Seasonal piRNA Expression Profile Changes in the Testes of Plateau Zokor (Eospalax baileyi)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. piRNA Extraction, Annotation, and Identification
2.3. Screening of DE piRNAs and Functional Enrichment of Target Genes
2.4. qPCR Validation
3. Results
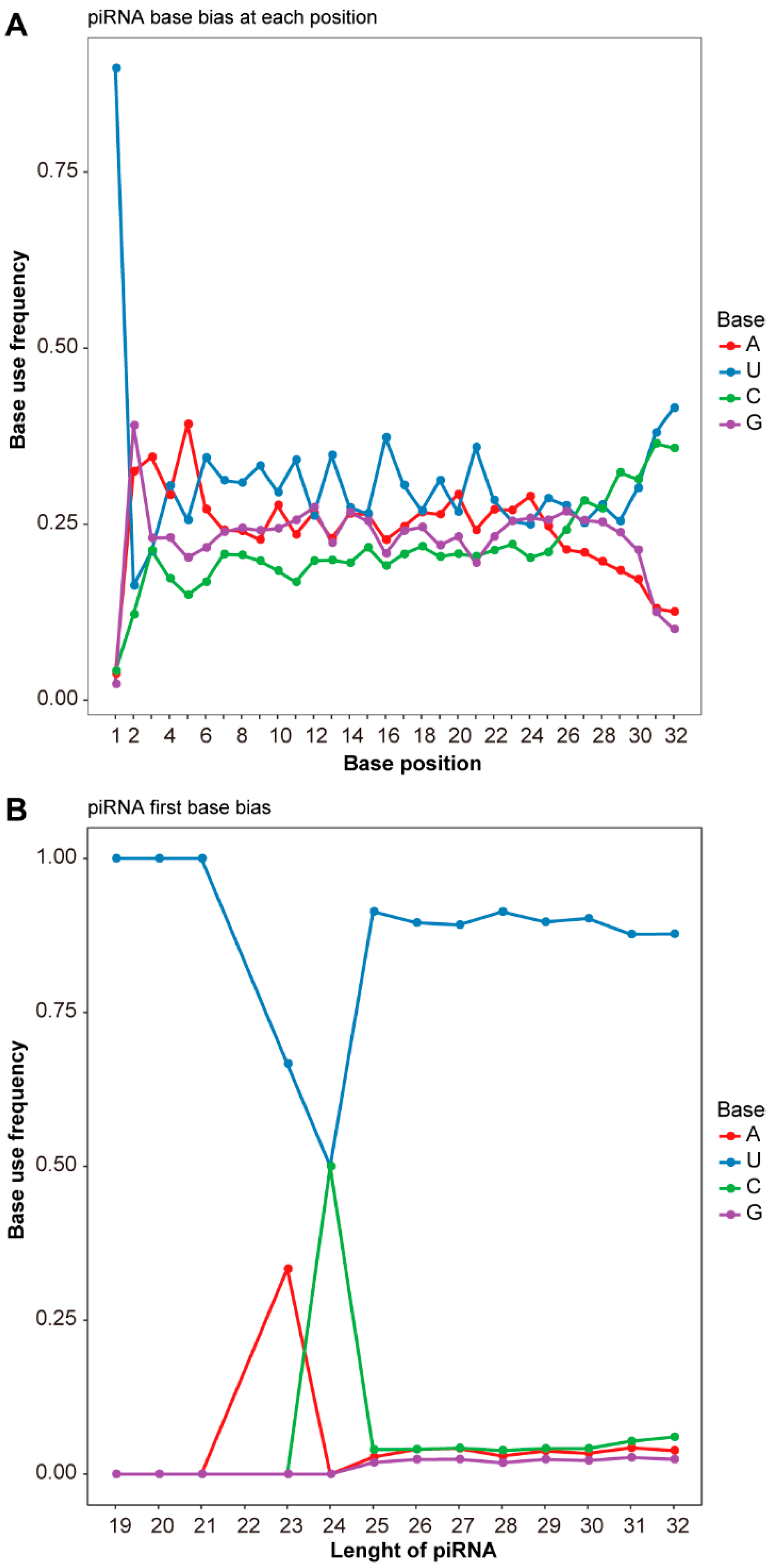
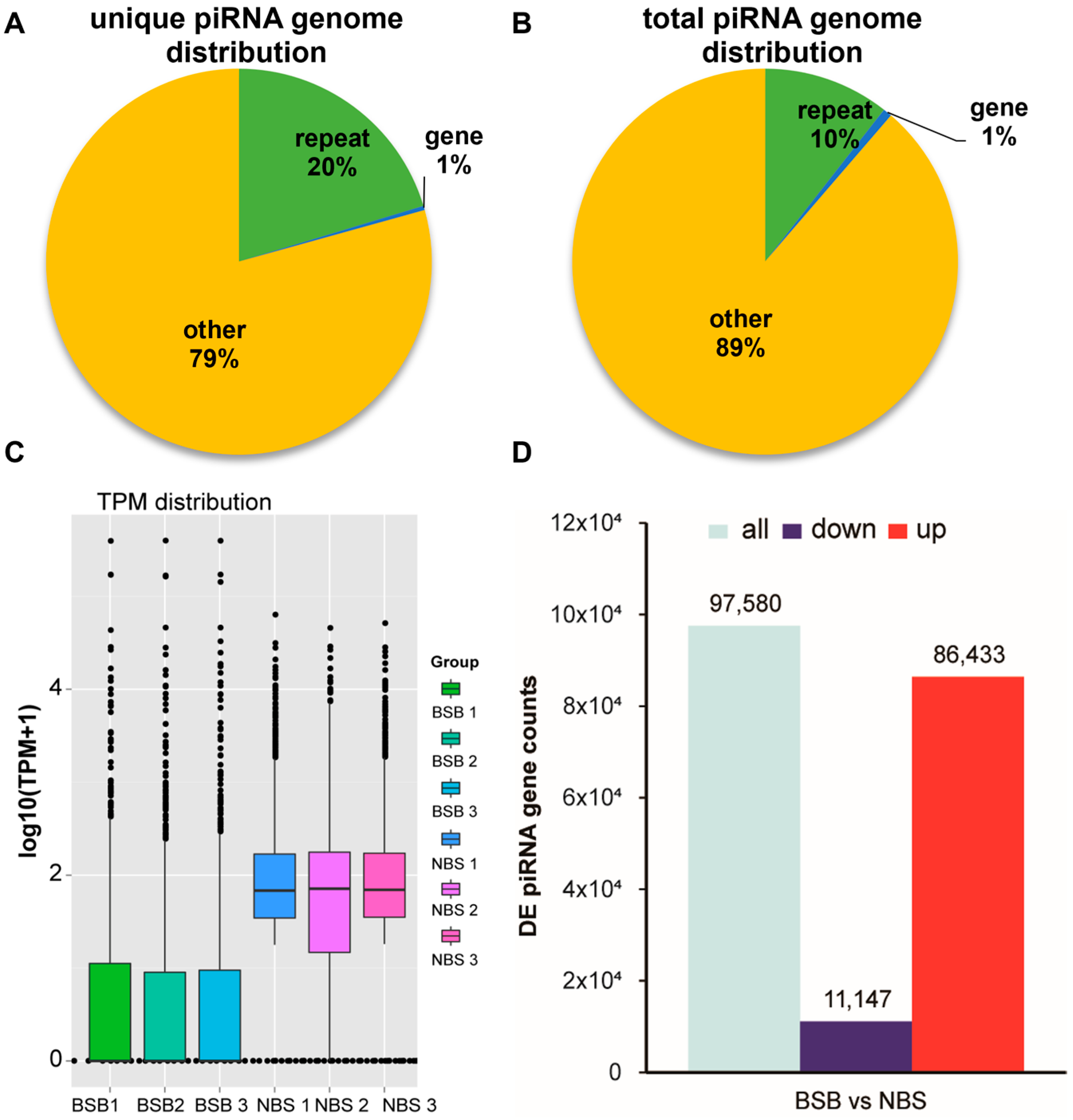
3.1. Expression of piRNA in the Testes of Plateau Zokors
3.2. DE piRNAs in the Testes of Plateau Zokors
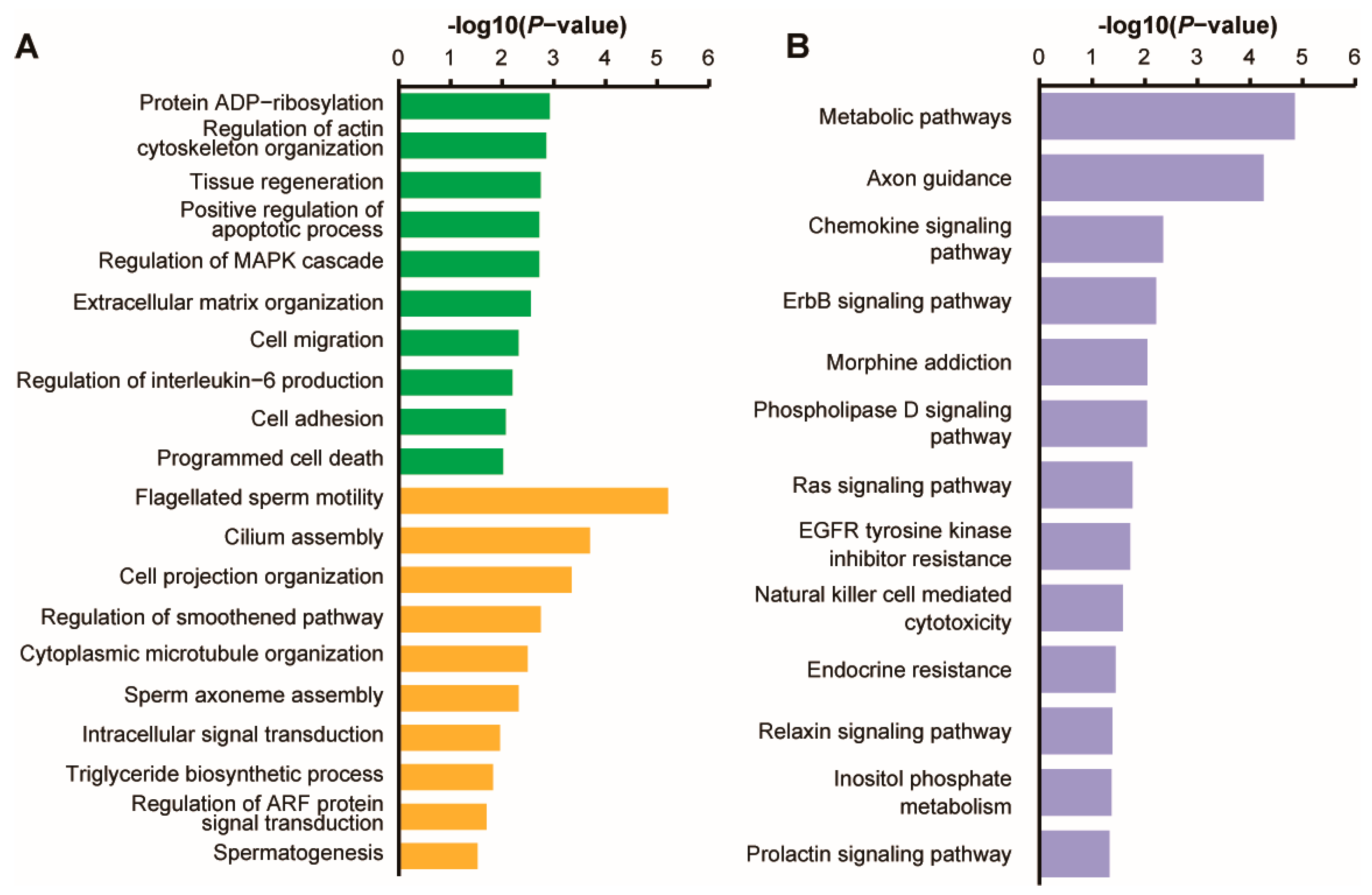
3.3. Prediction and Enrichment Analysis of Target mRNAs for DE piRNAs
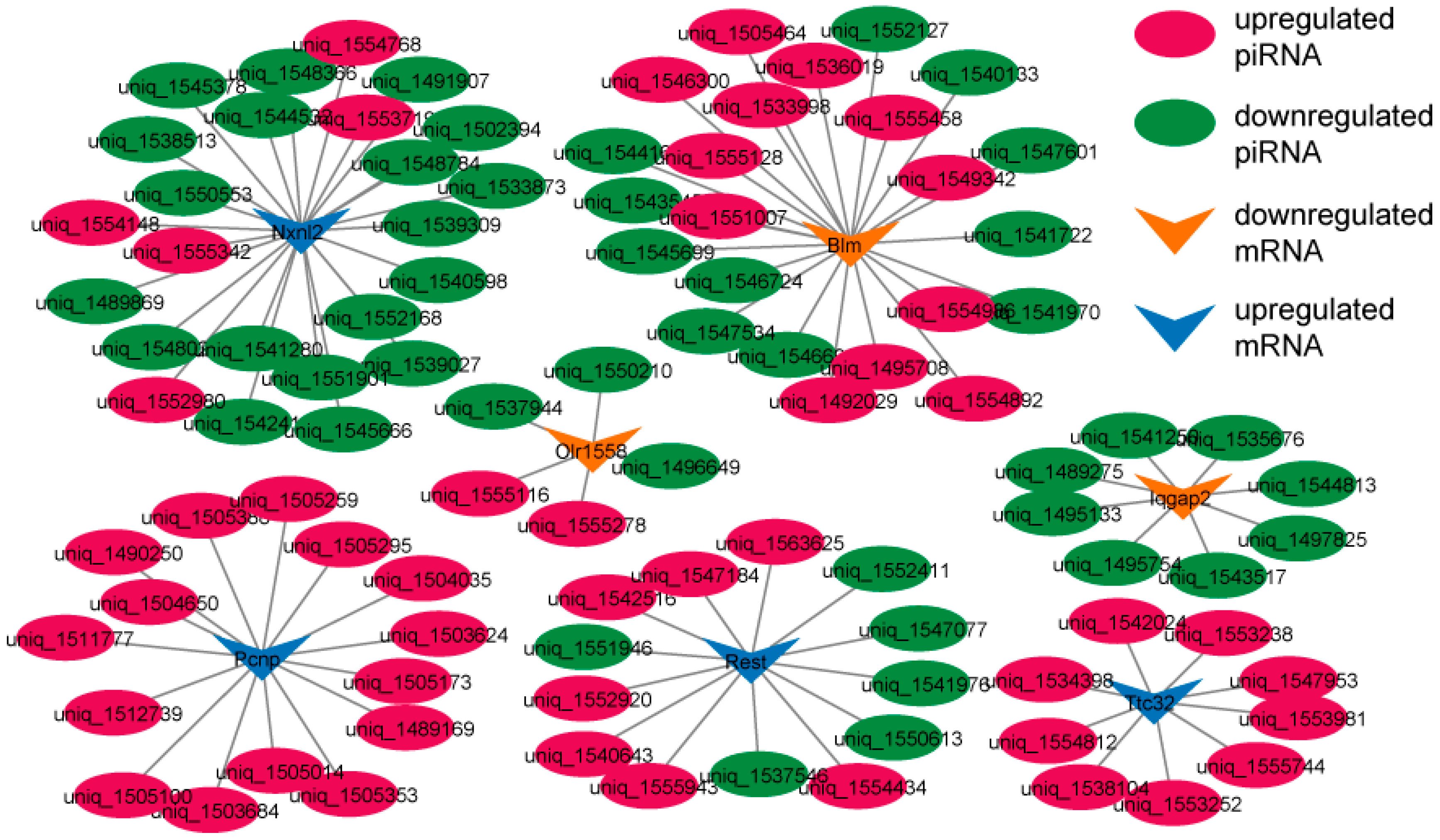
3.4. piRNA–mRNA Interaction Network
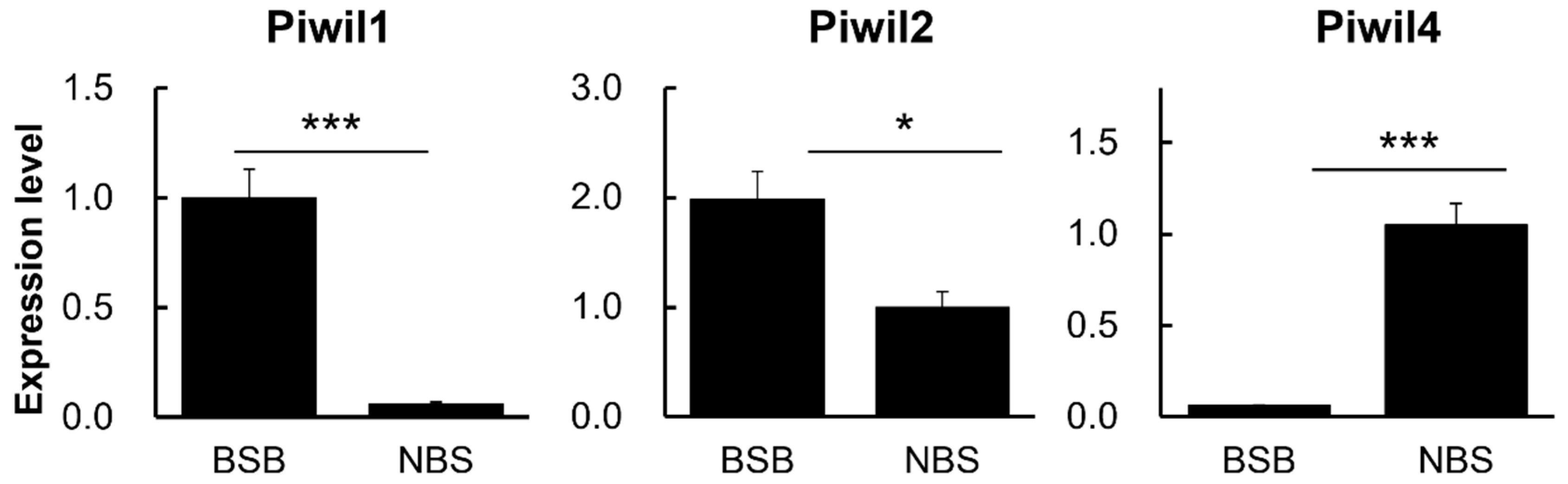
3.5. Relative mRNA Expression of PIWIL Family Was Measured by qPCR in Plateau Zokor Testes
3.6. qPCR
4. Discussion
4.1. DE piRNAs and Differential Gene Association Regulate Testicular Development
4.2. PiRNAs Participate in the Inhibitory Functions of Transposons in the Testes
4.3. Signaling Pathways of DE piRNAs Target mRNAs Involved in Reproduction
4.4. Accumulation of PIWIL4 Obstructs Sperm Meiosis in the NBS Group
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, R.; Badura, L.L.; Goldman, B.D. Mechanisms of seasonal cycles of behavior. Annu. Rev. Psychol. 1990, 41, 81–108. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Evolution of animal photoperiodism. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 1–25. [Google Scholar] [CrossRef]
- Ji, C.; Zhou, J.; Chu, B.; Zhou, R.; Wang, Z.; Wang, T.; Tian, Y.; Zhou, Y.; Hua, L. Activity pattern of plateau zokor (Eospalax baileyi) and its influences in eastern Qilian Mountain region. Acta Theriol. Sin. 2018, 38, 201–210. [Google Scholar]
- Andreychev, A.V. Daily and seasonal feeding activity of the greater mole-rat (Spalax microphtalmus, Rodentia, Spalacidae). Biol. Bull. 2019, 46, 1172–1181. [Google Scholar] [CrossRef]
- Paribello, P.; Manchia, M.; Bosia, M.; Pinna, F.; Carpiniello, B.; Comai, S. Melatonin and aggressive behavior: A systematic review of the literature on preclinical and clinical evidence. J. Pineal Res. 2022, 72, e12794. [Google Scholar] [CrossRef]
- Rhoads, M.L. Review: Reproductive consequences of whole-body adaptations of dairy cattle to heat stress. Animal 2023, 17, 100847. [Google Scholar] [CrossRef]
- Dadhich, R.K.; Real, F.M.; Zurita, F.; Barrionuevo, F.J.; Burgos, M.; Jiménez, R. Role of apoptosis and cell proliferation in the testicular dynamics of seasonal breeding mammals: A study in the Iberian mole, Talpa occidentalis. Biol. Reprod. 2010, 83, 83–91. [Google Scholar] [CrossRef]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Niu, C.M.; Xia, J.; Shen, X.Y.; Xia, M.M.; Hu, Y.Q.; Zheng, Y. Stimulated by retinoic acid gene 8 (Stra8) plays important roles in many stages of spermatogenesis. Asian J. Androl. 2018, 20, 479–487. [Google Scholar]
- Aravin, A.A.; Naumova, N.M.; Tulin, A.V.; Vagin, V.V.; Rozovsky, Y.M.; Gvozdev, V.A. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D melanogaster germline. Curr. Biol. 2001, 11, 1017–1027. [Google Scholar] [CrossRef]
- Czech, B.; Munafò, M.; Ciabrelli, F.; Eastwood, E.L.; Fabry, M.H.; Kneuss, E.; Hannon, G.J. piRNa-guided genome defense: From biogenesis to silencing. Annu. Rev. Genet. 2018, 52, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2019, 20, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Grivna, S.T.; Beyret, E.; Wang, Z.; Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006, 20, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; Van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Zhang, R.; Mwacharo, J.M.; Wang, X.; He, X.; Liu, Y.; Zhang, J.; Gong, Y.; Zhang, X.; Chu, M. Characteristics of piRNAs and their comparative profiling in testes of sheep with different fertility. Front. Genet. 2022, 13, 1078049. [Google Scholar] [CrossRef]
- Norris, R.W.; Zhou, K.; Zhou, C.; Yang, G.; William, K.C.; Honeycutt, R.L. The phylogenetic position of the zokors (Myospalacinae) and comments on the families of muroids (Rodentia). Mol. Phylogenet. Evol. 2004, 31, 972–978. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J. Effects of plateau zokors (Myospalax fontanierii) on plant community and soil in an alpine meadow. J. Mammal. 2003, 84, 644–651. [Google Scholar] [CrossRef]
- Tan, Y.; Yao, B.; Kang, Y.; Shi, S.; Shi, Z.; Su, J. Emerging role of the crosstalk between gut microbiota and liver metabolome of subterranean herbivores in response to toxic plants. Ecotoxicol. Environ. Saf. 2024, 269, 115902. [Google Scholar] [CrossRef]
- Yao, B.; An, K.; Kang, Y.; Tan, Y.; Zhang, D.; Su, J. Reproductive suppression caused by spermatogenic arrest: Transcriptomic evidence from a non-social animal. Int. J. Mol. Sci. 2023, 24, 4611. [Google Scholar] [CrossRef]
- An, K.; Yao, B.; Kang, Y.; Bao, M.; Tan, Y.; Pu, Q.; Su, J. Seasonal expression of gonadotropin genes in the pituitary and testes of male plateau zokor (Eospalax baileyi). Animals 2022, 12, 725. [Google Scholar] [CrossRef]
- Yao, B.; Kang, Y.; An, K.; Tan, Y.; Hou, Q.; Zhang, D.; Su, J. Comparative analysis of microRNA and messenger RNA expression profiles in plateau zokor testicular cells under reproductive suppression. Front. Vet. Sci. 2023, 10, 1184120. [Google Scholar] [CrossRef]
- Su, J.; Hegab, I.M.; Ji, W.; Nan, Z. Function-related drivers of skull morphometric variation and sexual size dimorphism in a subterranean rodent, Plateau zokor (Eospalax baileyi). Ecol. Evol. 2018, 8, 4631–4643. [Google Scholar] [CrossRef]
- Faulkes, C.G.; Bennett, N.C. Social evolution in African mole-rats—A comparative overview. Adv. Exp. Med. Biol. 2021, 1319, 1–33. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 1, 2498–2504. [Google Scholar] [CrossRef]
- Yan, Z.; Hu, H.Y.; Jiang, X.; Maierhofer, V.; Neb, E.; He, L.; Hu, Y.; Hu, H.; Li, N.; Chen, W.; et al. Widespread expression of piRNA-like molecules in somatic tissues. Nucleic Acids Res. 2011, 39, 6596–6607. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Q.; Wang, Q.; Ma, Y.; Du, J.; Zhang, Y.; Zhao, X. Identification and verification of potential piRNAs from domesticated yak testis. Reproduction 2018, 155, 117–127. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Fang, C.; Shi, L.; Tan, J.; Xiong, Y.; Bin, F.; Li, C. Genome-wide differential expression of genes and small RNAs in testis of two different porcine breeds and at two different ages. Sci. Rep. 2016, 6, 26852. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.W.; Siomi, M.C.; Siomi, H. PIWI-Interacting RNA: Its biogenesis and functions. Annu. Rev. Biochem. 2015, 84, 405–433. [Google Scholar] [CrossRef] [PubMed]
- Grimson, A.; Srivastava, M.; Fahey, B.; Woodcroft, B.J.; Chiang, H.R.; King, N.; Degnan, B.M.; Rokhsar, D.S.; Bartel, D.P. Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 2008, 455, 1193–1197. [Google Scholar] [CrossRef]
- Shirzad, H. PiRNA Biogenesis and their role in human cancers and other diseases: A narrative review. Iran. J. Public Health 2021, 50, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Song, J.; Wang, S.; Kapusta, A.; Feschotte, C.; Chen, K.C.; Xing, J. A comprehensive analysis of piRNAs from adult human testis and their relationship with genes and mobile elements. BMC Genom. 2014, 15, 545. [Google Scholar] [CrossRef] [PubMed]
- Weick, E.M.; Miska, E.A. piRNAs: From biogenesis to function. Development 2014, 141, 3458–3471. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.A.; Sachidanandam, R.; Girard, A.; Fejes-Toth, K.; Hannon, G.J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 2007, 316, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Vojtek, A.B.; Der, C.J. Increasing complexity of the Ras signaling pathway. J. Biol. Chem. 1998, 273, 19925–19928. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, M.; Gelb, B.D. Disorders of dysregulated signal traffic through the RAS-MAPK pathway: Phenotypic spectrum and molecular mechanisms. Ann. N. Y. Acad. Sci. 2010, 1214, 99–121. [Google Scholar] [CrossRef]
- Bruscoli, S.; Velardi, E.; Di Sante, M.; Bereshchenko, O.; Venanzi, A.; Coppo, M.; Berno, V.; Mameli, M.G.; Colella, R.; Cavaliere, A.; et al. Long glucocorticoid-induced leucine zipper (L-GILZ) protein interacts with ras protein pathway and contributes to spermatogenesis control. J. Biol. Chem. 2012, 287, 1242–1251. [Google Scholar] [CrossRef]
- Yang, Q.E.; Kim, D.; Kaucher, A.; Oatley, M.J.; Oatley, J.M. CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J. Cell Sci. 2013, 126, 1009–1020. [Google Scholar] [CrossRef]
- Diao, R.; Cai, X.; Liu, L.; Yang, L.; Duan, Y.; Cai, Z.; Gui, Y.; Mou, L. In vitro chemokine (C-C motif) receptor 6-dependent non-inflammatory chemotaxis during spermatogenesis. Biol. Res. 2018, 51, 12. [Google Scholar] [CrossRef]
- Gao, Q.; Frohman, M.A. Roles for the lipid-signaling enzyme MitoPLD in mitochondrial dynamics, piRNA biogenesis, and spermatogenesis. BMB Rep. 2012, 45, 7–13. [Google Scholar] [CrossRef]
- Watanabe, T.; Chuma, S.; Yamamoto, Y.; Kuramochi-Miyagawa, S.; Totoki, Y.; Toyoda, A.; Hoki, Y.; Fujiyama, A.; Shibata, T.; Sado, T.; et al. MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev. Cell 2011, 20, 364–375. [Google Scholar] [CrossRef]
- Peng, X.; Frohman, M.A. Mammalian phospholipase D physiological and pathological roles. Acta. Physiol. Oxf. 2012, 204, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Menegazzo, M.; Gianesello, L.; Selice, R.; Foresta, C. Effect of relaxin on human sperm functions. J. Androl. 2012, 33, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.S.; Tian, H.; Zhao, L.; Amento, E.P. Relaxin is a key mediator of prostate growth and male reproductive tract development. Lab. Investig. 2003, 83, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Anand-Ivell, R.; Heng, K.; Hafen, B.; Setchell, B.; Ivell, R. Dynamics of INSL3 peptide expression in the rodent testis. Biol. Reprod. 2009, 81, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Burnicka-Turek, O.; Mohamed, B.A.; Shirneshan, K.; Thanasupawat, T.; Hombach-Klonisch, S.; Klonisch, T.; Adham, I.M. INSL5-deficient mice display an alteration in glucose homeostasis and an impaired fertility. Endocrinology 2012, 153, 4655–4665. [Google Scholar] [CrossRef]
- Kimura, R.; Inoue, Y.U.; Kikkawa, T.; Tatehana, M.; Morimoto, Y.; Inada, H.; Oki, S.; Inoue, T.; Osumi, N. Detection of REST expression in the testis using epitope-tag knock-in mice generated by genome editing. Dev. Dyn. 2022, 251, 525–535. [Google Scholar] [CrossRef]
- Wang, H.; Huo, R.; Xu, M.; Lu, L.; Xu, Z.; Li, J.; Zhou, Z.; Sha, J. Cloning and characterization of a novel transcript variant of IQGAP2 in human testis. DNA Seq. 2004, 15, 319–325. [Google Scholar] [CrossRef]
- Sun, X.; Brieño-Enríquez, M.A.; Cornelius, A.; Modzelewski, A.J.; Maley, T.T.; Campbell-Peterson, K.M.; Holloway, J.K.; Cohen, P.E. FancJ (Brip1) loss-of-function allele results in spermatogonial cell depletion during embryogenesis and altered processing of crossover sites during meiotic prophase I in mice. Chromosoma 2016, 125, 237–252. [Google Scholar] [CrossRef]
- Kim, K.W. PIWI proteins and piRNAs in the nervous system. Mol. Cells 2019, 42, 828–835. [Google Scholar]
- Kang, H.J.; Moon, M.J.; Lee, H.Y.; Han, S.W. Testosterone alters testis function through regulation of piRNA expression in rats. Mol. Biol. Rep. 2014, 41, 6729–6735. [Google Scholar] [CrossRef]
- Wu, P.H.; Fu, Y.; Cecchini, K.; Özata, D.M.; Arif, A.; Yu, T.; Colpan, C.; Gainetdinov, I.; Weng, Z.; Zamore, P.D. The evolutionarily conserved piRNA-producing locus pi6 is required for male mouse fertility. Nat. Genet. 2020, 52, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Niu, X.; Huang, S.; Li, S.; Ran, X.; Wang, J.; Dai, X. The piRNAs present in the developing testes of Chinese indigenous Xiang pigs. Theriogenology 2022, 189, 92–106. [Google Scholar] [CrossRef] [PubMed]
- La, Y.; Ma, X.; Bao, P.; Chu, M.; Yan, P.; Guo, X.; Liang, C. Identification and characterization of Piwi-interacting RNAs for early testicular development in Yak. Int. J. Mol. Sci. 2022, 23, 12320. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef]
- Bortvin, A. PIWI-interacting RNAs (piRNAs)—A mouse testis perspective. Biochemistry 2013, 78, 592–602. [Google Scholar] [CrossRef]
- Gomes, F.M.; He, N.; Wang, F.; Van, I.L.; Eguizabal, C.; Matorras, R.; Roelen, B.A.J.; De Sousa Lopes, S.M.C. Human-specific subcellular compartmentalization of P-element induced wimpy testis-like (PIWIL) granules during germ cell development and spermatogenesis. Hum. Reprod. 2018, 33, 258–269. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, M.; Chen, X.; Jing, K.; Li, Y.; Liu, X.; Ran, H.; Qin, J.; Zhong, J.; Cai, X. Dysregulated genes in undifferentiated spermatogonia and Sertoli cells are associated with the spermatogenic arrest in cattle yak. Mol. Reprod. Dev. 2022, 89, 632–645. [Google Scholar] [CrossRef]


| Type | piRNAs | Sequences of Stem-Loop Primers and qPCR Amplification Primers |
|---|---|---|
| piRNA | piR_000965 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCAGAA |
| F: ATGTACCGAGGATCGCTTGAGTC | ||
| R: ATCCAGTGCAGGGTCCGAGG | ||
| piR_008812 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGAACA | |
| F: AACCGGTTTGAAGGGAGCATTTG | ||
| R: ATCCAGTGCAGGGTCCGAGG | ||
| piR_008957 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGAAGC | |
| F: ACCGAGGTTAACAGACTGAATTGTG | ||
| R: ATCCAGTGCAGGGTCCGAGG | ||
| piR_005854 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAGTGC | |
| F: AACAGTGGTGCGCTATGCCG | ||
| R: ATCCAGTGCAGGGTCCGAGG | ||
| piR_022980 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCTGAG | |
| F: ATGCGCGCTGATCCTTTTCAAC | ||
| R: ATCCAGTGCAGGGTCCGAGG | ||
| piR_013875 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCTGAG | |
| F: CGTGTAAAATAAGACATTTGTAGCCT | ||
| R: ATCCAGTGCAGGGTCCGAGG | ||
| U6 | RT: AACGCTTCACGAATTTGCGT | |
| F: GCTTCGGCAGCACATATACT | ||
| R: TTCACGAATTTGCGTGTCAT | ||
| mRNA | PIWIL1 | F: GAAGGCAGATGGCTCAGAGGTC |
| R: GCTACCTCGTTCCTCTGGATGTC | ||
| PIWIL2 | F: TCGGAATGACTCTGTGCTGGATG | |
| R: CGATTCTGTAGGTGCGGTTGTTG | ||
| PIWIL4 | F: GAAGGCAGATGGCTCAGAGGTC | |
| R: CCGCTTGGGCTGGCTCAC | ||
| Β-ACTIN | F: TTGTGCGTGACATCAAAGAG | |
| R: ATGCCAGAAGATTCCATACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Yao, B.; Tan, Y.; Liu, Y.; Su, J. Seasonal piRNA Expression Profile Changes in the Testes of Plateau Zokor (Eospalax baileyi). Animals 2024, 14, 2620. https://doi.org/10.3390/ani14172620
Cai Z, Yao B, Tan Y, Liu Y, Su J. Seasonal piRNA Expression Profile Changes in the Testes of Plateau Zokor (Eospalax baileyi). Animals. 2024; 14(17):2620. https://doi.org/10.3390/ani14172620
Chicago/Turabian StyleCai, Zhiyuan, Baohui Yao, Yuchen Tan, Yongjie Liu, and Junhu Su. 2024. "Seasonal piRNA Expression Profile Changes in the Testes of Plateau Zokor (Eospalax baileyi)" Animals 14, no. 17: 2620. https://doi.org/10.3390/ani14172620
APA StyleCai, Z., Yao, B., Tan, Y., Liu, Y., & Su, J. (2024). Seasonal piRNA Expression Profile Changes in the Testes of Plateau Zokor (Eospalax baileyi). Animals, 14(17), 2620. https://doi.org/10.3390/ani14172620






