Melatonin Regulates the Expression of VEGF and HOXA10 in Bovine Endometrial Epithelial Cells through the SIRT1/PI3K/AKT Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Reagents and Chemicals
2.3. Cell Culture and Treatment
2.4. CCK8
2.5. Real-Time Quantitative PCR Analysis
2.6. Western Blot Analysis
2.7. Immunofluorescence Assay
2.8. miRNA Mimic, miRNA Inhibitor, and Transfection
2.9. Statistical Analysis
3. Results
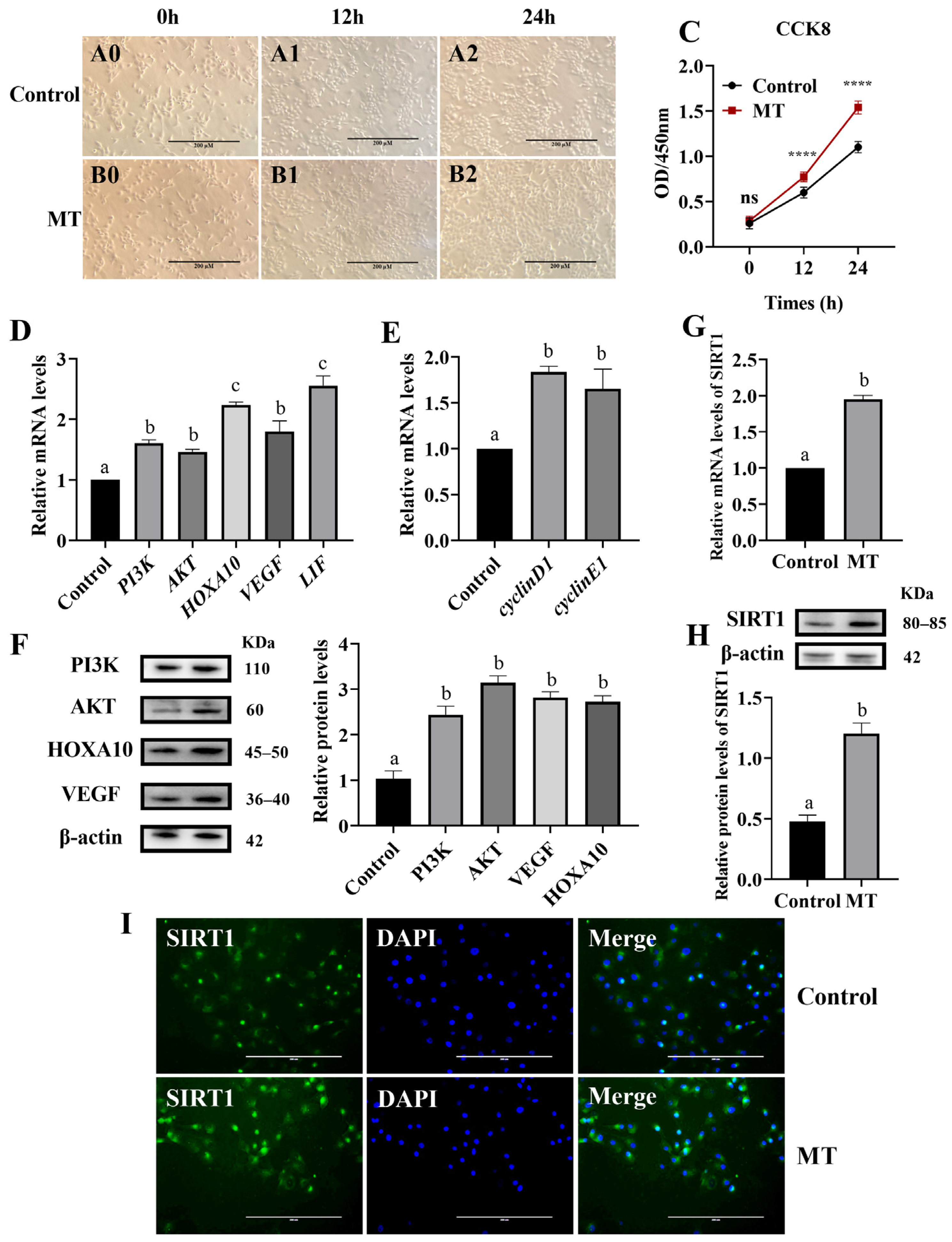
3.1. Effects of Melatonin on BEEC Proliferation and Genes Expression
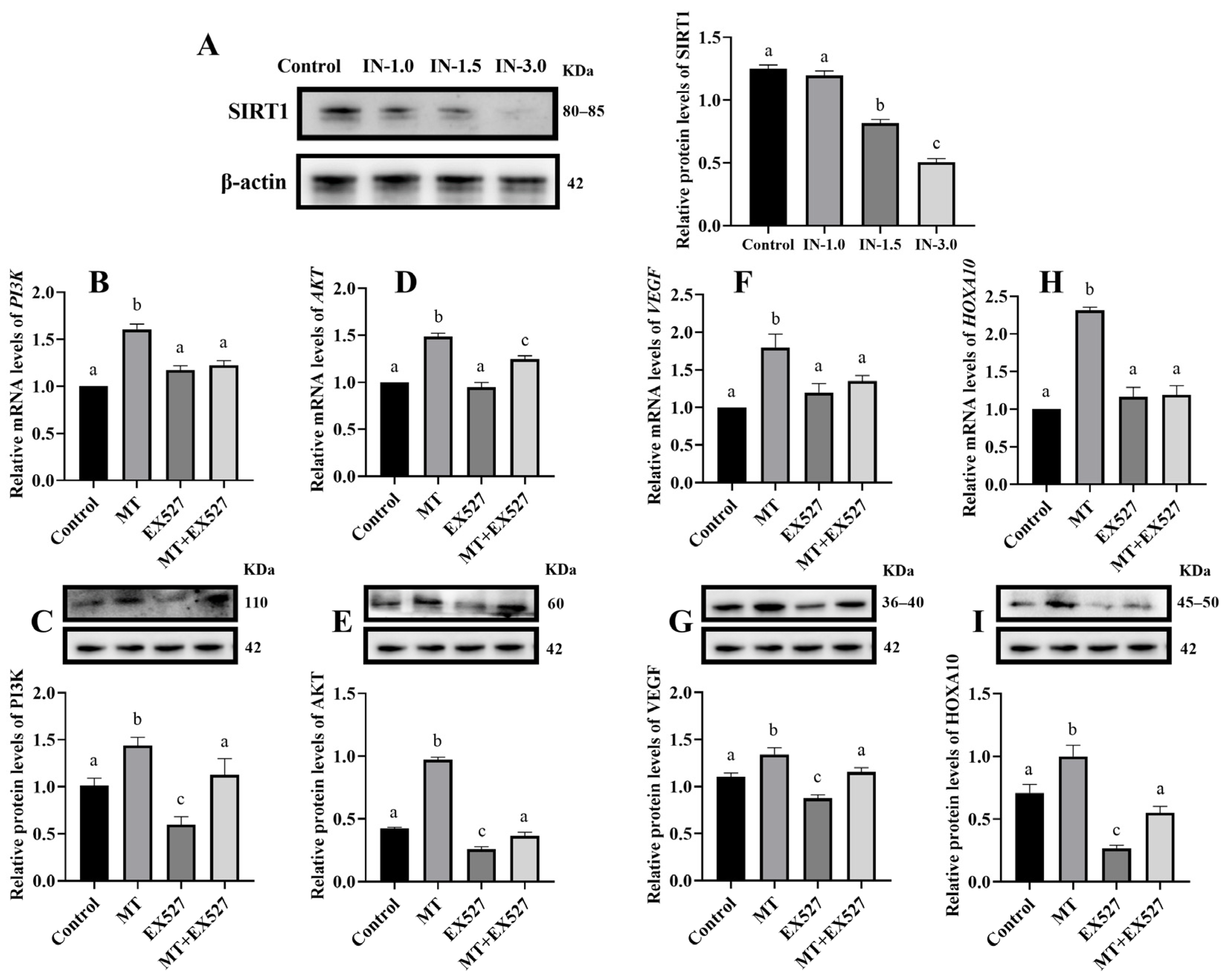
3.2. Melatonin Regulates Gene Expression in Cells via SIRT1
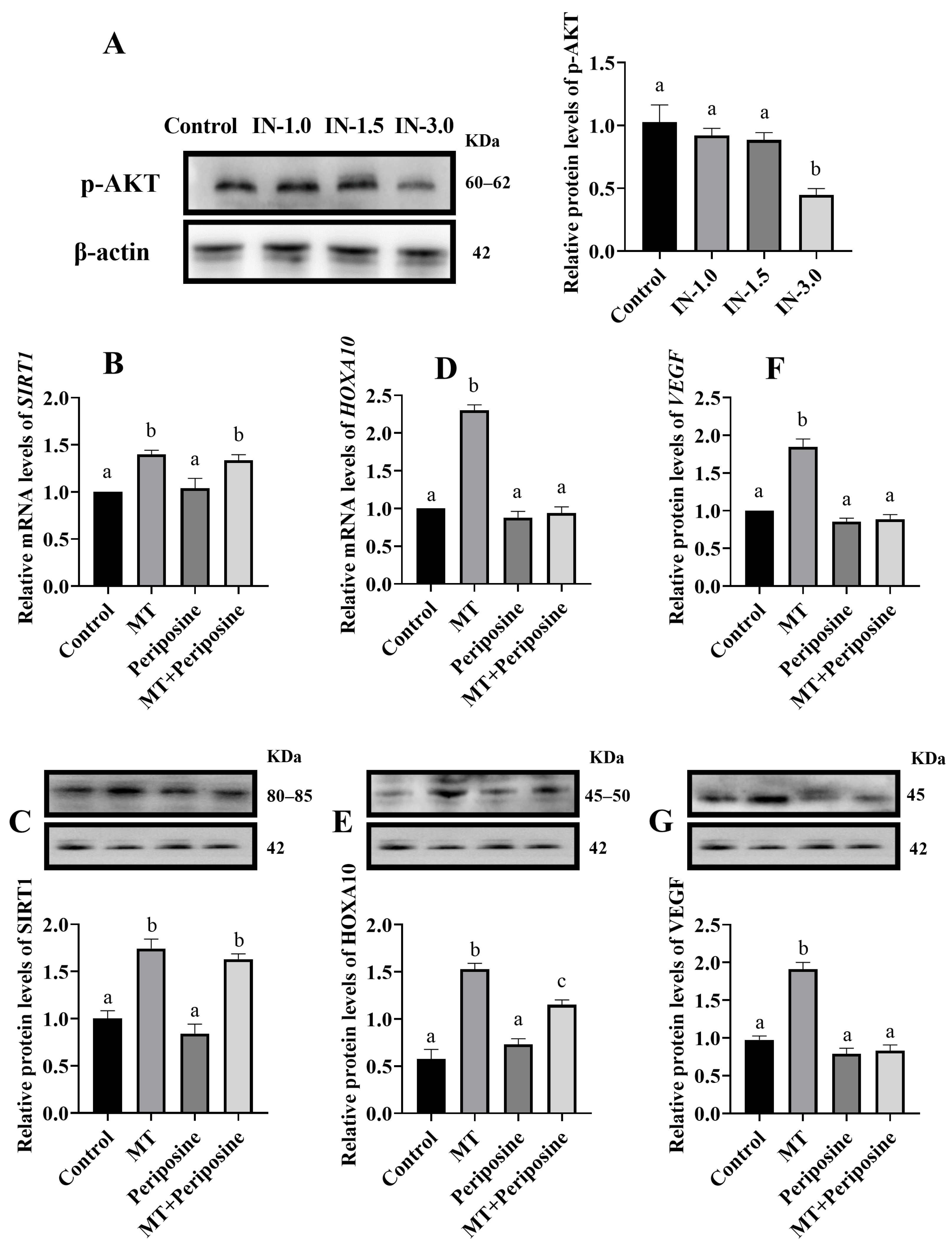
3.3. Melatonin Regulates Cellular VEGF and HOXA10 Gene Expression through AKT
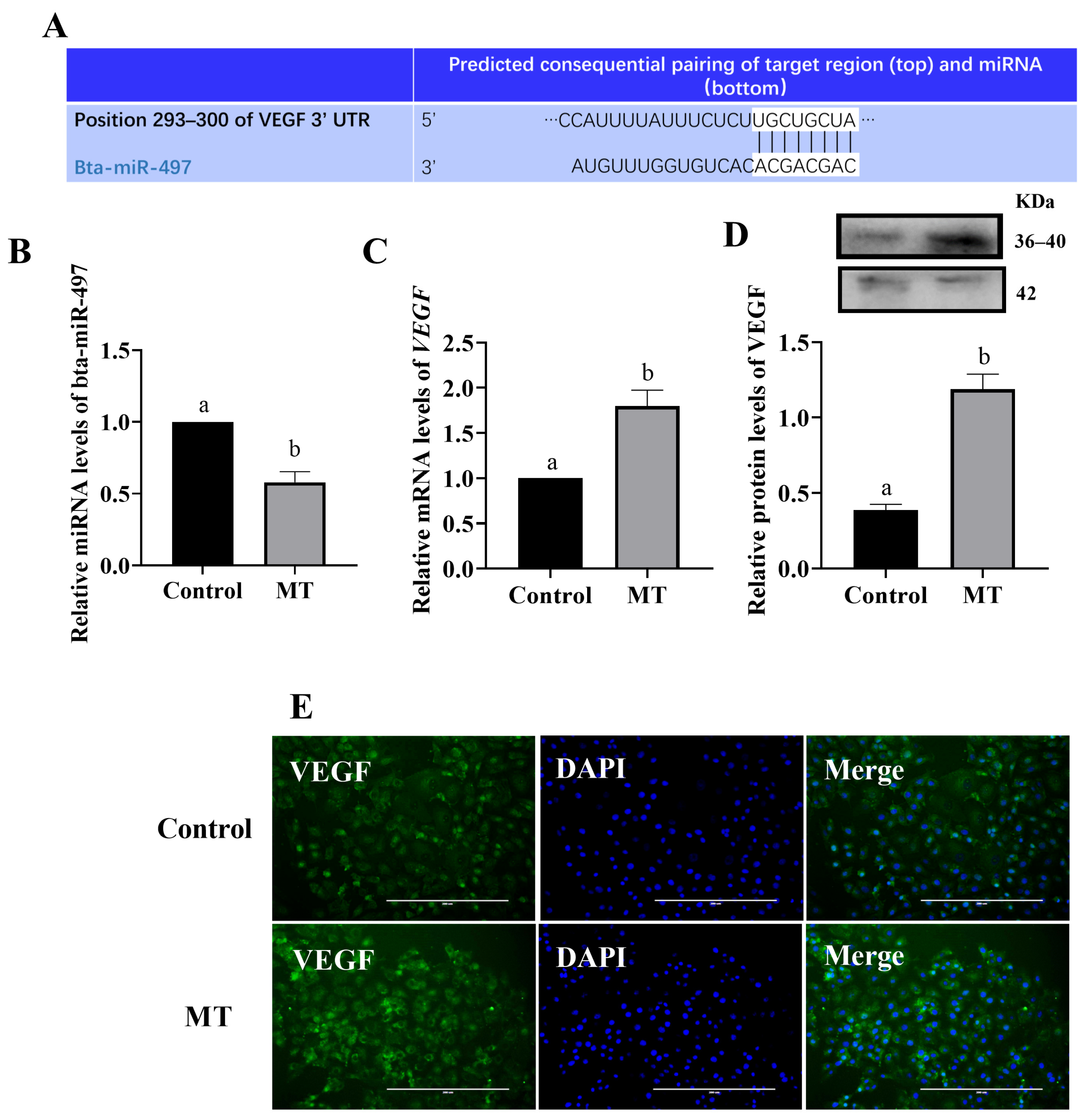
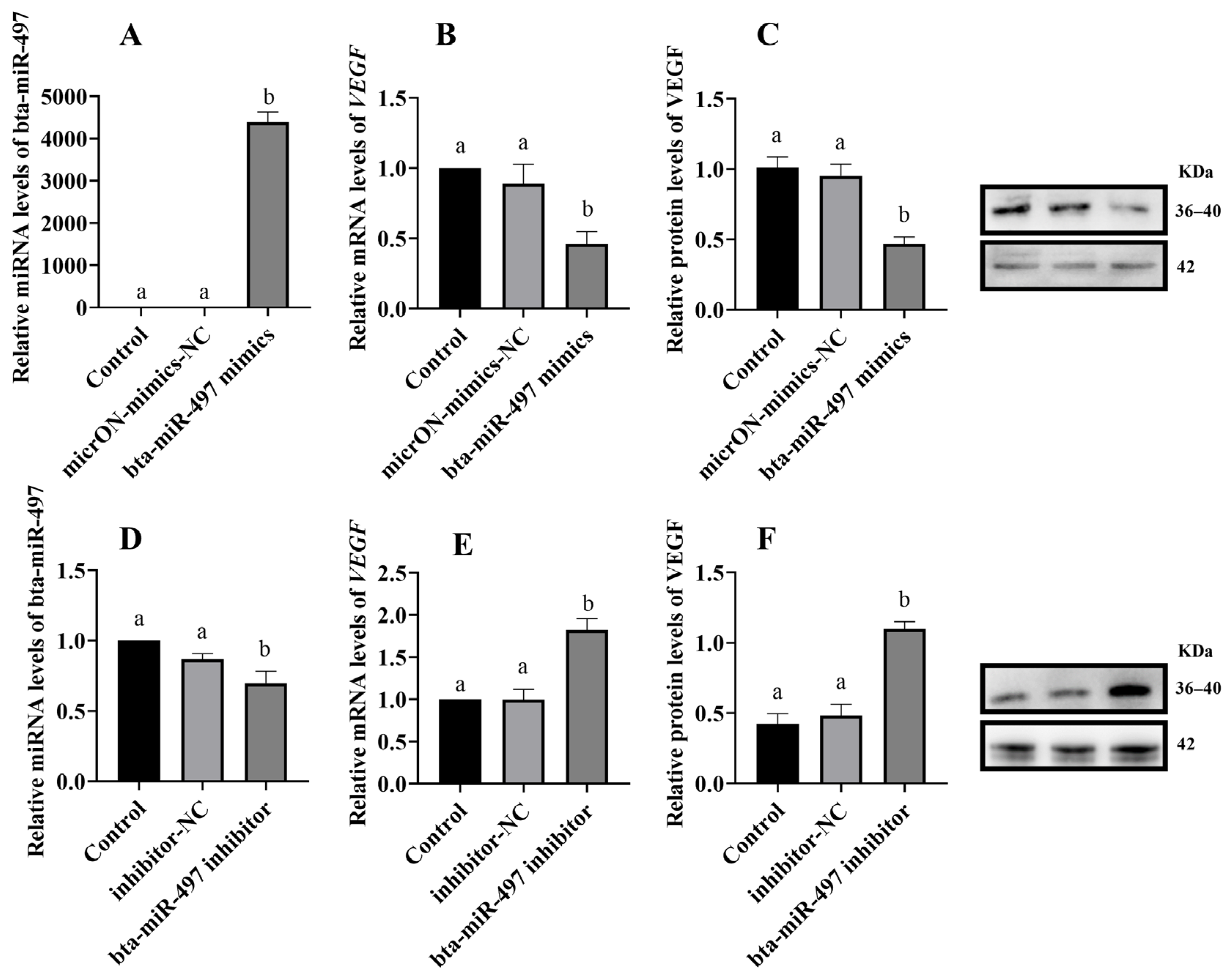
3.4. Melatonin Regulates VEGF Expression through miRNA
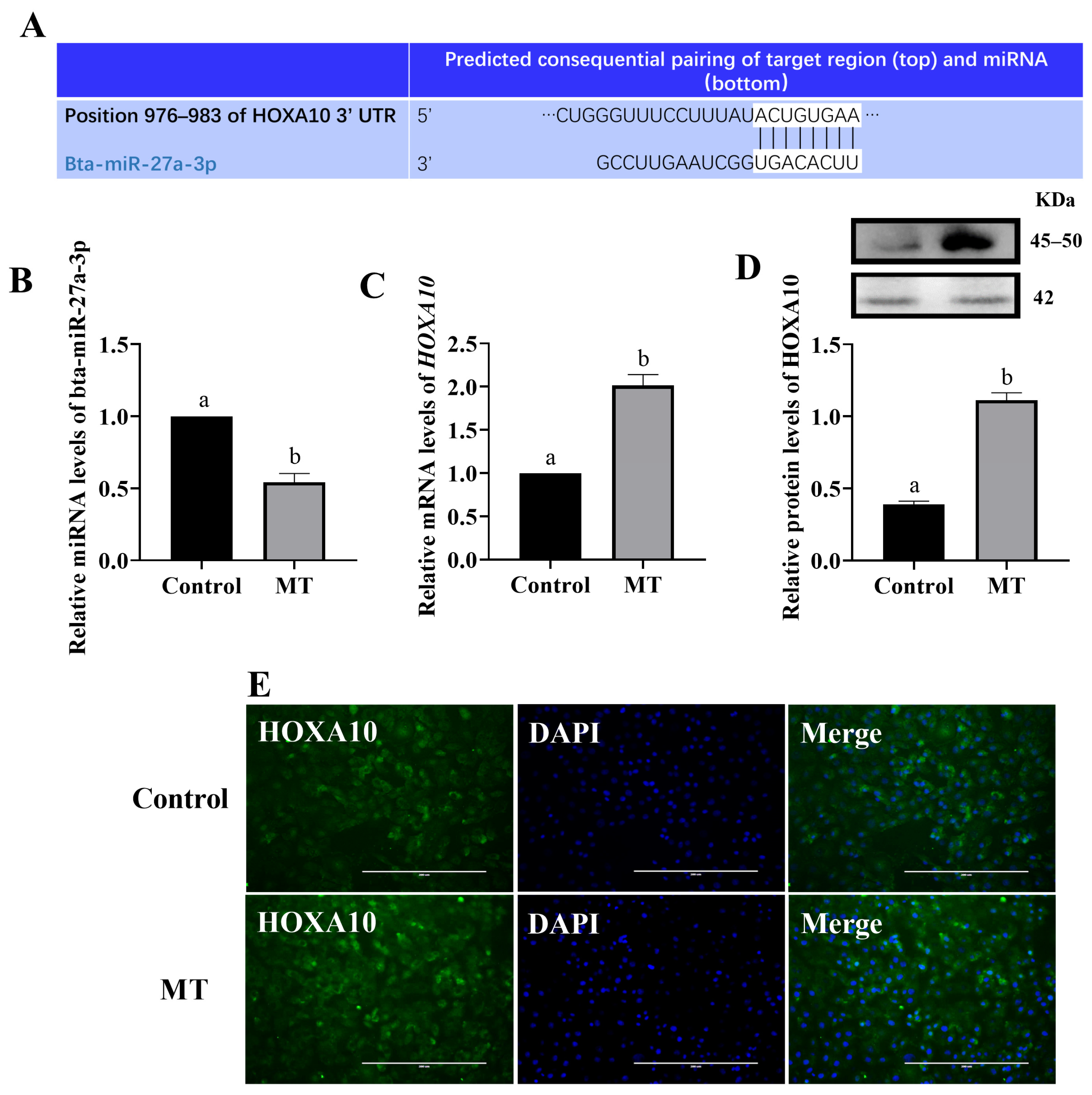
3.5. Melatonin Regulated HOXA10 Expression through miRNA
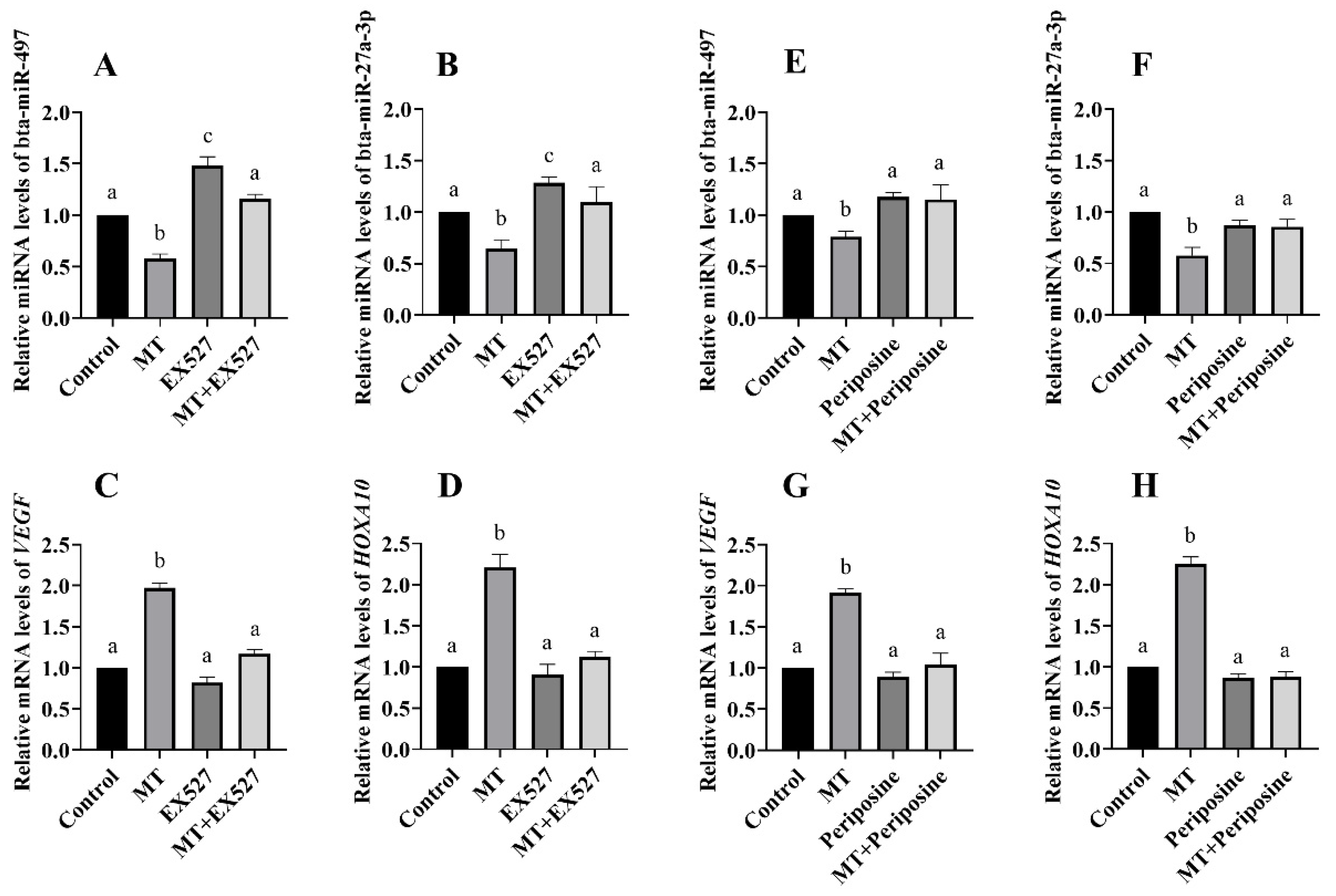
3.6. Regulatory Relationship between SIRT1, AKT, and miRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Chen, S.R.; Wang, Z.P.; Zhang, Y.; Tang, J.X.; Li, J.; Wang, X.X.; Cheng, J.M.; Jin, C.; Li, X.Y.; et al. Melatonin promotes development of haploid germ cells from early developing spermatogenic cells of Suffolk sheep under in vitro condition. J. Pineal Res. 2016, 60, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Take, G.; Kaplanoglu, I.; Erdogan, D.; Seymen, C.M. Therapeutic effects of melatonin in long-term exposure to 2100 MHz radiofrequency radiation on rat sperm characteristics. Rev. Int. Androl. 2023, 21, 100371. [Google Scholar] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Corral, S.A.R. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tamura, H.; Tan, D.X.; Xu, X.Y. Melatonin and the circadian system: Contributions to successful female reproduction. Fertil. Steril. 2014, 102, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, E.A.; Abo El-Maaty, A.M. Melatonin and CIDR improved the follicular and luteal haemodynamics, uterine and ovarian arteries vascular perfusion, ovarian hormones and nitric oxide in cyclic cows. Reprod. Domest. Anim. 2021, 56, 498–510. [Google Scholar] [CrossRef]
- Pillai, V.V.; Siqueira, L.G.; Das, M.; Kei, T.G.; Tu, L.N.; Herren, A.W.; Phinney, B.S.; Cheong, S.H.; Hansen, P.J.; Selvaraj, V. Physiological profile of undifferentiated bovine blastocyst-derived trophoblasts. Biol. Open 2019, 8, bio037937. [Google Scholar] [CrossRef]
- Turner, M.L.; Cronin, J.G.; Healey, G.D.; Sheldon, I.M. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014, 155, 1453–1465. [Google Scholar] [CrossRef]
- Chakraborty, I.; Das, S.K.; Dey, S.K. Differential expression of vascular endothelial growth factor and its receptor mRNAs in the mouse uterus around the time of implantation. J. Endocrinol. 1995, 147, 339–352. [Google Scholar] [CrossRef]
- Jakeman, L.B.; Armanini, M.; Phillips, H.S.; Ferrara, N. Developmental expression of binding sites and messenger ribonucleic acid for vascular endothelial growth factor suggests a role for this protein in vasculogenesis and angiogenesis. Endocrinology 1993, 133, 848–859. [Google Scholar]
- Krussel, J.S.; Behr, B.; Milki, A.A.; Hirchenhain, J.; Wen, Y.; Bielfeld, P.; Polan, M.L. Vascular endothelial growth factor (VEGF) mRNA splice variants are differentially expressed in human blastocysts. Mol. Hum. Reprod. 2001, 7, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.; Godbole, G. HOXA10 signals on the highway through pregnancy. J. Reprod. Immunol. 2009, 83, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Fazleabas, A.T. Uterine receptivity and implantation: The regulation and action of insulin-like growth factor binding protein-1 (IGFBP-1), HOXA10 and forkhead transcription factor-1 (FOXO-1) in the baboon endometrium. Reprod. Biol. Endocrinol. 2004, 2, 34. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Adeniran, S.O.; Qiu, Z.; Zhao, Q.; Zheng, P. LIF regulates the expression of miR-27a-3p and HOXA10 in bovine endometrial epithelial cells via STAT3 pathway. Theriogenology 2023, 210, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, A.D.G.L.; Lupi, A.L.; Cucielo, S.M.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2019, 21, 300. [Google Scholar] [CrossRef]
- Ma, W.; Song, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 2963–2968. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Li, Y.; Zhu, K.; Xu, Z.; Song, Y.; Song, Y.; Liu, G. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 2015, 58, 300–309. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, L.; Lu, Z.; Xiong, J.; Wu, M.; Shi, L.; Luo, A.; Wang, S. Are sirtuins markers of ovarian aging? Gene 2016, 575, 680–686. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, X.; Ge, S.Q.; Zhang, E.; Zhang, B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int. J. Clin. Exp. Pathol. 2015, 8, 8276–8283. [Google Scholar] [PubMed]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e1281. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhao, X.; Wu, H.; Li, J.; Cheng, Y.; Guo, Q.; Cao, X.; Liang, T.; Sun, L. METTL3-mediated m6A modification of SIRT1 mRNA inhibits progression of endometriosis by cellular senescence enhancing. J. Transl. Med. 2023, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Sung, G.J.; Marquardt, R.; Young, S.L.; Lessey, B.A.; Kim, T.H.; Cheon, Y.; Jeong, J. SIRT1 plays an important role in implantation and decidualization during mouse early pregnancy. Biol. Reprod. 2022, 106, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Huang, H.; Li, X.; Huang, H.; Adeniran, S.O.; Wang, Z.; Feng, R.; Zhang, G. LRH-A3 and HCG increase pregnancy rate during timed artificial insemination in dairy cows. Anim. Sci. J. 2021, 92, e13549. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Qin, X.; Li, Q.; Adeniran, S.O.; Huang, H.; Li, Y.; Zhao, Q.; Zheng, P. Progesterone regulates inflammation and receptivity of cells via the NF-kappaB and LIF/STAT3 pathways. Theriogenology 2022, 186, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Qin, X.; Feng, R.; Li, Q.; Huang, F.; Li, Y.; Zhao, Q.; Huang, H. Alleviative effect of melatonin on the decrease of uterine receptivity caused by blood ammonia through ROS/NF-κB pathway in dairy cow. Ecotoxicol. Environ. Saf. 2022, 231, 113166. [Google Scholar] [CrossRef]
- Dey, S.K. Reproductive biology: Fatty link to fertility. Nature 2005, 435, 34–35. [Google Scholar] [CrossRef]
- Mathew, D.J.; Peterson, K.D.; Senn, L.K.; Oliver, M.A.; Ealy, A.D. Ruminant conceptus-maternal interactions: Interferon-tau and beyond. J. Anim. Sci. 2022, 100, skac123. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatarre-Jimenez, M. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, F.; Tian, X.; Ji, P.; Liu, G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod. Biol. Endocrinol. 2017, 15, 78. [Google Scholar] [CrossRef]
- Santofimia-Castano, P.; Garcia-Sanchez, L.; Ruy, D.C.; Sanchez-Correa, B.; Fernandez-Bermejo, M.; Tarazona, R.; Salido, G.M.; Gonzalez, A. Melatonin induces calcium mobilization and influences cell proliferation independently of MT1/MT2 receptor activation in rat pancreatic stellate cells. Cell Biol. Toxicol. 2015, 31, 95–110. [Google Scholar] [CrossRef]
- Niu, B.; Li, B.; Wu, C.; Wu, J.; Yan, Y.; Shang, R.; Bai, C.; Li, G.; Hua, J. Melatonin promotes goat spermatogonia stem cells (SSCs) proliferation by stimulating glial cell line-derived neurotrophic factor (GDNF) production in Sertoli cells. Oncotarget 2016, 7, 77532–77542. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.G.; Hosoe, M.; Fujii, S.; Kanahara, H.; Sakumoto, R. Temporal expression and localization of vascular endothelial growth factor family members in the bovine uterus during peri-implantation period. Theriogenology 2019, 133, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ma, W.G.; Daikoku, T.; Zhao, X.; Paria, B.C.; Das, S.K.; Trzaskos, J.M.; Dey, S.K. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J. Biol. Chem. 2002, 277, 29260–29267. [Google Scholar] [CrossRef]
- Benson, G.V.; Lim, H.; Paria, B.C.; Dey, S.K.; Mass, R.L. Mechanisms of reduced fertility in Hoxa-10 mutant mice: Uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996, 122, 2687–2696. [Google Scholar] [CrossRef]
- Satokata, I.; Benson, G.; Maas, R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 1995, 374, 460–463. [Google Scholar] [CrossRef]
- Guan, X.; Liu, D.; Zhou, H.; Dai, C.; Wang, T.; Fang, Y.; Jia, Y.; Li, K. Melatonin improves pregnancy outcomes in adenomyosis mice by restoring endometrial receptivity via NF-kappaB/apoptosis signaling. Ann. Transl. Med. 2022, 10, 1317. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Menendezp, G.; Cepas, V.; Tan, D.-X.; Reiter, R.J. Melatonin and sirtuins: A “not-so unexpected” relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef]
- Lee, S.E.; Koh, H.; Joo, D.J.; Cepas, V.; Tian, D.; Reiter, R.J. Induction of SIRT1 by melatonin improves alcohol-mediated oxidative liver injury by disrupting the CRBN-YY1-CYP2E1 signaling pathway. J. Pineal Res. 2020, 68, e12638. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Dai, S.; Luo, X.; Zhu, J.; Li, F.; Liu, J.; Yao, G.; Sun, Y. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction 2018, 156, 81–92. [Google Scholar] [CrossRef]
- Lv, T.; Cao, B.; Qin, J.; Wei, Y.; Pan, B.; Ye, J.; Zhou, G. Melatonin promotes parthenogenetic development of vitrified-warmed mouse MII oocytes, potentially by reducing oxidative stress through SIRT1. Theriogenology 2023, 208, 132–141. [Google Scholar] [CrossRef]
- Yong, W.; Ma, H.; Na, M.; Gao, T.; Zhang, Y.; Hao, L.; Yu, H.; Yang, H.; Deng, X. Roles of melatonin in the field of reproductive medicine. Biomed. Pharmacother. 2021, 144, 112001. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.A.; Herlitz, K.; Troncoso, F.; Acurio, J.; Aguayo, C.; Roberts, J.M.; Truong, G.; Duncombe, G.; Rice, G.; Salomon, C. Role of Extracellular Vesicles and microRNAs on Dysfunctional Angiogenesis during Preeclamptic Pregnancies. Front. Physiol. 2016, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Hwang, S.L.; Lee, E.J.; Kim, H.M.; Chung, M.J.; Elfadl, A.K.; Lee, S.E.; Nedumaran, B.; Harris, R.A.; Jeong, K.S. Melatonin ameliorates alcohol-induced bile acid synthesis by enhancing miR-497 expression. J. Pineal Res. 2017, 62, e12386. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, S.; Xu, R.; Gao, S.; Yin, J. Melatonin Attenuates Endothelial-to-Mesenchymal Transition of Glomerular Endothelial Cells via Regulating miR-497/ROCK in Diabetic Nephropathy. Kidney Blood Press Res. 2018, 43, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, X.; Liu, J.; Chen, B.; Gao, Z.; Wang, Q. The role of miR-27b-3p/HOXA10 axis in the pathogenesis of endometriosis. Ann. Palliat. Med. 2021, 10, 3162–3170. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Zhao, X.; Ni, J.; Zhu, X.; Fu, Y.; Yang, F. SIRT1 enhances oxaliplatin resistance in colorectal cancer through microRNA-20b-3p/DEPDC1 axis. Cell Biol. Int. 2022, 46, 2107–2117. [Google Scholar] [CrossRef]
- Sun, L.N.; Zhi, Z.; Chen, L.Y.; Zhou, Q.; Li, X.M.; Gan, W.J.; Chen, S.; Yang, M.; Liu, Y.; Shen, T.; et al. SIRT1 suppresses colorectal cancer metastasis by transcriptional repression of miR-15b-5p. Cancer Lett. 2017, 409, 104–115. [Google Scholar] [CrossRef]

| Gene Name | Serial Number | Primer Sequence (5′→3′) | Product Length (bp) |
|---|---|---|---|
| β-actin | NM_173979.3 | F: GCGGCATTCACGAAACTACCTT R: TCCTGCTTGCTGATCCACATCT | 268 |
| PI3K | NM_001206047.2 | F: GGATGCTACCTTACGGCTGCTTAG R: CATCTTTGTTGAAGGCTGCTGCTG | 87 |
| AKT | NM_173986.2 | F: AGGACCTGGAGCAGCGTGAG R: GCAGGCAGCGGATGATGAAGG | 67 |
| SIRT1 | NM_001192980.3 | F: CAACGGTTTCCATTCGTGTG R: GTTCGAGGATCTGTGCCAAT | 138 |
| HOXA10 | NM_001105017.1 | F: TTTCCTTGGCAAAGAGAAGGGCTTG R: GACCTTACACAAACTGGAAGAGAC | 99 |
| VEGF | M32976 | F: CCCAGATGAGATTGAGTTCATTTT R: AGCAAGGCCCACAGGGATTT | 245 |
| LIF | NM_173931 | F: CATCCCTGTCCCAGCAACCTCATG R: CATCCCTGTCCCAGCAACCTCATG | 215 |
| cyclinD1 | NM_001046273.2 | F: CTGGTCCTGGTGAACAAACT R: ACAGAGGGCAACGAAGGT | 106 |
| cyclinE1 | NM_001192776.1 | F: AAGGAGAGGGATGCGAAGGA R: AATCAGGGAGCAGGGGTTTT | 170 |
| Gene Name | Primer Sequence (5′→3′) |
|---|---|
| U6 | F: CTCGCTTCGGCAGCACA R: AACGCTTCACGAATTTGCGT |
| URP | TGGTGTCGTGGAGTCG |
| Bta-miR-27a-3p | acactccagctgggTTCACAGTGGCTAA |
| Bta-miR-497 | acactccagctgggCAGCAGCACACTGTGG |
| Bta-miR-16b | acactccagctgggTAGCAGCACGTAAAT |
| Bta-miR-29b | acactccagctgggTAGCACCATTTGAAATC |
| Bta-miR-29c | acactccagctgggTAGCACCATTTGAAAT |
| Bta-miR-27a-3p (RT-primer) | ctcaactggtgtcgtggagtcggcaattcagttgagCGGAACTT |
| Bta-miR-497 (RT-primer) | ctcaactggtgtcgtggagtcggcaattcagttgagTACAAACC |
| Bta-miR-16b (RT-primer) | ctcaactggtgtcgtggagtcggcaattcagttgagGCCAATAT |
| Bta-miR-29b (RT-primer) | ctcaactggtgtcgtggagtcggcaattcagttgagTTCTCTGT |
| Bta-miR-29c (RT-primer) | ctcaactggtgtcgtggagtcggcaattcagttgagTAACCGAT |
| Name | Dilution Rate | Manufacturer | Product Number |
|---|---|---|---|
| β-actin rabbit antibody | 1:5000 | Bioss, Shanghai, China | bs-0061R |
| SIRT1 rabbit Polyclonal antibody | 1:1000 | Proteintech, Wuhan, China | 13161-1-AP |
| PI3K mouse monoclonal antibody | 1:1000 | Proteintech, Wuhan, China | 67071-1-Ig |
| Phospho-AKT(Ser473) mouse Monoclonal antibody | 1:5000 | Proteintech, Wuhan, China | 66444-1-Ig |
| AKT rabbit polyclonal antibody | 1:1000 | Affinity, Shanghai, China | AF0908 |
| HOXA10 rabbit Polyclonal antibody | 1:1000 | Proteintech, Wuhan, China | 26497-1-AP |
| VEGFA rabbit Polyclonal antibody | 1:1000 | Proteintech, Wuhan, China | 19003-1-AP |
| HRP Goat Anti-Rabbit IgG H&L | 1:10,000 | Immunoway, China | RS0002 |
| HRP Goat Anti-Mouse IgG H&L | 1:10,000 | Bioss, Shanghai, China | bs-0296G |
| Name | Dilution Rate | Manufacturer | Product Number |
|---|---|---|---|
| SIRT1 rabbit Polyclonal antibody | 1:200 | Proteintech, Wuhan, China | 13161-1-AP |
| Rabbit Anti-HOXA10 Polyclonal Antibody | 1:200 | Bioss, Shanghai, China | bs-2502R |
| VEGFA rabbit Polyclonal antibody | 1:200 | Proteintech, Wuhan, China | 19003-1-AP |
| DAPI | 1:500 | Biosharp, Heifei, China | BL105A |
| Goat Anti-rabbit IgG H&L/FITC | 1:500 | Bioss, Shanghai, China | bs-0295G-FITC |
| Name | Primer Sequence (5′→3′) |
|---|---|
| NC inhibitor (cel-miR-67-3p) | Antisense strand: UCUACUCUUUCUAGGAGGUUGUGA |
| NC mimics (cel-miR-67-3p) | Sense strand: UCACAACCUCCUAGAAAGAGUAGA Antisense strand: UCUACUCUUUCUAGGAGGUUGUGA |
| Bta-miR-27a-3p inhibitor | Antisense strand: CGGAACUUAGCCACUGUGAA |
| Bta-miR-27a-3p mimics | Sense strand: UUCACAGUGGCUAAGUUCCG Antisense strand: CGGAACUUAGCCACUGUGAA |
| Bta-miR-497 inhibitor | Antisense strand: UACAAACCACAGUGUGCUGCUG |
| Bta-miR-497 mimics | Sense strand: CAGCAGCACACUGUGGUUUGUA Antisense strand: UACAAACCACAGUGUGCUGCUG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Tang, Y.; Chen, Y.; Li, B.; Wang, H.; Liu, S.; Adeniran, S.O.; Zheng, P. Melatonin Regulates the Expression of VEGF and HOXA10 in Bovine Endometrial Epithelial Cells through the SIRT1/PI3K/AKT Pathway. Animals 2024, 14, 2771. https://doi.org/10.3390/ani14192771
Li Q, Tang Y, Chen Y, Li B, Wang H, Liu S, Adeniran SO, Zheng P. Melatonin Regulates the Expression of VEGF and HOXA10 in Bovine Endometrial Epithelial Cells through the SIRT1/PI3K/AKT Pathway. Animals. 2024; 14(19):2771. https://doi.org/10.3390/ani14192771
Chicago/Turabian StyleLi, Qi, Ying Tang, Yanru Chen, Bo Li, Hongzhan Wang, Shicheng Liu, Samson O. Adeniran, and Peng Zheng. 2024. "Melatonin Regulates the Expression of VEGF and HOXA10 in Bovine Endometrial Epithelial Cells through the SIRT1/PI3K/AKT Pathway" Animals 14, no. 19: 2771. https://doi.org/10.3390/ani14192771
APA StyleLi, Q., Tang, Y., Chen, Y., Li, B., Wang, H., Liu, S., Adeniran, S. O., & Zheng, P. (2024). Melatonin Regulates the Expression of VEGF and HOXA10 in Bovine Endometrial Epithelial Cells through the SIRT1/PI3K/AKT Pathway. Animals, 14(19), 2771. https://doi.org/10.3390/ani14192771






