Effects of Microencapsulated Essential Oils on Growth and Intestinal Health in Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Micro-Encapsulated Essential Oils
2.2. Experimental Design
2.3. Sample Collection
2.4. Growth Performance, Diarrhea, and Organ Index
2.5. Intestinal Tissue Morphology
2.6. Analysis of Antioxidant Capacity in Small Intestine
2.7. Determination of Short-Chain Fatty Acids
2.8. Bacterial DNA Extraction and 16S rDNA Gene Sequencing
2.9. Statistical Analysis
3. Results
3.1. Growth Performance, Diarrhea Score, and Organ Index
3.2. Intestinal Tissue Morphology and Structure
3.3. Intestinal Antioxidant Capacity
3.4. SCFA Concentrations in Colonic Content
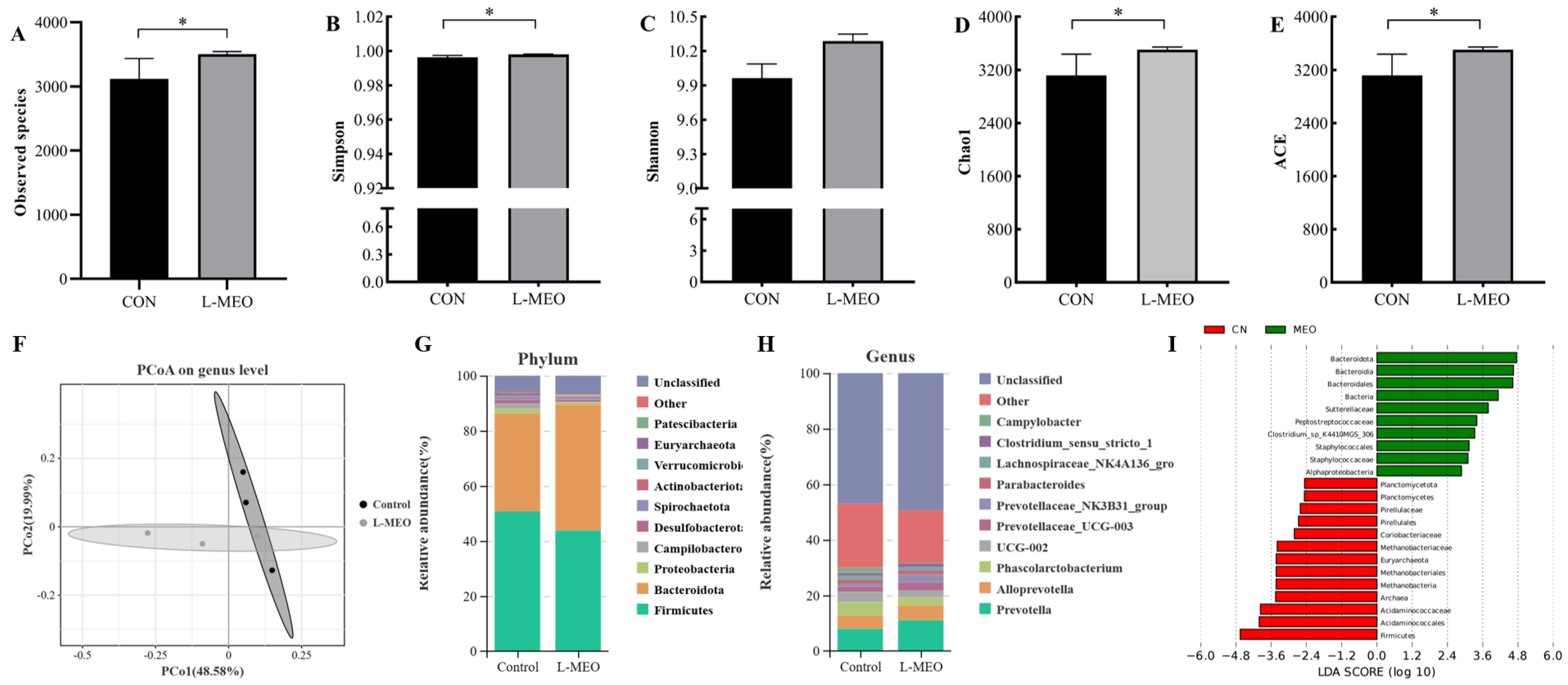
3.5. The Composition of Colonic Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peace, R.M.; Campbell, J.; Polo, J.; Crenshaw, J.; Russell, L.; Moeser, A. Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J. Nutr. 2011, 141, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, B.; Nyachoti, C.M. Husbandry practices and gut health outcomes in weaned piglets: A review. Anim. Nutr. 2017, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Cao, H.; Zhang, D.; Huang, J.; Li, J.; Wang, S.; Lu, J.; Li, X.; Yang, G.; Shi, X. Cordyceps militaris Modulates Intestinal Barrier Function and Gut Microbiota in a Pig Model. Front. Microbiol. 2022, 13, 810230–810243. [Google Scholar] [CrossRef]
- Xun, W.; Ji, M.; Ma, Z.; Deng, T.; Yang, W.; Hou, G.; Shi, L.; Cao, T. Dietary emodin alleviates lipopolysaccharide-induced intestinal mucosal barrier injury by regulating gut microbiota in piglets. Anim. Nutr. 2023, 14, 152–162. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; Alagawany, M.; Abdel-Moneim, A.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210–222. [Google Scholar] [CrossRef]
- Parameswari, M.N.; Kumar, P.S.; Latha, J.N.L. Antimicrobial activity of essential plant oils and their major components. Heliyon 2021, 7, e06835–e06849. [Google Scholar]
- Mo, K.; Yu, W.; Li, J.; Zhang, Y.; Xu, Y.; Huang, X.; Ni, H. Dietary supplementation with the microencapsulated complex of thymol, carvacrol, and cinnamaldehyde improves intestinal barrier function in weaning piglets. J. Sci. Food. Agr. 2022, 103, 1994–2003. [Google Scholar] [CrossRef]
- Zahra, K.F.; Dumitrita, S.G.; Lefter, R.; Cotea, V.V.; Niculaua, M.; Ababei, D.C.; Ciobica, A.; Ech-Chahad, A. Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil. Antioxidants 2022, 11, 347–365. [Google Scholar] [CrossRef]
- Dumitrescu, E.; Muselin, F.; Tîrziu, E.; Folescu, M.; Dumitrescu, C.S.; Orboi, D.M.; Cristina, R.T. Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants 2023, 12, 2428–2441. [Google Scholar] [CrossRef]
- Asadi-Yousefabad, S.H.; Mohammadi, S.; Ghasemi, S.; Saboktakin-Rizi, K.; Sahraeian, S.; Asadi, S.S.; Hashemi, M.; Ghaffari, H.R. Development of fortified milk with gelled-oil nanoparticles incorporated with cinnamaldehyde and tannic acid. LWT 2022, 154, 112652–112661. [Google Scholar] [CrossRef]
- Sun, J.; Leng, X.; Zang, J.; Zhao, G. Bio-based antibacterial food packaging films and coatings containing cinnamaldehyde: A review. Crit. Rev. Food. Sci. 2022, 64, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, T.; Hu, H.; Duan, X.; Wu, K.; Chai, X.; He, D. Trans-cinnamaldehyde fumigation inhibits Escherichia coli by affecting the mechanism of intracellular biological macromolecules. Nat. Prod. Res. 2024, 22, 1–12. [Google Scholar] [CrossRef]
- Field, D.; Baghou, I.; Rea, M.C.; Gardiner, G.E.; Ross, R.P.; Hill, C. Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia coli Strains of Swine Origin. Antibiotics 2017, 6, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Angélica, E.; Miriam, P.; Gustavo, R.; Guillermo, B. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961–990. [Google Scholar] [CrossRef]
- Cicalău, G.I.P.; Babes, P.A.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.M.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899–6928. [Google Scholar] [CrossRef]
- Froehlich, K.A.; Abdelsalam, K.W.; Chase, C.; Koppien-Fox, J.; Casper, D.P. Evaluation of essential oils and prebiotics for newborn dairy calves. J. Anim. Sci. 2017, 95, 3772–3782. [Google Scholar] [CrossRef]
- Swedzinski, C.; Froehlich, K.A.; Abdelsalam, K.W.; Chase, C.; Greenfield, T.J.; Koppien-Fox, J.; Casper, D.P. Evaluation of essential oils and a prebiotic for newborn dairy calves. Translational Anim. Sci. 2020, 4, 75–83. [Google Scholar] [CrossRef]
- Zhao, B.C.; Wang, T.H.; Chen, J.; Qiu, B.H.; Xu, Y.R.; Li, J.L. Essential oils improve nursery pigs’ performance and appetite via modulation of intestinal health and microbiota. Anim. Nutr. 2024, 16, 174–188. [Google Scholar] [CrossRef]
- Isabel, S.V.; Filipa, P.J.; Filipa, M.J.; Adriana, F.M.; José, T.C. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730–1772. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends. Food. Sci. Tech. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; de Sá Costa, B.; Siqueira, R.P.; Garcia-Rojas, E.E. Complex coacervates of β-lactoglobulin/sodium alginate for the microencapsulation of black pepper (Piper nigrum L.) essential oil: Simulated gastrointestinal conditions and modeling release kinetics. Int. J. Biol. Macromol. 2020, 160, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Bhandari, B.; Bai, B. Fennel essential oil loaded porous starch-based microencapsulation as an efficient delivery system for the quality improvement of ground pork. Int. J. Biol. Macromol. 2021, 172, 464–474. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends. Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Le Flocʹh, N.; Achard, C.S.; Eugenio, F.A.; Apper, E.; Combes, S.; Quesnel, H. Effect of live yeast supplementation in sow diet during gestation and lactation on sow and piglet fecal microbiota, health and performance. J. Anim. Sci. 2022, 100, 209–223. [Google Scholar] [CrossRef]
- Su, G.; Zhou, X.; Wang, Y.; Chen, D.; Chen, G.; Li, Y.; He, J. Dietary supplementation of plant essential oil improves growth performance, intestinal morphology and health in weaned pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 579–589. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef]
- Tan, B.F.; Lim, T.; Boontiam, W. Effect of dietary supplementation with essential oils and a Bacillus probiotic on growth performance, diarrhoea and blood metabolites in weaned pigs. Anim. Prod. Sci. 2021, 61, 64–71. [Google Scholar] [CrossRef]
- Van Noten, N.; Degroote, J.; Van Liefferinge, E.; Taminiau, B.; De Smet, S.; Desmet, T.; Michiels, J. Effects of Thymol and Thymol α-D-Glucopyranoside on Intestinal Function and Microbiota of Weaned Pigs. Animals 2020, 10, 329–350. [Google Scholar] [CrossRef]
- Zhai, H.; Luo, Y.; Ren, W.; Schyns, G.; Guggenbuhl, P. The effects of benzoic acid and essential oils on growth performance, nutrient digestibility, and colonic microbiota in nursery pigs. Anim. Feed. Sci. Tech. 2020, 262, 114426–114436. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, N.; Zhang, T.; Zhang, Y.; Dong, S.; Wang, H.; Xu, C.; Wang, C. Effects of Tetrabasic Zinc Chloride on the Diarrhea Rate, Intestinal Morphology, Immune Indices and Microflora of Weaned Piglets. Animals 2024, 14, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of Adding Essential Oil to the Diet of Weaned Pigs on Performance, Nutrient Utilization, Immune Response and Intestinal Health. Asian-Australas. J. Anim. Sci. 2012, 25, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Wang, L.; Liu, S.; Lu, P.; Zhao, X.; Liu, H.; Lahaye, L.; Santin, E.; Liu, S.; Nyachoti, M.; et al. Effects of a microencapsulated formula of organic acids and essential oils on nutrient absorption, immunity, gut barrier function, and abundance of enterotoxigenic Escherichia coli F4 (ETEC F4) in weaned piglets challenged with ETEC F4. J. Anim. Sci. 2020, 98, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Parma, L.; Yúfera, M.; Navarro-Guillén, C.; Moyano, F.J.; Soverini, M.; Amico, F.D.; Candela, M.; Fontanillas, R.; Gatta, P.P.; Bonaldo, A. Effects of calcium carbonate inclusion in low fishmeal diets on growth, gastrointestinal pH, digestive enzyme activity and gut bacterial community of European sea bass (Dicentrarchus labrax L.) juveniles. Aquaculture 2019, 510, 283–292. [Google Scholar] [CrossRef]
- Ying, M.; Zheng, B.; Yu, Q.; Hou, K.; Wang, H.; Zhao, M.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice. Food. Chem. Toxicol. 2020, 138, 111244–111253. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, H.; Mi, Y.; Xue, Y.; Li, J.; Ma, Y. Effects of essential oil coated with glycerol monolaurate on growth performance, intestinal morphology, and serum profiles in weaned piglets. Anim. Biosci. 2022, 36, 753–761. [Google Scholar] [CrossRef]
- Jiang, X.R.; Awati, A.; Agazzi, A.; Vitari, F.; Ferrari, A.; Bento, H.; Crestani, M.; Domeneghini, C.; Bontempo, V. Effects of a blend of essential oils and an enzyme combination on nutrient digestibility, ileum histology and expression of inflammatory mediators in weaned piglets. Animal 2014, 9, 417–426. [Google Scholar] [CrossRef]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384–1405. [Google Scholar] [CrossRef]
- Novais, A.K.; Deschêne, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.P.; Lessard, M.; Matte, J.; Lapointe, J. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PloS. One. 2021, 16, e0247188–e0247209. [Google Scholar] [CrossRef]
- Zou, Y.; Xiang, Q.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Improves Intestinal Morphology and Expression of Tight Junction Proteins Associated with Modulation of Selected Intestinal Bacteria and Immune Status in a Pig Model. Biomed. Res. Int. 2016, 2016, 5436738–5436749. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.Y.; Piao, X. Essential Oil Blend Could Decrease Diarrhea Prevalence by Improving Antioxidative Capability for Weaned Pigs. Animals 2019, 9, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Long, S.; Wang, J.; Gao, J.; Piao, X. Microencapsulated essential oils combined with organic acids improves immune antioxidant capacity and intestinal barrier function as well as modulates the hindgut microbial community in piglets. J. Anim. Sci. Biotechno. 2022, 13, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Chen, Q.; Luo, L.; Ma, M.; Xiao, B.; Zeng, L. Camellia sinensis and Litsea coreana Ameliorate Intestinal Inflammation and Modulate Gut Microbiota in Dextran Sulfate Sodium-Induced Colitis Mice. Mol. Nutr. Food. Res. 2020, 64, e1900943–e1900954. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2020, 98, 133–143. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Yin, Y.; Huang, P.; Jiang, Q.; Liu, Z.; Yin, Y.; Chen, J. Dietary Litsea cubeba essential oil supplementation improves growth performance and intestinal health of weaned piglets. Anim. Nutr. 2023, 13, 9–18. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320–354. [Google Scholar] [CrossRef]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poultry. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Gao, J.; Ma, J.; Li, T.; Tan, B.; Huang, X.; Yin, J. Opportunities of prebiotics for the intestinal health of monogastric animals. Anim. Nutr. 2020, 6, 379–388. [Google Scholar] [CrossRef]
- Mo, K.; Li, J.; Liu, F.; Xu, Y.; Huang, X.; Ni, H. Superiority of Microencapsulated Essential Oils Compared With Common Essential Oils and Antibiotics: Effects on the Intestinal Health and Gut Microbiota of Weaning Piglet. Front. Nutr. 2022, 8, 808106–808120. [Google Scholar] [CrossRef]
- Peng, W.; He, C.X.; Li, R.L.; Qian, D.; Wang, L.Y.; Chen, W.W.; Wu, C.J.; Zhang, Q. Zanthoxylum bungeanum amides ameliorates nonalcoholic fatty liver via regulating gut microbiota and activating AMPK/Nrf2 signaling. J. Ethnopharmacol. 2023, 318, 116848–116863. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liang, J.; Li, X.; Wang, Y.; Zhang, X.; Chen, D.; Wu, L.; Wang, S. Dahuang zhechong pill ameliorates hepatic fibrosis by regulating gut microbiota and metabolites. J. Ethnopharmacol. 2023, 321, 117402–117422. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Lantz, H.; Munyaka, P.M.; Willing, B.P. Prevotella in Pigs: The Positive and Negative Associations with Production and Health. Microorganisms 2020, 8, 1584–1606. [Google Scholar] [CrossRef]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.R.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Micr. 2013, 63, 1214–1218. [Google Scholar] [CrossRef]
- Yang, X.; He, Z.; Hu, R.; Yan, J.; Zhang, Q.; Li, B.; Yuan, X.; Zhang, H.; He, J.; Wu, S. Dietary β-Carotene on Postpartum Uterine Recovery in Mice: Crosstalk Between Gut Microbiota and Inflammation. Front. Immunol. 2021, 12, 744425–744435. [Google Scholar] [CrossRef]
- Yu, D.; Zhu, W.; Hang, S. Effects of low-protein diet on the intestinal morphology, digestive enzyme activity, blood urea nitrogen, and gut microbiota and metabolites in weaned pigs. Arch. Anim. Nutr. 2019, 73, 287–305. [Google Scholar] [CrossRef]

| Items | Content | Items | Content |
|---|---|---|---|
| Composition of ingredients | Nutrient levels 2 | ||
| Corn | 59 | Net energy/(MJ·kg−1) 3 | 11.07 |
| Soybean meal | 6 | Crude protein | 17.59 |
| Extruded soybean | 14 | Crude fat | 6.63 |
| Soybean oil | 1.5 | Crude ash | 5.97 |
| Dried whey | 5 | Total calcium | 0.55 |
| Fermented soybean meal | 4 | Total phosphorus | 0.55 |
| Montmorillonite | 0.5 | Digestible lysine | 1.22 |
| Premix 1 | 10 | ||
| Total | 100 |
| Item | Treatments 1 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| CON | L-MEO | M-MEO | H-MEO | |||
| ADG(g/d) 2 | 212.02 b | 281.25 a | 254.4 ab | 256.55 ab | 10.28 | 0.045 |
| ADFI(g/d) 2 | 383.65 | 431.12 | 402.73 | 403.17 | 9.26 | 0.120 |
| FCR2 | 1.81 a | 1.54 b | 1.61 ab | 1.57 b | 0.04 | 0.037 |
| Diarrhea scores (%) | 26.36 a | 14.03 b | 10b | 11.52 b | 2.23 | 0.001 |
| Organ index (g/kg) | ||||||
| Heart index | 5.04 | 5.05 | 5.08 | 5.21 | 0.12 | 0.677 |
| Liver index | 22.31 b | 22.33 b | 25.65 a | 25.71 a | 0.58 | 0.023 |
| Spleen index | 1.76 | 1.77 | 1.75 | 2.22 | 0.09 | 0.100 |
| Kidneys index | 5.59 ab | 5.27 ab | 5.2 b | 6.93 a | 0.3 | 0.018 |
| Pancreas index | 2.62 | 2.5 | 2.52 | 2.82 | 0.1 | 0.335 |
| Lungs index | 15.37 | 14.32 | 17.92 | 15.19 | 1.21 | 0.395 |
| Item | Treatments 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| CON | L-MEO | M-MEO | H-MEO | |||
| Intestine length, cm | ||||||
| Duodenum length | 35.33 | 37.77 | 37.63 | 36.83 | 2.7 | 0.803 |
| Jejunum length | 310.1 b | 397.53 a | 368.2 a | 350.57 ab | 11.73 | 0.027 |
| Ileum length | 420.7 | 480.93 | 478.8 | 437.73 | 11.15 | 0.063 |
| Small intestine length | 766.13 b | 916.23 a | 884.63 a | 825.13 ab | 22.46 | 0.032 |
| Villus height, μm | ||||||
| Duodenum | 322.16 | 346.23 | 335.64 | 322.57 | 5.05 | 0.130 |
| Jejunum | 310.21 b | 367.1 a | 369.9 a | 329.54 ab | 9.6 | 0.039 |
| Ileum | 268.14 b | 375.33 a | 331.64 a | 349.37 a | 14.06 | 0.013 |
| Crypt depth, μm | ||||||
| Duodenum | 254.16 | 228.88 | 223.79 | 227.32 | 5.72 | 0.088 |
| Jejunum | 231.34 | 197.24 | 224.04 | 218.81 | 7.98 | 0.205 |
| Ileum | 198.75 | 202.46 | 186.93 | 239.66 | 9.09 | 0.062 |
| Villi height/Crypt depth | ||||||
| Duodenum | 1.27 b | 1.52 a | 1.50 a | 1.42 a | 0.03 | 0.006 |
| Jejunum | 1.35 b | 1.86 a | 1.68 ab | 1.53 sb | 0.08 | 0.047 |
| Ileum | 1.35 b | 1.86 a | 1.79 ab | 1.48 ab | 0.08 | 0.033 |
| Item | Treatments 1 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| CON | L-MEO | M-MEO | H-MEO | |||
| Duodenum | ||||||
| T-SOD (U/mg prot) 2 | 67.22 | 59.32 | 66.22 | 64.03 | 2.68 | 0.398 |
| MDA (nmol/mg prot) 2 | 2.13 | 0.91 | 2.02 | 2.18 | 0.27 | 0.146 |
| T-AOC (nmol/mg prot) 2 | 0.07 | 0.07 | 0.08 | 0.08 | 0.003 | 0.078 |
| CAT (U/mg prot) 2 | 2.38 | 3.02 | 2.97 | 3.18 | 0.16 | 0.115 |
| Jejunum | ||||||
| T-SOD (U/mg prot) | 37.12 | 48.71 | 44.83 | 44.94 | 2.09 | 0.083 |
| MDA (nmol/mg prot) | 1.69 b | 0.74 c | 1.72 b | 4.4 a | 0.43 | p < 0.001 |
| T-AOC (nmol/mg prot) | 0.035 b | 0.058 a | 0.049 ab | 0.044 ab | 0.003 | 0.011 |
| CAT (U/mg prot) | 1.95 | 3.12 | 2.9 | 2.93 | 0.27 | 0.198 |
| Ileum | ||||||
| T-SOD (U/mg prot) | 54.14 | 69.04 | 58.52 | 58.94 | 3.15 | 0.154 |
| MDA (nmol/mg prot) | 2.92 a | 2.56 ab | 1.8 b | 2.10 ab | 0.19 | 0.039 |
| T-AOC (nmol/mg prot) | 0.037 b | 0.083 a | 0.06 ab | 0.055 ab | 0.007 | 0.015 |
| CAT (U/mg prot) | 1.91 b | 2.91 a | 2.67 a | 2.66 a | 0.14 | 0.033 |
| Item | Treatments 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| Con | L-MEO | M-MEO | H-MEO | |||
| Acetic acid, mg/g | 0.255 | 0.262 | 0.288 | 0.319 | 0.019 | 0.333 |
| Propionic acid, mg/g | 0.185 | 0.153 | 0.179 | 0.234 | 0.015 | 0.098 |
| Butyric acid, mg/g | 0.075 | 0.062 | 0.062 | 0.095 | 0.009 | 0.279 |
| Isobutyric acid, mg/g | 0.047 b | 0.069 ab | 0.086 ab | 0.116 a | 0.01 | 0.012 |
| Valeric acid, mg/g | 0.013 | 0.011 | 0.013 | 0.019 | 0.01 | 0.184 |
| Isovaleric acid, mg/g | 0.077 | 0.055 | 0.068 | 0.090 | 0.007 | 0.096 |
| Total SCFAs, mg/g | 0.651 | 0.612 | 0.696 | 0.872 | 0.055 | 0.148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Dai, Z.; Zhang, Y.; Wu, S.; Liu, L.; Wang, K.; Shen, D.; Li, C. Effects of Microencapsulated Essential Oils on Growth and Intestinal Health in Weaned Piglets. Animals 2024, 14, 2705. https://doi.org/10.3390/ani14182705
Chen K, Dai Z, Zhang Y, Wu S, Liu L, Wang K, Shen D, Li C. Effects of Microencapsulated Essential Oils on Growth and Intestinal Health in Weaned Piglets. Animals. 2024; 14(18):2705. https://doi.org/10.3390/ani14182705
Chicago/Turabian StyleChen, Ketian, Zhiqi Dai, Yijian Zhang, Sheng Wu, Le Liu, Kai Wang, Dan Shen, and Chunmei Li. 2024. "Effects of Microencapsulated Essential Oils on Growth and Intestinal Health in Weaned Piglets" Animals 14, no. 18: 2705. https://doi.org/10.3390/ani14182705
APA StyleChen, K., Dai, Z., Zhang, Y., Wu, S., Liu, L., Wang, K., Shen, D., & Li, C. (2024). Effects of Microencapsulated Essential Oils on Growth and Intestinal Health in Weaned Piglets. Animals, 14(18), 2705. https://doi.org/10.3390/ani14182705







