Bovine Respiratory Disease (BRD) in Post-Weaning Calves with Different Prevention Strategies and the Impact on Performance and Health Status
Abstract
:Simple Summary
Abstract
1. Introduction
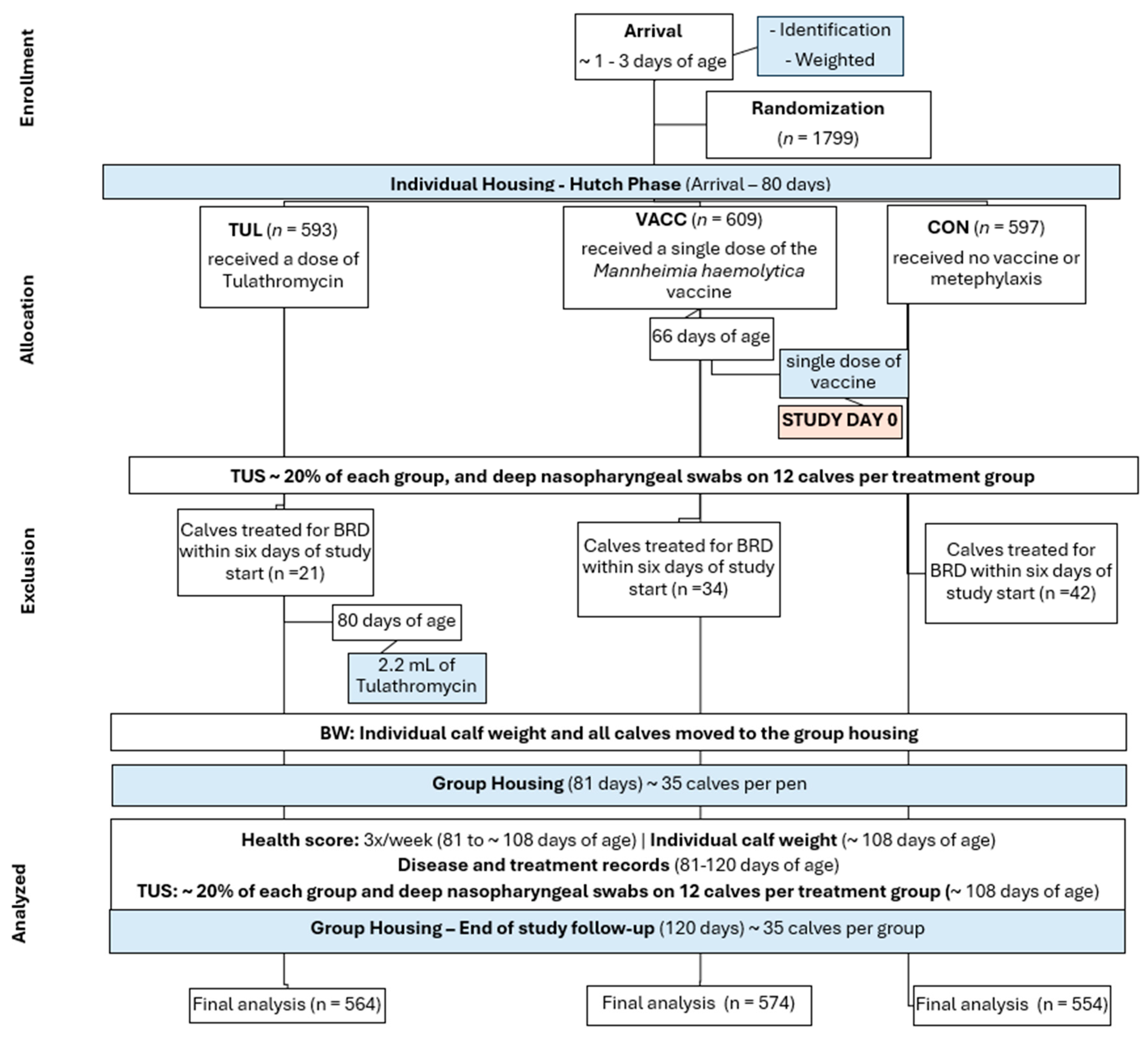
2. Materials and Methods
2.1. Sample Size Calculations
2.2. Animals, Housing, and Management
2.3. Weights and Average Daily Gain
2.4. Thoracic Ultrasound and Nasal Swab
2.5. Health Scores and Treatment Records
2.6. Weather Data
2.7. Statistical Analysis
3. Results
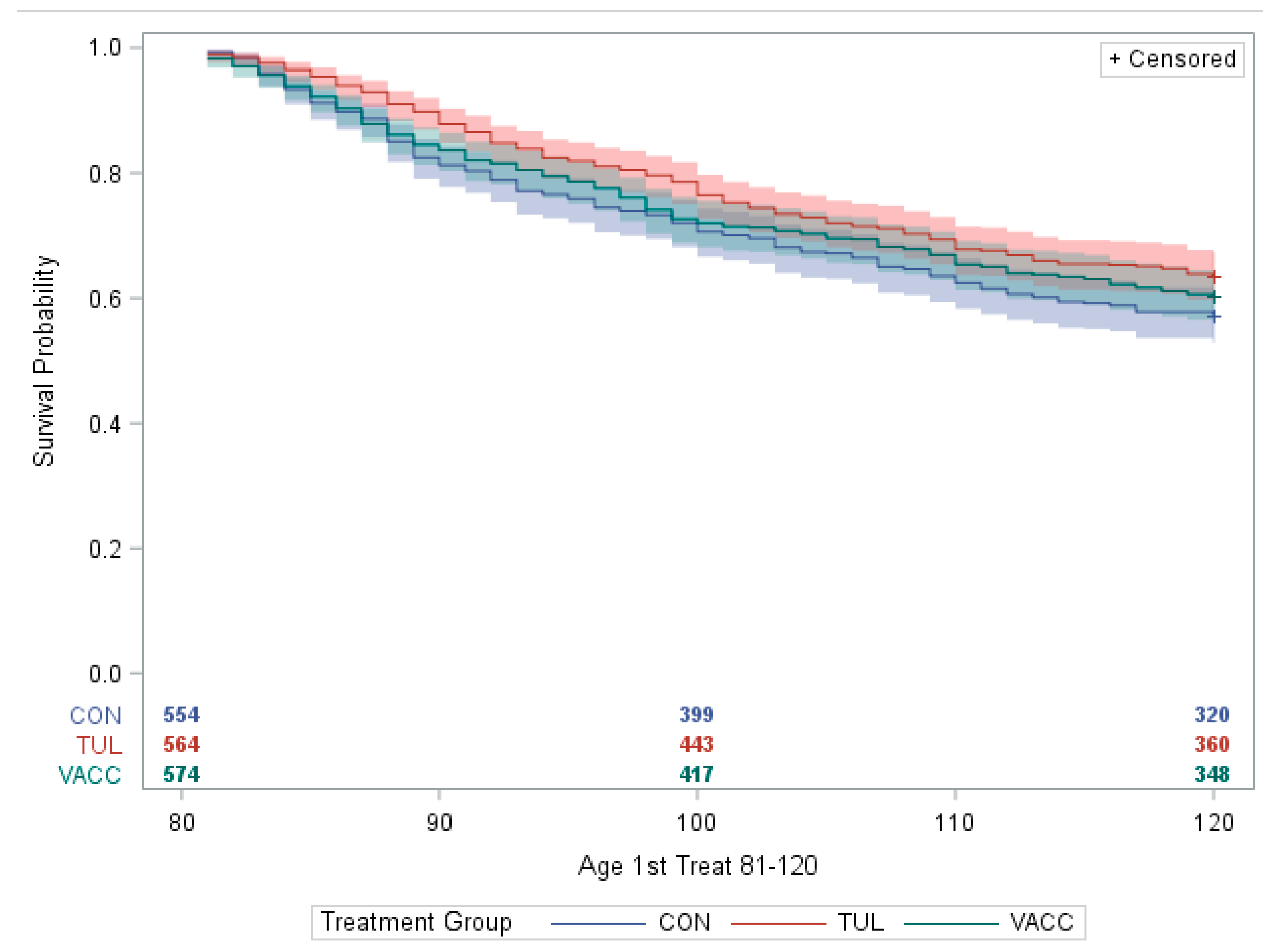
3.1. Respiratory Morbidity, Mortality, and Growth
3.2. Thoracic Ultrasound and Nasopharyngeal Swabs
3.3. Calf Scoring Results
3.4. Weather Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Dairy 2014, “Trends in Dairy Cattle Health and Management Practices in the United States, 1991–2014” USDA–APHIS–VS–CEAH-NAHMS. Fort Collins, CO #711.0821 2021; USDA: Washington, DC, USA, 2021.
- Dubrovsky, S.A.; Van Eenennaam, A.L.; Aly, S.S.; Karle, B.M.; Rossitto, P.V.; Overton, M.W.; Lehenbauer, T.W.; Fadel, J.G. Preweaning Cost of Bovine Respiratory Disease (BRD) and Cost-Benefit of Implementation of Preventative Measures in Calves on California Dairies: The BRD 10K Study. J. Dairy Sci. 2020, 103, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Peel, D.S. The Effect of Market Forces on Bovine Respiratory Disease. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 497–508. [Google Scholar] [CrossRef]
- Buczinski, S.; Achard, D.; Timsit, E. Effects of Calfhood Respiratory Disease on Health and Performance of Dairy Cattle: A Systematic Review and Meta-Analysis. J. Dairy Sci. 2021, 104, 8214–8227. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.L.; Kelton, D.F.; LeBlanc, S.J.; Wormuth, J.; Leslie, K.E. The Effect of Respiratory Disease and a Preventative Antibiotic Treatment on Growth, Survival, Age at First Calving, and Milk Production of Dairy Heifers. J. Dairy Sci. 2012, 95, 4950–4960. [Google Scholar] [CrossRef] [PubMed]
- Overton, M.W. Economics of Respiratory Disease in Dairy Replacement Heifers. Anim. Health Res. Rev. 2020, 21, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Preview: Economic Effects of Bovine Respiratory Disease. Journal of Animal Science 2020, 98, skaa042. [CrossRef]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An Evaluation of the Economic Effects of Bovine Respiratory Disease on Animal Performance, Carcass Traits, and Economic Outcomes in Feedlot Cattle Defined Using Four BRD Diagnosis Methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef]
- Aly, S.S.; Love, W.J.; Blanchard, P.C.; Crossley, B.; Van Eenennaam, A.L.; Lehenbauer, T.W. Etiology and Risk Factors for Bovine Respiratory Disease in Pre-Weaned Calves on California Dairies and Calf Ranches. Prev. Vet. Med. 2021, 197, 105506. [Google Scholar] [CrossRef]
- Cummings, D.B.; Meyer, N.F.; Step, D.L. Bovine Respiratory Disease Considerations in Young Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2022, 38, 93–105. [Google Scholar] [CrossRef]
- Snyder, E.; Credille, B. Mannheimia Haemolytica and Pasteurella Multocida in Bovine Respiratory Disease. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 253–268. [Google Scholar] [CrossRef]
- Booker, C.W.; Abutarbush, S.M.; Morley, P.S.; Jim, G.K.; Pittman, T.J.; Schunicht, O.C.; Perrett, T.; Wildman, B.K.; Fenton, R.K.; Guichon, P.T.; et al. Microbiological and Histopathological Findings in Cases of Fatal Bovine Respiratory Disease of Feedlot Cattle in Western Canada. Can. Vet. J. 2008, 49, 473–481. [Google Scholar] [PubMed]
- Poonsuk, K.; Kordik, C.; Hille, M.; Cheng, T.-Y.; Crosby, W.B.; Woolums, A.R.; Clawson, M.L.; Chitko-McKown, C.; Brodersen, B.; Loy, J.D. Detection of Mannheimia Haemolytica-Specific IgG, IgM and IgA in Sera and Their Relationship to Respiratory Disease in Cattle. Animals 2023, 13, 1531. [Google Scholar] [CrossRef] [PubMed]
- DelCurto, T.; Murphy, T.; Moreaux, S. Demographics and Long-Term Outlook for Western Us Beef, Sheep, and Horse Industries and Their Importance for the Forage Industry. In Proceedings of the 47th Western Alfalfa & Forage Symposium, Reno, NV, USA, 28–30 November 2017; UC Cooperative Extension, Plant Sciences Department, University of California: Davis, CA, USA, 2017. [Google Scholar]
- De Vries, A.; Overton, M.; Fetrow, J.; Leslie, K.; Eicker, S.; Rogers, G. Exploring the Impact of Sexed Semen on the Structure of the Dairy Industry. J. Dairy Sci. 2008, 91, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Overton, M.W.; Dhuyvetter, K.C. Symposium Review: An Abundance of Replacement Heifers: What Is the Economic Impact of Raising More than Are Needed? J. Dairy Sci. 2020, 103, 3828–3837. [Google Scholar] [CrossRef]
- Berry, D.P. Invited Review: Beef-on-Dairy—The Generation of Crossbred Beef × Dairy Cattle. J. Dairy Sci. 2021, 104, 3789–3819. [Google Scholar] [CrossRef] [PubMed]
- McCabe, E.D.; King, M.E.; Fike, K.E.; Odde, K.G. Effects of Holstein and Beef-Dairy Cross Breed Description on the Sale Price of Feeder and Weaned Calf Lots Sold through Video Auctions. Appl. Anim. Sci. 2022, 38, 70–78. [Google Scholar] [CrossRef]
- Timsit, E.; Tison, N.; Booker, C.W.; Buczinski, S. Association of Lung Lesions Measured by Thoracic Ultrasonography at First Diagnosis of Bronchopneumonia with Relapse Rate and Growth Performance in Feedlot Cattle. Vet. Intern. Med. 2019, 33, 1540–1546. [Google Scholar] [CrossRef]
- Crawford, D.M.; Richeson, J.T.; Perkins, T.L.; Samuelson, K.L. Feeding a High-Energy Finishing Diet upon Arrival to High-Risk Feedlot Calves: Effects on Health, Performance, Ruminal pH, Rumination, Serum Metabolites, and Carcass Traits. J. Anim. Sci. 2022, 100, skac194. [Google Scholar] [CrossRef]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 507–534. [Google Scholar] [CrossRef]
- Walker, W.L.; Epperson, W.B.; Wittum, T.E.; Lord, L.K.; Rajala-Schultz, P.J.; Lakritz, J. Characteristics of Dairy Calf Ranches: Morbidity, Mortality, Antibiotic Use Practices, and Biosecurity and Biocontainment Practices. J. Dairy Sci. 2012, 95, 2204–2214. [Google Scholar] [CrossRef]
- Word, A.B.; Ellis, G.B.; Holland, B.P.; Streeter, M.N.; Hutcheson, J.P. Effects of Antimicrobial Metaphylaxis Using No Antimicrobial, Tilmicosin, or Tildipirosin and 2 Different Days on Feed on the Health and Growth Performance of Lightweight Beef Steer Calves Originating from Mexico. Appl. Anim. Sci. 2021, 37, 207–216. [Google Scholar] [CrossRef]
- Nickell, J.S.; White, B.J. Metaphylactic Antimicrobial Therapy for Bovine Respiratory Disease in Stocker and Feedlot Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 285–301. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.M.; Hu, D.; Totton, S.C.; Scott, N.; Winder, C.B.; Wang, B.; Wang, C.; Glanville, J.; Wood, H.; White, B.; et al. A Systematic Review and Network Meta-Analysis of Injectable Antibiotic Options for the Control of Bovine Respiratory Disease in the First 45 Days Post Arrival at the Feedlot. Anim. Health Res. Rev. 2019, 20, 163–181. [Google Scholar] [CrossRef]
- Stanton, A.L.; Kelton, D.F.; LeBlanc, S.J.; Millman, S.T.; Wormuth, J.; Dingwell, R.T.; Leslie, K.E. The Effect of Treatment with Long-Acting Antibiotic at Postweaning Movement on Respiratory Disease and on Growth in Commercial Dairy Calves. J. Dairy Sci. 2010, 93, 574–581. [Google Scholar] [CrossRef]
- Binversie, E.S.; Ruegg, P.L.; Combs, D.K.; Ollivett, T.L. Randomized Clinical Trial to Assess the Effect of Antibiotic Therapy on Health and Growth of Preweaned Dairy Calves Diagnosed with Respiratory Disease Using Respiratory Scoring and Lung Ultrasound. J. Dairy Sci. 2020, 103, 11723–11735. [Google Scholar] [CrossRef]
- Holschbach, C.L.; Raabis, S.M.; Ollivett, T.L. Effect of Antibiotic Treatment in Preweaned Holstein Calves after Experimental Bacterial Challenge with Pasteurella Multocida. J. Dairy Sci. 2019, 102, 11359–11369. [Google Scholar] [CrossRef]
- Ollivett, T.L.; Buczinski, S. On-Farm Use of Ultrasonography for Bovine Respiratory Disease. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Goecke, N.B.; Nielsen, B.H.; Petersen, M.B.; Larsen, L.E. Design of a High-Throughput Real-Time PCR System for Detection of Bovine Respiratory and Enteric Pathogens. Front. Vet. Sci. 2021, 8, 677993. [Google Scholar] [CrossRef]
- Apley, M.D. Treatment of Calves with Bovine Respiratory Disease. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 441–453. [Google Scholar] [CrossRef]
- Collier, R.J.; Laun, W.H.; Rungruang, S.; Zimbleman, R.B. Quantifying Heat Stress and Its Impact on Metabolism and Performance; University of Florida: Gainesville, FL, USA, 2012; pp. 74–83. [Google Scholar]
- Stull, C.; Reynolds, J. Calf Welfare. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 191–203. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, F.; Xiao, J.; Wang, Y.; Yang, H.; Li, S.; Cao, Z. Heat Stress on Calves and Heifers: A Review. J. Anim. Sci Biotechnol. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.V.; Altier, C.; Siler, J.D.; Mann, S.; Jordan, D.; Warnick, L.D. Longitudinal Effects of Enrofloxacin or Tulathromycin Use in Preweaned Calves at High Risk of Bovine Respiratory Disease on the Shedding of Antimicrobial-Resistant Fecal Escherichia Coli. J. Dairy Sci. 2020, 103, 10547–10559. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.L.; Step, D.L. Evidence-Based Effectiveness of Vaccination Against Mannheimia Haemolytica, Pasteurella Multocida, and Histophilus Somni in Feedlot Cattle for Mitigating the Incidence and Effect of Bovine Respiratory Disease Complex. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 97–106.e7. [Google Scholar] [CrossRef]
- Comerford, J. Causes of Vaccine Failure in Beef Cattle. Available online: https://extension.psu.edu/causes-of-vaccine-failure-in-beef-cattle (accessed on 3 December 2023).
- Richeson, J.T. Vaccinating High-Risk Calves against BRD. In Proceedings of the Forty-Eighth Annual Conference. American Association of Bovine Practitioners, New Orleans, LA, USA, 17–19 September 2015; pp. 172–175. [Google Scholar] [CrossRef]
- Arthington, J.D.; Cooke, R.F.; Maddock, T.D.; Araujo, D.B.; Moriel, P.; DiLorenzo, N.; Lamb, G.C. Effects of Vaccination on the Acute-Phase Protein Response and Measures of Performance in Growing Beef Calves1. J. Anim. Sci. 2013, 91, 1831–1837. [Google Scholar] [CrossRef]
- Nowakowski, M.A.; Inskeep, P.B.; Risk, J.E.; Skogerboe, T.L.; Benchaoui, H.A.; Meinert, T.R.; Sherington, J.; Sunderland, S.J. Pharmacokinetics and Lung Tissue Concentrations of Tulathromycin, a New Triamilide Antibiotic, in Cattle. Vet. Ther. 2004, 5, 60–74. [Google Scholar] [PubMed]
- Crosby, S.; Credille, B.; Giguère, S.; Berghaus, R. Comparative Efficacy of Enrofloxacin to That of Tulathromycin for the Control of Bovine Respiratory Disease and Prevalence of Antimicrobial Resistance in Mannheimia Haemolytica in Calves at High Risk of Developing Bovine Respiratory Disease1. J. Anim. Sci. 2018, 96, 1259–1267. [Google Scholar] [CrossRef]
- Munoz, V.I.; Samuelson, K.L.; Tomczak, D.J.; Seiver, H.A.; Smock, T.M.; Richeson, J.T. Comparative Efficacy of Metaphylaxis with Tulathromycin and Pentavalent Modified-Live Virus Vaccination in High-Risk, Newly Received Feedlot Cattle. Appl. Anim. Sci. 2020, 36, 799–807. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The Epidemiology of Bovine Respiratory Disease: What Is the Evidence for Predisposing Factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar]
- Cusack, P.; McMeniman, N.; Lean, I. The Medicine and Epidemiology of Bovine Respiratory Disease in Feedlots. Aust Vet. J 2003, 81, 480–487. [Google Scholar] [CrossRef]
- Padalino, B.; Cirone, F.; Zappaterra, M.; Tullio, D.; Ficco, G.; Giustino, A.; Ndiana, L.A.; Pratelli, A. Factors Affecting the Development of Bovine Respiratory Disease: A Cross-Sectional Study in Beef Steers Shipped From France to Italy. Front. Vet. Sci. 2021, 8, 627894. [Google Scholar] [CrossRef]
- Cusack, P.; McMeniman, N.; Lean, I. Feedlot Entry Characteristics and Climate: Their Relationship with Cattle Growth Rate, Bovine Respiratory Disease and Mortality. Aust. Vet. J. 2007, 85, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Diesel, D.A.; Lebel, J.L.; Tucker, A. Pulmonary Particle Deposition and Airway Mucociliary Clearance in Cold-Exposed Calves. Am. J. Vet. Res. 1991, 52, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Backgrounding Feeder Cattle Nutrition | Cattle. Available online: https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/livestock/cattle-poultry-and-other-livestock/cattle/backgrounding-and-feeder-cattle-nutrition (accessed on 15 January 2024).
- Wang, S.; Li, Q.; Peng, J.; Niu, H. Effects of Long-Term Cold Stress on Growth Performance, Behavior, Physiological Parameters, and Energy Metabolism in Growing Beef Cattle. Animals 2023, 13, 1619. [Google Scholar] [CrossRef]
- Committee on Nutrient Requirements of Dairy Cattle; Board on Agriculture and Natural Resources; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Dairy Cattle: Eighth Revised Edition; National Academies Press: Washington, DC, USA, 2021; p. 25806. ISBN 978-0-309-67777-6. [Google Scholar]
- Roland, L.; Drillich, M.; Klein-Jöbstl, D.; Iwersen, M. Invited Review: Influence of Climatic Conditions on the Development, Performance, and Health of Calves. J. Dairy Sci. 2016, 99, 2438–2452. [Google Scholar] [CrossRef]
- Patterson, D.J.; Bellows, R.A.; Burfening, P.J.; Carr, J.B. Occurrence of Neonatal and Postnatal Mortality in Range Beef Cattle. I. Calf Loss Incidence from Birth to Weaning, Backward and Breech Presentations and Effects of Calf Loss on Subsequent Pregnancy Rate of Dams. Theriogenology 1987, 28, 557–571. [Google Scholar] [CrossRef]
- Cuevas-Gómez, I.; McGee, M.; Sánchez, J.M.; O’Riordan, E.; Byrne, N.; McDaneld, T.; Earley, B. Association between Clinical Respiratory Signs, Lung Lesions Detected by Thoracic Ultrasonography and Growth Performance in Pre-weaned Dairy Calves. Ir. Vet. J. 2021, 74, 7. [Google Scholar] [CrossRef]
- Sáadatnia, A.; Mohammadi, G.R.; Azizzadeh, M.; Mirshahi, A.; Mohieddini, A.A.; Buczinski, S. Effect of Ultrasonographic Lung Consolidation on Health and Growth in Dairy Calves: A Longitudinal Study. J. Dairy Sci. 2023, 106, 8047–8059. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, V.; Ryan, E.G.; Hayes, C.J.; McAloon, C.; O’Grady, L.; Hoey, S.; Mee, J.F.; Pardon, B.; Earley, B.; McAloon, C.G. Diagnosis of Respiratory Disease in Preweaned Dairy Calves Using Sequential Thoracic Ultrasonography and Clinical Respiratory Scoring: Temporal Transitions and Association with Growth Rates. J. Dairy Sci. 2021, 104, 11165–11175. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.; Credille, B.; Berghaus, R.; Giguère, S. Prevalence of Multi Drug Antimicrobial Resistance in Mannheimia Haemolytica Isolated from High-Risk Stocker Cattle at Arrival and Two Weeks after Processing1. J. Anim. Sci. 2017, 95, 1124–1131. [Google Scholar] [CrossRef]
- Woolums, A.R.; Karisch, B.B.; Frye, J.G.; Epperson, W.; Smith, D.R.; Blanton, J.; Austin, F.; Kaplan, R.; Hiott, L.; Woodley, T.; et al. Multidrug Resistant Mannheimia Haemolytica Isolated from High-Risk Beef Stocker Cattle after Antimicrobial Metaphylaxis and Treatment for Bovine Respiratory Disease. Vet. Microbiol. 2018, 221, 143–152. [Google Scholar] [CrossRef]
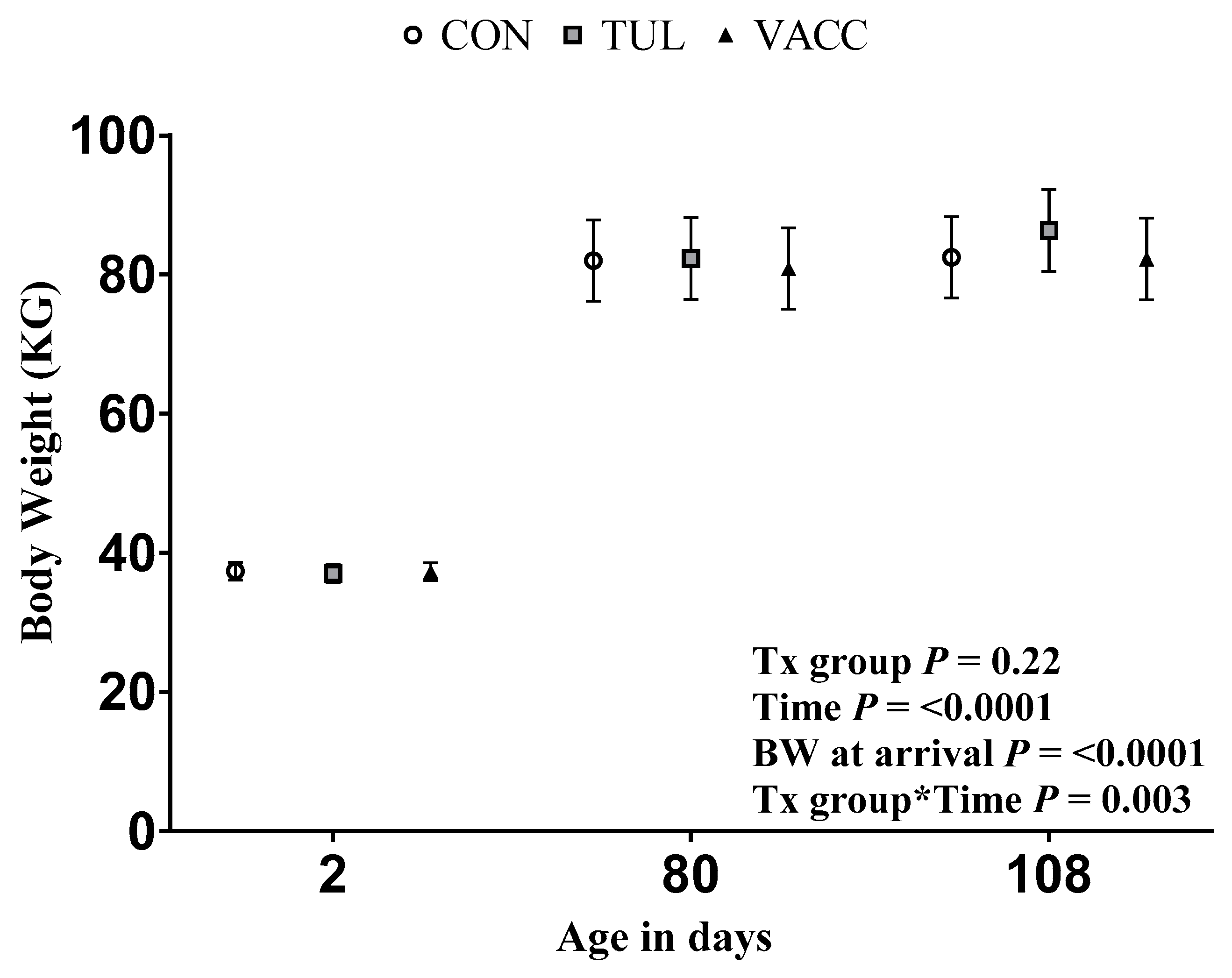

| 1 Treatment Groups | |||||
|---|---|---|---|---|---|
| Characteristics | CON | TUL | VACC | p-Value | |
| Sex | Female | 225 | 221 | 227 | 0.98 |
| Male | 372 | 372 | 382 | ||
| Breed | Angus | 224 | 227 | 231 | 0.90 |
| Charolais | 169 | 162 | 168 | ||
| Holstein | 72 | 71 | 69 | ||
| Limousine | 132 | 133 | 141 | ||
| 2 Body weight at arrival ± SE, (kg) | 37.3 ± 0.5 | 37.0 ± 0.5 | 37.1 ± 0.5 | 0.60 | |
| Treatment Groups 1 | p-Value | |||
|---|---|---|---|---|
| Event | CON | TUL | VACC | Tx |
| Calves included in the analysis | 554 | 564 | 574 | |
| LSM ± SE BRD treatment 81–120 days 2 | 0.53 ± 0.03 | 0.45 ± 0.03 | 0.47 ± 0.03 | 0.10 |
| Mortality (n, %) | 35 (6.3%) | 26 (4.6%) | 35 (6.1%) | 0.40 |
| 1 Treatment Groups | ||||||
|---|---|---|---|---|---|---|
| p-Value | ||||||
| Time of Ultrasound Collection | CON | TUL | VACC | Tx | Time | |
| 2 66 days of age | Score < 3 | 71 (67.6%) | 80 (64.5%) | 70 (63.1%) | ||
| Score ≥ 3 | 34 (32.4%) | 44 (35.5%) | 41 (36.9%) | |||
| 0.77 | 0.001 | |||||
| 3 108 days of age | ||||||
| Score < 3 | 30 (61.2%) | 39 (65.0%) | 29 (60.4%) | |||
| Score ≥ 3 | 19 (38.2%) | 21 (35.0%) | 19 (39.6%) | |||
| 1 Treatment Groups | ||||||
|---|---|---|---|---|---|---|
| Time of Swab Collection | p-Value | |||||
| CON (n, %) | TUL (n, %) | VACC (n, %) | Tx | Time | ||
| 2 66 days of age | Positive | 8 (66.6%) | 7 (53.8%) | 5 (41.6%) | ||
| Negative | 4 (33.3%) | 6 (46.2%) | 7 (58.3%) | |||
| 0.48 | 0.97 | |||||
| 3 108 days of age | ||||||
| Positive | 10 (100.0%) | 11 (91.6%) | 11 (100%) | |||
| Negative | 0 (0.0%) | 1 (8.4%) | 0 (0.0%) | |||
| Died | 2 | 1 | 1 | |||
| Total Counts of Eye, Nose, and Ear Scores | 1 Treatment Groups | p-Value | |||
|---|---|---|---|---|---|
| CON | TUL | VACC | Tx | Time | |
| Score < 5 | 6915 (32.4%) | 7040 (32.9%) | 6983 (32.7%) | 0.50 | <0.0001 |
| Score ≥ 5 | 141 (0.7%) | 136 (0.7%) | 169 (0.8%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madureira Ferreira, M.; Santos, B.; Skarbek, A.; Mills, C.; Thom, H.; Prentice, D.; McConnel, C.; Leal Yepes, F.A. Bovine Respiratory Disease (BRD) in Post-Weaning Calves with Different Prevention Strategies and the Impact on Performance and Health Status. Animals 2024, 14, 2807. https://doi.org/10.3390/ani14192807
Madureira Ferreira M, Santos B, Skarbek A, Mills C, Thom H, Prentice D, McConnel C, Leal Yepes FA. Bovine Respiratory Disease (BRD) in Post-Weaning Calves with Different Prevention Strategies and the Impact on Performance and Health Status. Animals. 2024; 14(19):2807. https://doi.org/10.3390/ani14192807
Chicago/Turabian StyleMadureira Ferreira, Marina, Bruna Santos, Agata Skarbek, Carley Mills, Hannah Thom, David Prentice, Craig McConnel, and Francisco A. Leal Yepes. 2024. "Bovine Respiratory Disease (BRD) in Post-Weaning Calves with Different Prevention Strategies and the Impact on Performance and Health Status" Animals 14, no. 19: 2807. https://doi.org/10.3390/ani14192807
APA StyleMadureira Ferreira, M., Santos, B., Skarbek, A., Mills, C., Thom, H., Prentice, D., McConnel, C., & Leal Yepes, F. A. (2024). Bovine Respiratory Disease (BRD) in Post-Weaning Calves with Different Prevention Strategies and the Impact on Performance and Health Status. Animals, 14(19), 2807. https://doi.org/10.3390/ani14192807






