Faecal Glucocorticoid Metabolites and Hair Cortisone/Cortisol Measurements in Domestic Pigs Exposed to Road Transportation and Dexamethasone Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Pigs and Housing Conditions
2.2. Experimental Design
2.3. Treatment 1: Road Transportation
2.4. Treatment 2: Dexamethasone Injection
2.5. Sample Collection and Steroid Extraction
2.6. Measurement of Faecal Glucocorticoid Metabolites
2.7. Measurement of Hair Glucocorticoids
2.8. Statistical Analysis
3. Results
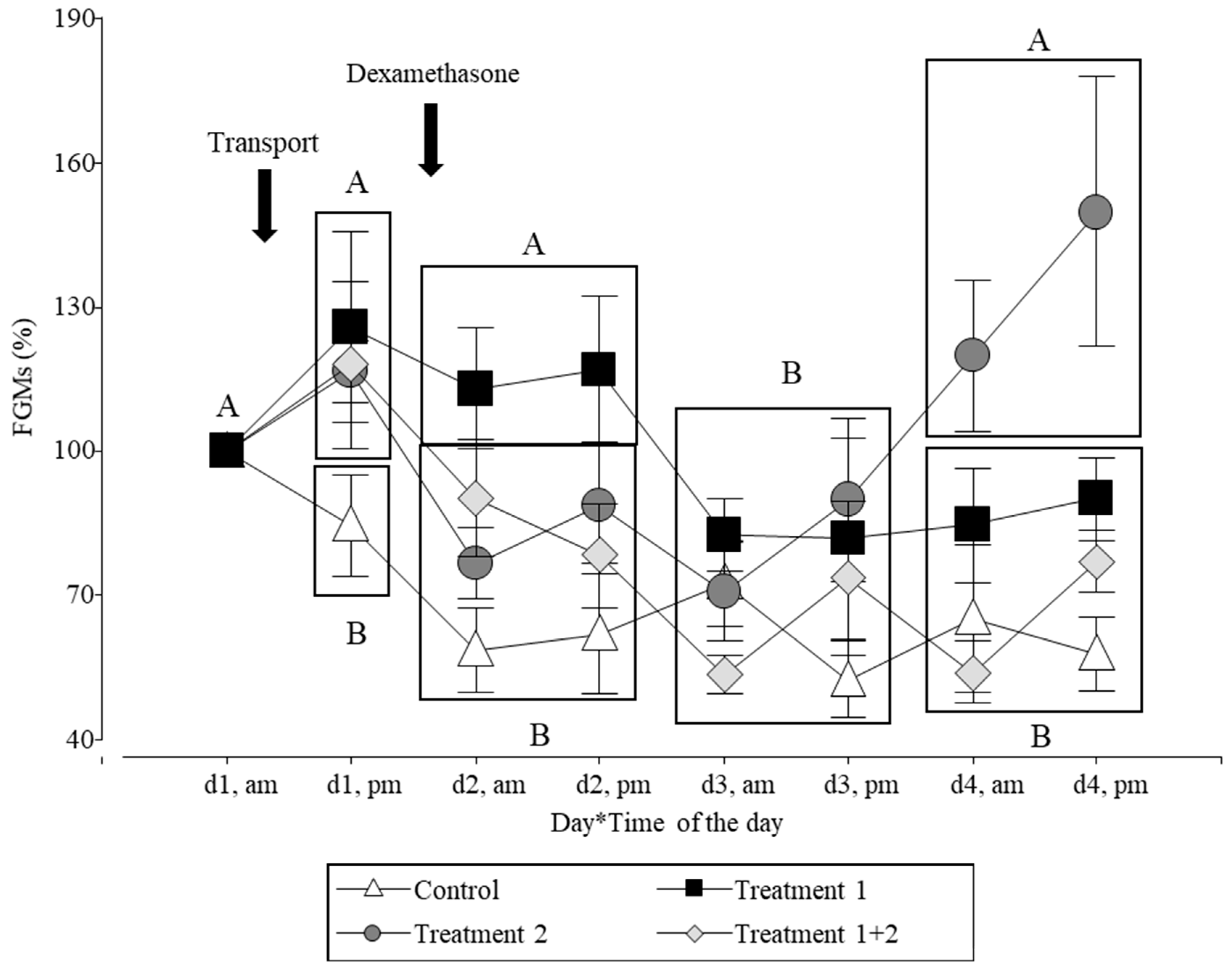
3.1. Measurement of Faecal Glucocorticoid Metabolites
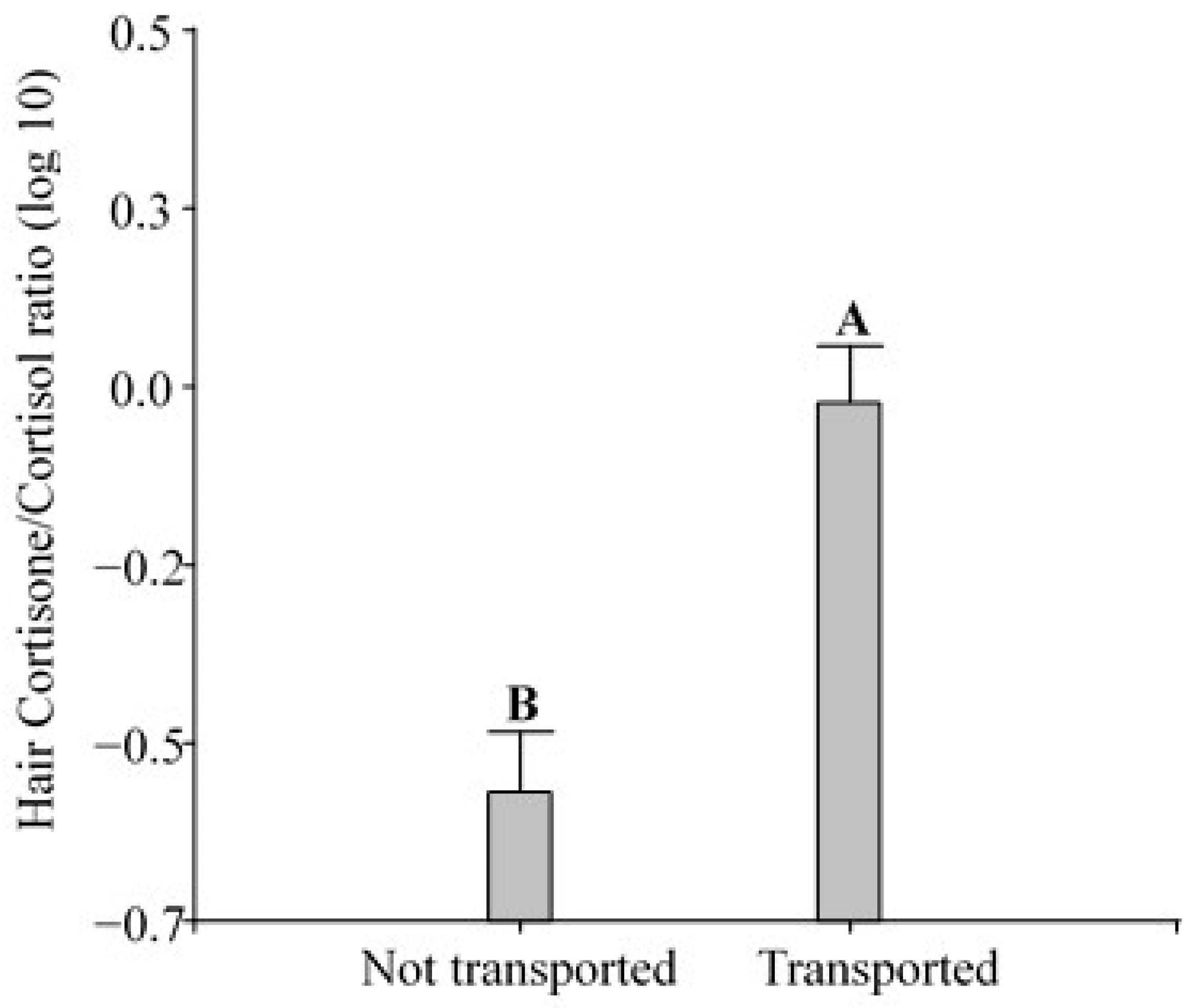
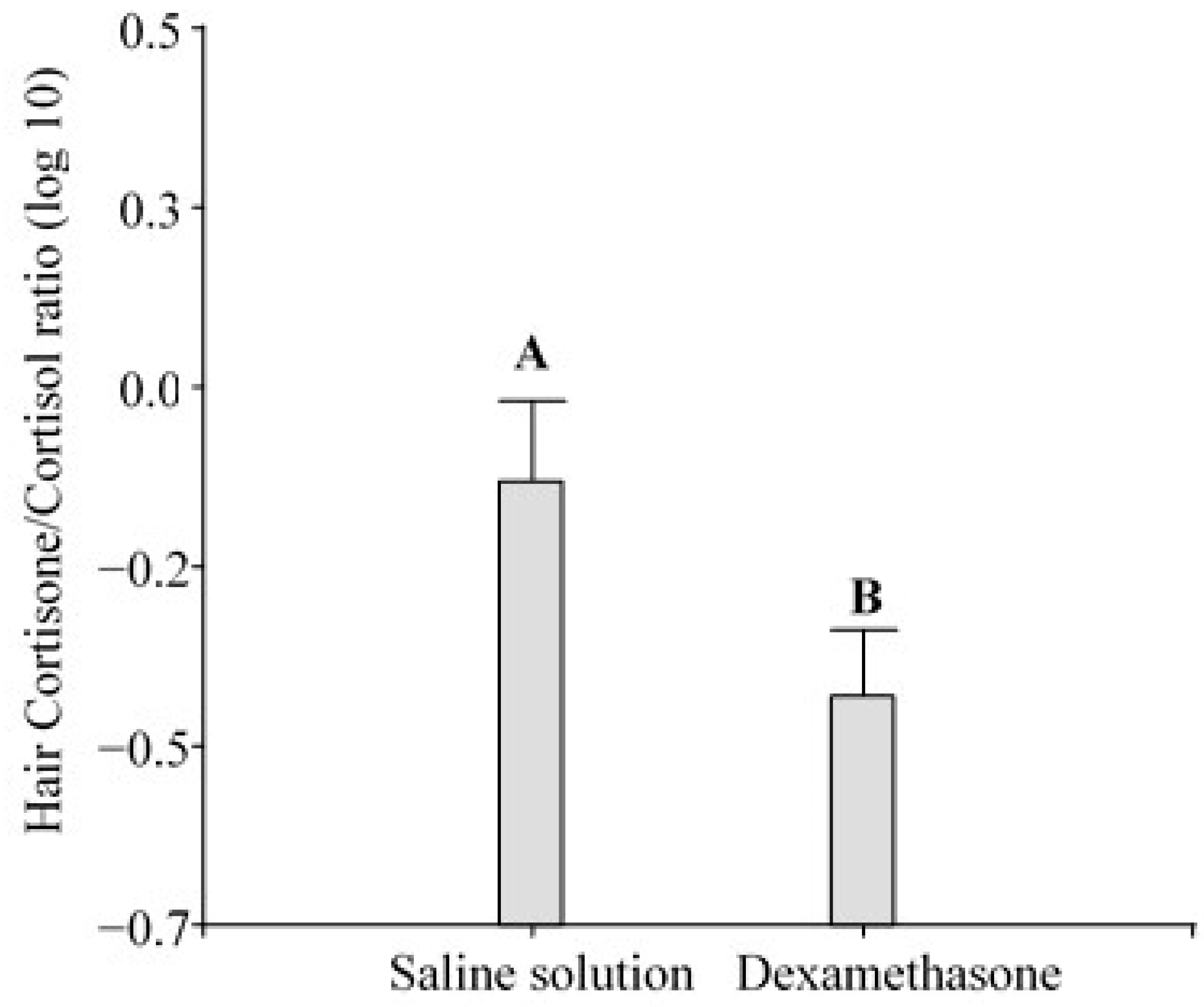
3.2. Measurement of Glucocorticoids in Hair and Cortisone/Cortisol Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Effect of Dexamethasone on Adrenocortical Activity Revealed by Faecal Glucocorticoid Metabolites
Appendix A.1. Statistical Analyses
Appendix A.2. Results and Conclusions

References
- Dahl-Pedersen, K.; Herskin, M.S. Transportation of cattle and pigs between EU member states 2014–2018–can data from TRACES be used to create overview and inform about potential welfare consequences? J. Appl. Anim. Welf. Sci. 2023, 26, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, P. The effects of handling, transport, slaughter and chilling on meat quality and yield in pigs: A review. Ir. J. Food Sci. Technol. 1989, 13, 79–107. [Google Scholar]
- Guàrdia, M.D.; Estany, J.; Balasch, S.; Oliver, M.A.; Gispert, M.; Diestre, A. Risk assessment of skin damage due to pre-slaughter conditions and RYR1 gene in pigs. Meat Sci. 2009, 81, 745–751. [Google Scholar] [CrossRef]
- World Organization for Animal Health. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_aw_land_transpt.htm (accessed on 3 August 2021).
- Manrique, J.; Ballerio, M.; Racciati, D.; Rodriguez Vazquez, G. Animal Welfare Workshop: Transportation of Standing Animals and Distances. National Service of Agri-Food Health and Quality (SENASA). 2019. Available online: https://www.argentina.gob.ar/senasa (accessed on 21 May 2021).
- Merlot, E.; Mounier, A.M.; Prunier, A. Endocrine response of gilts to various common stressors: A comparison of indicators and methods of analysis. Physiol. Behav. 2011, 102, 259–265. [Google Scholar] [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef]
- Ortín-Bustillo, A.; Escribano, D.; López-Arjona, M.; Botia, M.; Fuentes, P.; Martínez-Miró, S.; Rubio, C.P.; Garcia-Manzanilla, E.; Franco-Martinez, L.; Pardo-Marin, L.; et al. Changes in a comprehensive profile of saliva analytes in fattening pigs during a complete productive cycle: A longitudinal study. Animals 2022, 12, 1865. [Google Scholar] [CrossRef]
- Kumar, P.; Ahmed, M.A.; Abubakar, A.A.; Hayat, M.N.; Kaka, U.; Ajat, M.; Goh, Y.M.; Sazili, A.Q. Improving animal welfare status and meat quality through assessment of stress biomarkers: A critical review. Meat Sci. 2023, 197, 109048. [Google Scholar] [CrossRef]
- Prims, S.; Hole, C.V.; Van Cruchten, S.; Van Ginneken, C.; Van Ostade, X.; Casteleyn, C. Hair or salivary cortisol analysis to identify chronic stress in piglets? Vet. J. 2019, 252, 105357. [Google Scholar] [CrossRef]
- Wolf, T.E.; Mangwiro, N.; Fasina, F.O.; Ganswindt, A. Non-invasive monitoring of adrenocortical function in female domestic pigs using saliva and faeces as sample matrices. PLoS ONE 2020, 15, e0234971. [Google Scholar] [CrossRef]
- Bergamin, C.; Comin, A.; Corazzin, M.; Faustini, M.; Peric, T.; Scollo, A.; Gottardo, F.; Montillo, M.; Prandi, A. Cortisol, DHEA, and sexual steroid concentrations in fattening pigs’ hair. Animals 2019, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.H.; Nath, A.; Thomas, R.; Kumar, S.; Banik, S.; Das, R.K.; Sarma, D.K. Relationship between plasma, saliva, urinary and faecal cortisol levels in pigs. Indian J. Anim. Sci. 2020, 90, 768–772. [Google Scholar] [CrossRef]
- Parois, S.P.; Van Der Zande, L.E.; Knol, E.F.; Kemp, B.; Rodenburg, T.B.; Bolhuis, J.E. A multi-suckling system combined with an enriched housing environment during the growing period promotes resilience to various challenges in pigs. Sci. Rep. 2022, 12, 6804. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Ghaffari, M.H.; Ataallahi, M.; Jo, J.H.; Lee, H.G. Stress concepts and applications in various matrices with a focus on hair cortisol and analytical methods. Animals 2022, 12, 3096. [Google Scholar] [CrossRef]
- Svoboda, M.; Nemeckova, M.; Medkova, D.; Sardi, L.; Hodkovicova, N. Non-invasive methods for analysing pig welfare biomarkers. Vet. Med. 2024, 69, 137. [Google Scholar] [CrossRef]
- Botía, M.; Escribano, D.; Ortín-Bustillo, A.; López-Martínez, M.J.; Fuentes, P.; Jiménez-Caparrós, F.J.; Hernández-Gómez, J.L.; Avellaneda, A.; Cerón, J.J.; Rubio, C.P.; et al. Comparison of the effect of two different handling conditions at slaughter in saliva analytes in pigs. Metabolites 2024, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Ann. N. Y. Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Palme, R. Measuring fecal steroids: Guidelines for practical application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef]
- Palme, R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim. Welf. 2012, 21, 331–337. [Google Scholar] [CrossRef]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, C.F.; Kauter, K.G.; McFarlane, J.R. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: Latency, localization and independence effects. Physiol. Res. 2009, 58, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi Nejad, J.G.; Lohakare, J.D.; Son, J.K.; Kwon, E.G.; West, J.W.; Sung, K.I. Wool cortisol is a better indicator of stress than blood cortisol in ewes exposed to heat stress and water restriction. Animal 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Is it getting in the hair? Cortisol concentrations in native, regrown and segmented hairs of cattle and pigs after repeated ACTH administrations. Gen. Comp. Endocrinol. 2020, 295, 113534. [Google Scholar] [CrossRef]
- Eerdenburg, F.J.C.M.v.E.; Hof, T.; Doeve, B.; Ravesloot, L.; Zeinstra, E.C.; Nordquist, R.E.; van Der Staay, F.J. The relation between hair-cortisol concentration and various welfare assessments of Dutch dairy farms. Animals 2021, 11, 821. [Google Scholar] [CrossRef]
- Casal, N.; Manteca, X.; Peña, R.; Bassols, A.; Fàbrega, E. Analysis of cortisol in hair samples as an indicator of stress in pigs. J. Vet. Behav. 2017, 19, 1–6. [Google Scholar] [CrossRef]
- Levallois, P.; Leblanc-Maridor, M.; Lehébel, A.; Gavaud, S.; Lieubeau, B.; Hervé, J.; Fourichon, C.; Belloc, C. Hair cortisol concentration in finishing pigs on commercial farms: Variability between pigs, batches, and farms. Front. Vet. Sci. 2024, 10, 1298756. [Google Scholar] [CrossRef]
- Bechshøft, T.Ø.; Sonne, C.; Dietz, R.; Born, E.W.; Novak, M.A.; Henchey, E.; Meyer, J.S. Cortisol levels in hair of East Greenland polar bears. Sci. Total Environm. 2011, 409, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.; Thomson, S.; Rieder, M.; Van Uum, S.; Koren, G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci. Internal. 2010, 196, 32–37. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Vanaelst, B.; Michels, N.; De Vriendt, T.; Huybrechts, I.; Vyncke, K.; Sioen, I.; Bammann, K.; Rivet, N.; Raul, J.S.; Molnar, D.; et al. Cortisone in hair of elementary school girls and its relationship with childhood stress. Eur. J. Pediatr. 2013, 172, 843–846. [Google Scholar] [CrossRef]
- Raul, J.S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Perogamvros, I.; Keevil, B.G.; Ray, D.W.; Trainer, P.J. Salivary cortisone is a potential biomarker for serum free cortisol. J. Clin. Endocr. Metab. 2010, 95, 4951–4958. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Alexander, N.; Bornstein, S.R.; Gao, W.; Miller, R.; Stark, S.; Bosch, J.A.; Fischer, J.E. Cortisol in hair and the metabolic syndrome. J. Clin. Endocr. Metab. 2013, 98, 2573–2580. [Google Scholar] [CrossRef]
- Stubsjøen, S.M.; Bohlin, J.; Dahl, E.; Knappe-Poindecker, M.; Fjeldaas, T.; Lepschy, M.; Palme, R.; Langbein, J.; Ropstad, E. Assessment of chronic stress in sheep (part I): The use of cortisol and cortisone in hair as non-invasive biological markers. Small Ruminant Res. 2015, 132, 25–31. [Google Scholar] [CrossRef]
- Botía, M.; Escribano, D.; Tecles, F.; Martínez-Sbuiela, S.; Cerón, J.J.; López-Arjona, M. Changes in cortisol, cortisone and 11β-hydrozysteroid dehydrogenase type II activity in saliva during pregnancy and lactation in sow. Dom. Anim. Endocr. 2024, 89, 106875. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Within a hair’s breadth–Factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocr. 2020, 288, 113359. [Google Scholar] [CrossRef]
- Kalliokoski, O.; Jellestad, F.K.; Murison, R. A systematic review of studies utilizing hair glucocorticoids as a measure of stress suggests the marker is more appropriate for quantifying short-term stressors. Sci. Rep. 2019, 9, 11997. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Warriss, P.D. Stress physiology of animals during transport. In Livestock Handling and Transport, 2nd ed.; Grandin, T., Ed.; CAB International: Oxon, UK, 2000; pp. 385–407. [Google Scholar]
- Fazio, E.; Ferlazzo, A. Evaluation of stress during transport. Vet. Res. Commun. 2003, 27, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J. Effects of transport on health of farm animals. Vet. Res. Commun. 2003, 27, 525–527. [Google Scholar] [CrossRef]
- Nielsen, B.L.; Dybkjær, L.; Herskin, M.S. Road transport of farm animals: Effects of journey duration on animal welfare. Animal 2011, 5, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T.; Shivley, C. How farm animals react and perceive stressful situations such as handling, restraint, and transport. Animals 2015, 5, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M.; Dickens, M.J.; Cyr, N.E. The reactive scope model—A new model integrating homeostasis, allostasis, and stress. Horm. Behav. 2009, 55, 375–389. [Google Scholar] [CrossRef]
- Eguizábal, G.V.; Superina, M.; Palme, R.; Asencio, C.J.; Villarreal, D.P.; Borrelli, L.; Busso, J.M. Non-invasive assessment of the seasonal stress response to veterinary procedures and transportation of zoo-housed lesser anteater (Tamandua tetradactyla). Animals 2022, 12, 75. [Google Scholar] [CrossRef]
- Vitousek, M.N.; Taff, C.C.; Ryan, T.A.; Zimmer, C. Stress resilience and the dynamic regulation of glucocorticoids. Integr. Comp. Biol. 2019, 59, 251–263. [Google Scholar] [CrossRef]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien. Tierärztl. Monat.–Vet. Med. Austria 2013, 100, 238–246. [Google Scholar]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocr. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Palme, R.; Möstl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Int. J. Mammal. Biol. 1997, 62, 192–197. [Google Scholar]
- Rettenbacher, S.; Möstl, E.; Hackl, R.; Ghareeb, K.; Palme, R. Measurement of corticosterone metabolites in chicken droppings. Brit. Poultry Sci. 2004, 45, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G. InfoStat Version. 2019. InfoStat Group, FCA, National University of Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 26 August 2023).
- Möstl, E.; Messmann, S.; Bagu, E.; Robia, C.; Palme, R. Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. J. Vet. Med. Series A 1999, 46, 621–631. [Google Scholar]
- Werner, C.; Reiners, K.; Wicke, M. Short as well as long transport duration can affect the welfare of slaughter pigs. Anim. Welf. 2007, 16, 385–389. [Google Scholar] [CrossRef]
- Goymann, W. Noninvasive monitoring of hormones in bird droppings: Physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Annals N. Y. Acad. Sci. 2005, 1046, 35–53. [Google Scholar] [CrossRef]
- Keckeis, K.; Lepschy, M.; Schöpper, H.; Moser, L.; Troxler, J.; Palme, R. Hair cortisol: A parameter of chronic stress? Insights from a radiometabolism study in guinea pigs. J. Comp. Physiol. B 2012, 182, 985–996. [Google Scholar] [CrossRef]
- Ito, N.; Ito, T.; Kromminga, A.; Bettermann, A.; Takigawa, M.; Kees, F.; Straub, R.H.; Paus, R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. FASEB J. 2005, 19, 1332–1334. [Google Scholar] [CrossRef]
- Skarlandtová, H.; Bičíková, M.; Neužil, P.; Mlček, M.; Hrachovina, V.; Svoboda, T.; Medová, E.; Kudlička, J.; Dohnalová, A.; Havránek, S.; et al. The Cortisol to Cortisone Ratio during Cardiac Catheterisation in Sows. Prague Med. Rep. 2015, 116, 279–289. [Google Scholar] [CrossRef]
- Wiechers, D.H.; Brunner, S.; Herbrandt, S.; Kemper, N.; Fels, M. Analysis of hair cortisol as an indicator of chronic stress in pigs in two different farrowing systems. Front. Vet. Sci. 2021, 8, 605078. [Google Scholar] [CrossRef]
- Stubsjøen, S.M.; Sørheim, K.; Chincarini, M.; Bohlin, J.; Brunberg, E.; Fuchs, B.; Palme, R.; Grøva, L. Exploring hair cortisone concentration as a novel tool to assess chronic stress in sheep with tick-borne fever. Small Ruminant Res. 2018, 164, 110–119. [Google Scholar] [CrossRef]
- Botía, M.; Escribano, D.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Tecles, F.; López-Arjona, M.; Cerón, J.J. Different types of glucocorticoids to evaluate stress and welfare in animals and humans: General concepts and examples of combined use. Metabolites 2023, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Escribano, D.; Contreras-Jodar, A.; López-Arjona, M.; Cerón, J.J.; Fàbrega, E.; Aymerich, P.; Dalmau, A. Changes in cortisol and cortisone in hair of pigs reared under heat stress conditions. Front. Vet. Sci. 2023, 10, 1156480. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Novak, M.A. Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 2012, 153, 4120–4127. [Google Scholar] [CrossRef] [PubMed]
- Piérard-Franchimont, C.; Quatresooz, P.; Piérard, G.E. Effect of UV radiation on scalp and hair growth. In Aging Hair; Trüeb, R.M., Tobin, D.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 113–121. [Google Scholar]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and mechanistic approach to the hair growth cycle and hair loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Otten, W.; Bartels, T.; Heimbürge, S.; Tuchscherer, A.; Kanitz, E. The dark side of white hair? Artificial light irradiation reduces cortisol concentrations in white but not black hairs of cattle and pigs. Animal 2021, 15, 100230. [Google Scholar] [CrossRef]
- Uetake, K.; Morita, S.; Sakagami, N.; Yamamoto, K.; Hashimura, S.; Tanaka, T. Hair cortisol levels of lactating dairy cows in cold-and warm-temperate regions in Japan. Anim. Sci. J. 2018, 89, 494–497. [Google Scholar] [CrossRef]
- Banse, H.E.; Getachew, F.; Levy, M.; Smits, J. Influence of season and pituitary pars intermedia dysfunction on hair cortisol concentration in horses. Domest. Anim. Endocrinol. 2020, 72, 106375. [Google Scholar] [CrossRef]
- Olvera-Maneu, S.; Carbajal, A.; Gardela, J.; Lopez-Bejar, M. Hair cortisol, testosterone, dehydroepiandrosterone sulfate and their ratios in stallions as a retrospective measure of hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes activity: Exploring the influence of seasonality. Animals 2021, 11, 2202. [Google Scholar] [CrossRef]
- Palme, R.; Robia, C.; Baumgartner, W.; Möstl, E. Transport stress in caftle as reflected by an increase in faecal cortisol metabolite concentrations. Vet. Rec. 2000, 146, 108. [Google Scholar] [CrossRef]
- Möstl, E.; Maggs, J.L.; Schrötter, G.; Besenfelder, U.; Palme, R. Measurement of cortisol metabolites in faeces of ruminants. Vet. Res. Commun. 2002, 26, 127–139. [Google Scholar] [CrossRef]
- Schmidt, A.; Möstl, E.; Wehnert, C.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release and heart rate variability in horses during road transport. Horm. Behav. 2010, 57, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Meunier-Salaün, M.C.; Brulaud, F.; Monnier, M.; Mormède, P. Assessment of hypothalamic-pituitary-adrenal axis and sympathetic nervous system activity in pregnant sows through the measurement of glucocorticoids and catecholamines in urine. J. Anim. Sci. 2000, 78, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.L.; Carter, C.S.; Garner, J.P.; Marchant-Forde, J.N.; Richert, B.T.; Lay, D.C., Jr. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav. 2013, 112, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.L.; Plush, K.; Yawno, T.; Langendijk, P. Allopregnanolone and social stress: Regulation of the stress response in early pregnancy in pigs. Stress 2015, 18, 569–577. [Google Scholar] [CrossRef]
- Bradshaw, R.H.; Parrott, R.F.; Forsling, M.L.; Goode, J.A.; Lloyd, D.M.; Rodway, R.G.; Broom, D.M. Stress and travel sickness in pigs: Effects of road transport on plasma concentrations of cortisol, beta-endorphin and lysine vasopressin. Anim. Sci. 1996, 63, 507–516. [Google Scholar] [CrossRef]

| Treatment 1 | ||||
| Transported (T) | Not Transported (NT) | |||
| Treatment 2 | Dexamethasone (D) | 5♀ & 5♂ = treatments 1 + 2 group | 5♀ & 5♂ = treatment 2 group | 20 pigs |
| Saline solution (SS) | 4♀ & 4♂ = treatment 1 group | 4♀ & 4♂ = control group | 16 pigs | |
| 18 pigs | 18 pigs | 36 pigs | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asencio, C.J.; Palme, R.; Ferrari, H.R.; Lattanzi, M.L.; Eguizábal, G.V.; Busso, J.M. Faecal Glucocorticoid Metabolites and Hair Cortisone/Cortisol Measurements in Domestic Pigs Exposed to Road Transportation and Dexamethasone Treatment. Animals 2024, 14, 2700. https://doi.org/10.3390/ani14182700
Asencio CJ, Palme R, Ferrari HR, Lattanzi ML, Eguizábal GV, Busso JM. Faecal Glucocorticoid Metabolites and Hair Cortisone/Cortisol Measurements in Domestic Pigs Exposed to Road Transportation and Dexamethasone Treatment. Animals. 2024; 14(18):2700. https://doi.org/10.3390/ani14182700
Chicago/Turabian StyleAsencio, Camila J., Rupert Palme, Héctor R. Ferrari, Mariano L. Lattanzi, Gabina V. Eguizábal, and Juan M. Busso. 2024. "Faecal Glucocorticoid Metabolites and Hair Cortisone/Cortisol Measurements in Domestic Pigs Exposed to Road Transportation and Dexamethasone Treatment" Animals 14, no. 18: 2700. https://doi.org/10.3390/ani14182700
APA StyleAsencio, C. J., Palme, R., Ferrari, H. R., Lattanzi, M. L., Eguizábal, G. V., & Busso, J. M. (2024). Faecal Glucocorticoid Metabolites and Hair Cortisone/Cortisol Measurements in Domestic Pigs Exposed to Road Transportation and Dexamethasone Treatment. Animals, 14(18), 2700. https://doi.org/10.3390/ani14182700







