34.06 | 33.64 | 3.346 | 0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, E.; Liu, L.; Zhang, Z.; Qu, M.; Xue, F. An Automated Sprinkler Cooling System Effectively Alleviates Heat Stress in Dairy Cows. Animals 2024, 14, 2586. https://doi.org/10.3390/ani14172586
Liu E, Liu L, Zhang Z, Qu M, Xue F. An Automated Sprinkler Cooling System Effectively Alleviates Heat Stress in Dairy Cows. Animals. 2024; 14(17):2586. https://doi.org/10.3390/ani14172586
Chicago/Turabian StyleLiu, En, Liping Liu, Zhili Zhang, Mingren Qu, and Fuguang Xue. 2024. "An Automated Sprinkler Cooling System Effectively Alleviates Heat Stress in Dairy Cows" Animals 14, no. 17: 2586. https://doi.org/10.3390/ani14172586
APA StyleLiu, E., Liu, L., Zhang, Z., Qu, M., & Xue, F. (2024). An Automated Sprinkler Cooling System Effectively Alleviates Heat Stress in Dairy Cows. Animals, 14(17), 2586. https://doi.org/10.3390/ani14172586




