The Impact of a Hypoallergenic Diet on the Control of Oral Lesions in Cats: A Case Report
Abstract
:Simple Summary
Abstract
1. Introduction
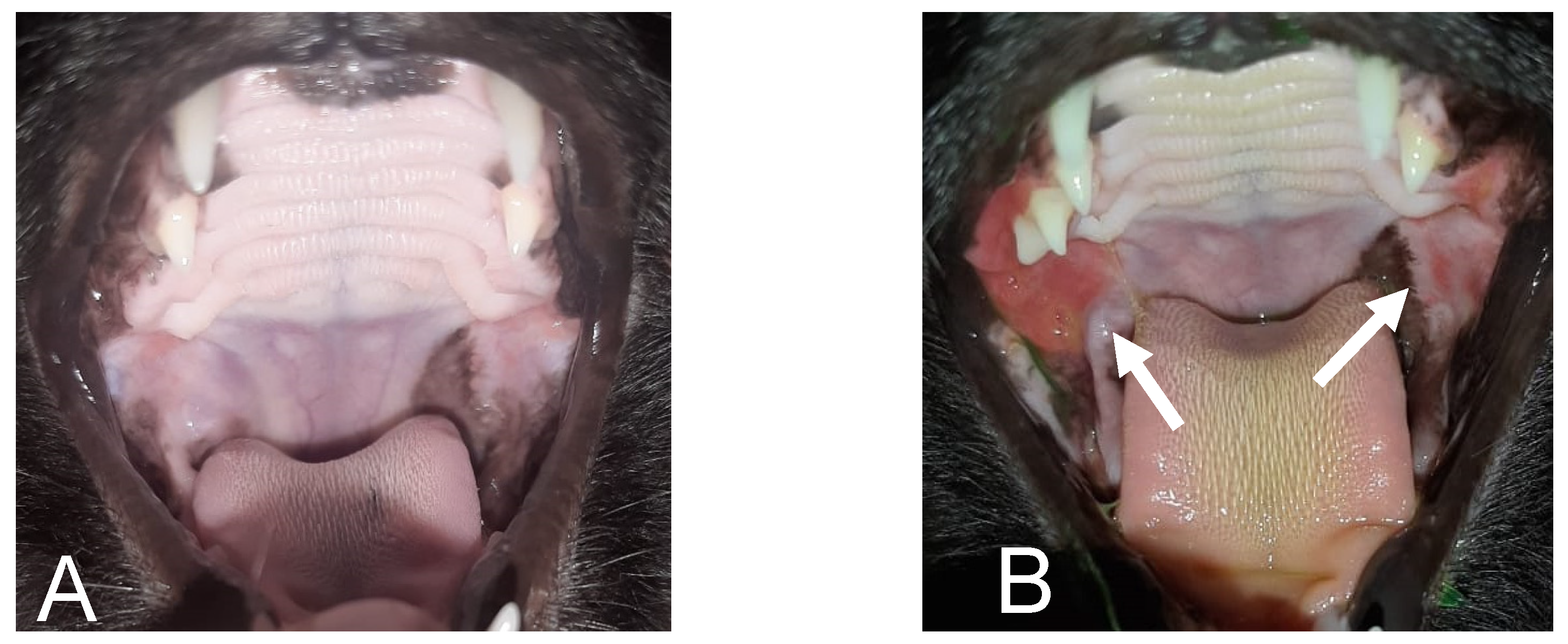
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diehl, K.; Rosychuk, R.A.W. Feline Gingivitis-Stomatitis-Pharyngitis. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 139–153. [Google Scholar] [CrossRef]
- Niza, M.M.R.E.; Mestrinho, L.A.; Vilela, C.L. Gengivo-estomatite crônica felina—Um desafio clínico. Rev. Port. Ciênc. Vet. 2004, 99, 127–135. [Google Scholar]
- Lyon, K.F. Gingivostomatitis. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 891–911. [Google Scholar] [CrossRef]
- Soltero-Rivera, M.M.; Reiter, A.M. Diseases of the Oral Cavity and Salivary Glands. In Clinical Small Animal Internal Medicine; Bruyette, D.S., Bexfield, N., Chretin, J.D., Kidd, L., Kube, S., Langston, C., Owen, T.J., Oyama, M.A., Peterson, N., Reiter, L.V., et al., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 533–546. [Google Scholar] [CrossRef]
- Williams, C.A.; Aller, M.S. Gingivitis/Stomatitis in Cats. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 1361–1383. [Google Scholar] [CrossRef]
- Healey, K.A.; Dawson, S.; Burrow, R.; Cripps, P.; Gaskell, C.J.; Hart, C.A.; Pinchbeck, G.L.; Radford, A.D.; Gaskell, R.M. Prevalence of Feline Chronic Gingivo-Stomatitis in First Opinion Veterinary Practice. J. Feline Med. Surg. 2007, 9, 373–381. [Google Scholar] [CrossRef]
- Robson, D. Review of the Properties and Mechanisms of Action of Cyclosporine with an Emphasis on Dermatological Therapy in Dogs, Cats and People. Vet. Rec. 2003, 152, 768–772. [Google Scholar] [CrossRef]
- Lommer, M.J. Efficacy of Cyclosporine for Chronic, Refractory Stomatitis in Cats: A Randomized, Placebo-Controlled, Double-Blinded Clinical Study. J. Vet. Dent. 2013, 30, 8–17. [Google Scholar] [CrossRef]
- Hennet, P. Chronic Gingivo-Stomatitis in Cats: Long-Term Follow-up of 30 Cases Treated by Dental Extractions. J. Vet. Dent. 1997, 14, 15–21. [Google Scholar] [CrossRef]
- Crystal, M.A. Gingivitis/stomatitis/faringitis. In El Paciente Felino: Bases del Diagnóstico y Tratamiento; Norsworthy, G.D., Crystal, M.A., Foochee, S.R., Tilley, L.P., Eds.; Inter-Médica Editorial: Buenos Aires, Argentina, 1998; pp. 228–231. [Google Scholar]
- Hofmann-Appollo, F.; Carvalho, V.G.G.; Gioso, M.A. Complexo Gengivite-Estomatite-Faringite Dos Felinos. Clínica Veterinária 2010, 15, 44–52. [Google Scholar]
- Laflamme, D.P. Development and Validation of a Body Condition Score System for Dogs: A Clinical Tool; Canine Practice: Santa Barbara, CA, USA, 1997; Volume 22, pp. 10–15. [Google Scholar]
- Michel, K.E.; Anderson, W.; Cupp, C.; Laflamme, D.P. Correlation of a Feline Muscle Mass Score with Body Composition Determined by Dual-Energy X-Ray Absorptiometry. Br. J. Nutr. 2011, 106 (Suppl. 1), S57–S59. [Google Scholar] [CrossRef]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; European Pet Food Industry Federation: Brussels, Belgium, 2021. [Google Scholar]
- Westropp, J.L.; Tony Buffington, C.A. Feline Idiopathic Cystitis: Current Understanding of Pathophysiology and Management. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 1043–1055. [Google Scholar] [CrossRef]
- Johnston, N. An updated approach to chronic feline gingivitis stomatitis syndrome. Vet. Pract. 2012, 44, 34–38. [Google Scholar]
- Carlotti, D.N.; Remy, I.; Prost, C. Food Allergy in Dogs And Cats. A Review and Report of 43 Cases. Vet. Dermatol. 1990, 1, 55–62. [Google Scholar] [CrossRef]
- Olivry, T.; Mueller, R.S. Critically Appraised Topic on Adverse Food Reactions of Companion Animals (7): Signalment and Cutaneous Manifestations of Dogs and Cats with Adverse Food Reactions. BMC Vet. Res. 2019, 15, 140. [Google Scholar] [CrossRef]
- White, S.D.; Sequoia, D. Food hypersensitivity in cats: 14 cases (1982–1987). J. Am. Vet. Med. Assoc. 1989, 194, 692–695. [Google Scholar]
- Hobi, S.; Linek, M.; Marignac, G.; Olivry, T.; Beco, L.; Nett, C.; Fontaine, J.; Roosje, P.; Bergvall, K.; Belova, S.; et al. Clinical Characteristics and Causes of Pruritus in Cats: A Multicentre Study on Feline Hypersensitivity- associated Dermatoses. Vet. Dermatol. 2011, 22, 406–413. [Google Scholar] [CrossRef]
- Mazzeranghi, F.; Zanotti, C.; Di Cerbo, A.; Verstegen, J.P.; Cocco, R.; Guidetti, G.; Canello, S. Clinical Efficacy of Nutraceutical Diet for Cats with Clinical Signs of Cutaneus Adverse Food Reaction (CAFR). Pol. J. Vet. Sci. 2017, 20, 269–276. [Google Scholar] [CrossRef]
- Mueller, R.S.; Olivry, T. Critically appraised topic on adverse food reactions of companion animals (4): Can we diagnose adverse food reactions in dogs and cats with in vivo or in vitro tests? BMC Vet. Res. 2017, 13, 275. [Google Scholar] [CrossRef]
- Olivry, T.; Mueller, R.S. Critically Appraised Topic on Adverse Food Reactions of Companion Animals (9): Time to Flare of Cutaneous Signs after a Dietary Challenge in Dogs and Cats with Food Allergies. BMC Vet. Res. 2020, 16, 158. [Google Scholar] [CrossRef]
- Moriello, K.A.; Kahn, C.M.; Line, S. The Merck Veterinary Manual; Merck & Co: Whitehouse Station, NJ, USA, 2010; pp. 649–852. [Google Scholar]
- Tiffany, S.; Parr, J.M.; Templeman, J.; Shoveller, A.K.; Manjos, R.; Yu, A.; Verbrugghe, A. Assessment of Dog Owners’ Knowledge Relating to the Diagnosis and Treatment of Canine Food Allergies. Can. Vet. J. 2019, 60, 268–274. [Google Scholar]
- Olivry, T.; Bizikova, P. A Systematic Review of the Evidence of Reduced Allergenicity and Clinical Benefit of Food Hydrolysates in Dogs with Cutaneous Adverse Food Reactions. Vet. Dermatol. 2010, 21, 32–41. [Google Scholar] [CrossRef]
- Woo, S.-B.; Sonis, S.T. Recurrent Aphthous Ulcers: A Review of Diagnosis and Treatment. J. Am. Dent. Assoc. 1996, 127, 1202–1213. [Google Scholar] [CrossRef]
- Zunt, S.L. Recurrent aphthous stomatitis. Dermatol. Clin. 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Chavan, M.; Jain, H.; Diwan, N.; Khedkar, S.; Shete, A.; Durkar, S. Recurrent Aphthous Stomatitis: A Review. J. Oral Pathol. Med. 2012, 41, 577–583. [Google Scholar] [CrossRef]
- Eversole, L.R.; Shopper, T.P.; Chambers, D.W. Effects of Suspected Foodstuff Challenging Agents in the Etiology of Recurrent Aphthous Stomatitis. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 33–38. [Google Scholar] [CrossRef]
- Wardhana, D.E.A. Recurrent aphthous stomatitis caused by food allergy. Acta Med. Indones. 2010, 42, 236–240. [Google Scholar] [PubMed]
- Miller, J.; Simpson, A.; Bloom, P.; Diesel, A.; Friedeck, A.; Paterson, T.; Wisecup, M.; Yu, C.M. AAHA Management of Allergic Skin Diseases in Dogs and Cats Guidelines. J. Am. Anim. Hosp. Assoc. 2023, 59, 255–284. [Google Scholar] [CrossRef]
- Camy, G.; Fahrenkrug, P.; Gracis, M.; Hennet, P.; Johnston, N.; Mihaljevic, S.; Schreyer, J. Proposed Guidelines on the Management of Feline Chronic Gingivostomatitis (FCGS) Syndrome: A Consensus Statement Consultation Version September. J. Feline Med. Surg. 2010, 20, 244–255. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.d.; Martins, T.; Porsani, M.Y.H.; Teixeira, F.A. The Impact of a Hypoallergenic Diet on the Control of Oral Lesions in Cats: A Case Report. Animals 2024, 14, 2656. https://doi.org/10.3390/ani14182656
Silva Ld, Martins T, Porsani MYH, Teixeira FA. The Impact of a Hypoallergenic Diet on the Control of Oral Lesions in Cats: A Case Report. Animals. 2024; 14(18):2656. https://doi.org/10.3390/ani14182656
Chicago/Turabian StyleSilva, Luiza da, Taís Martins, Mariana Yukari Hayasaki Porsani, and Fabio Alves Teixeira. 2024. "The Impact of a Hypoallergenic Diet on the Control of Oral Lesions in Cats: A Case Report" Animals 14, no. 18: 2656. https://doi.org/10.3390/ani14182656




