Effect of Different Early Weaning Diets on Survival, Growth, and Digestive Ontogeny of Channa striatus (Bloch, 1793) Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diet Formulation
2.2. Diet Preparation
2.3. Experimental Animal Source and Husbandry
2.4. Sampling of Larvae
2.5. Proximate Composition
2.6. Sample Preparation and Enzyme Assay
2.7. Histomorphological Analysis of Digestive System Development
2.8. Calculations and Statistical Analyses
- Average daily growth (ADG) = (Final weight (mg)−Initial weight (mg)/Trial period in days)
- Mean weight gain (MWG) = Final mean weight (mg)/Initial mean weight (mg)
- Feed conversion ratio (FCR) = Dry matter feed intake (g)/Wet weight gain (g)
- Protein efficiency ratio (PER) = Wet weight gain (g)/Dry weight of total protein fed (g)
- Specific growth rate (SGR) = (Ln of wet weight at the end of the trial−Ln of wet weight at the beginning of the trial)/Trial period in days × 100
- Survival = ((Live number of larvae left in the tank−number of sampled larvae)/initial number of larvae) × 100
- Cumulative mortality (CM) = (Total number of dead larvae/Initial number of larvae) × 100
3. Results
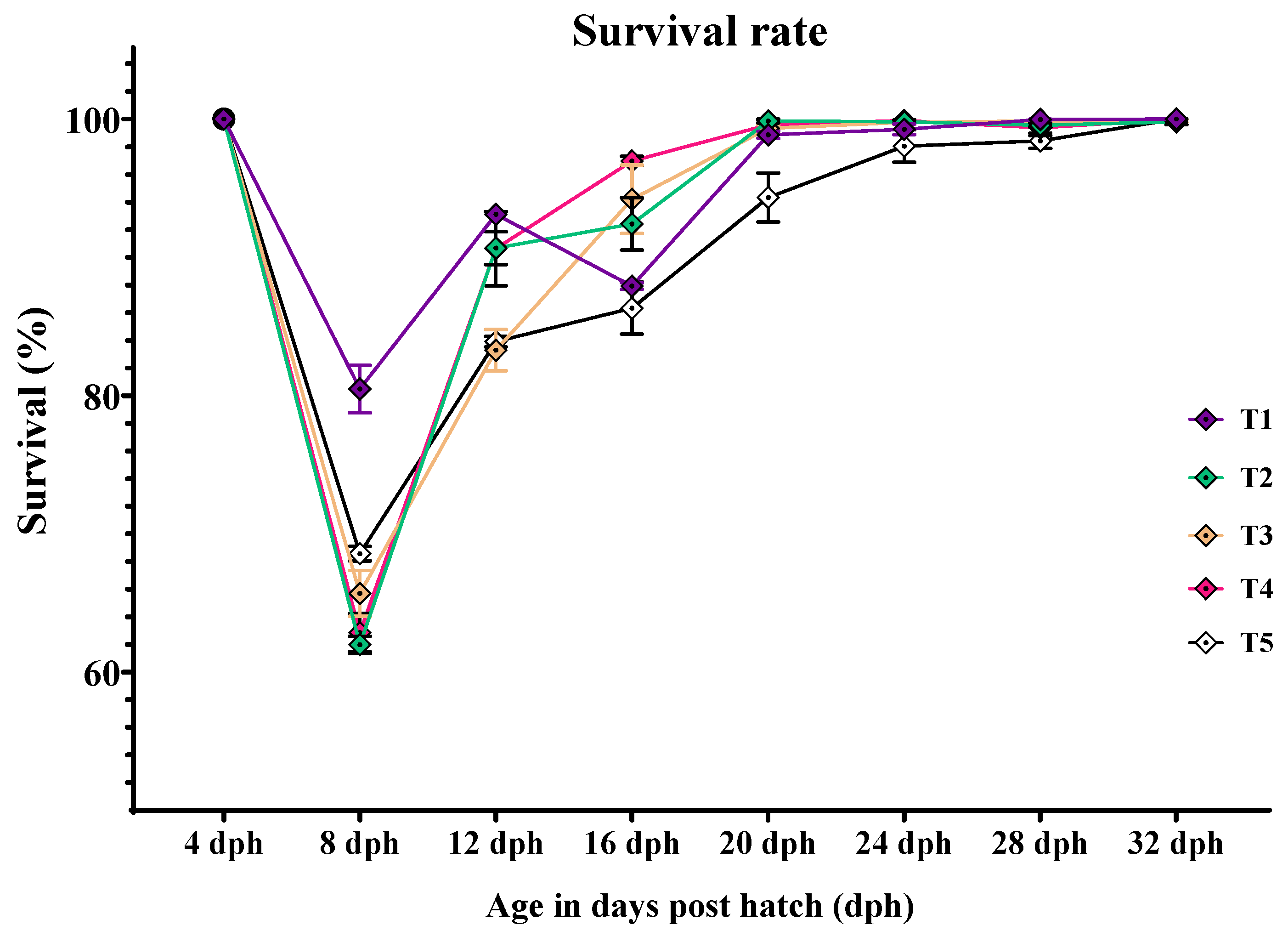
3.1. Larval Survival and Cumulative Mortality RATE
3.2. Growth Performance
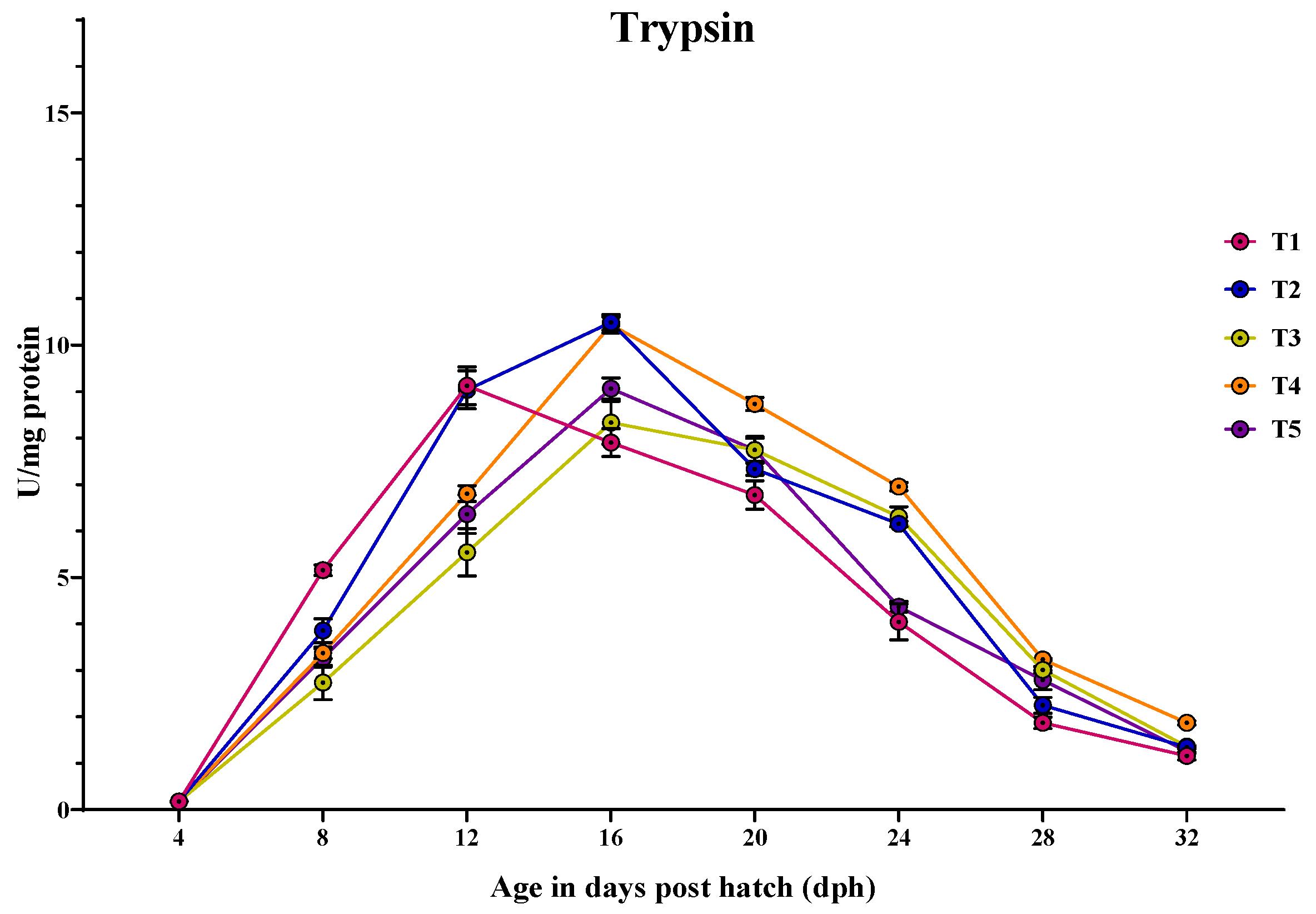
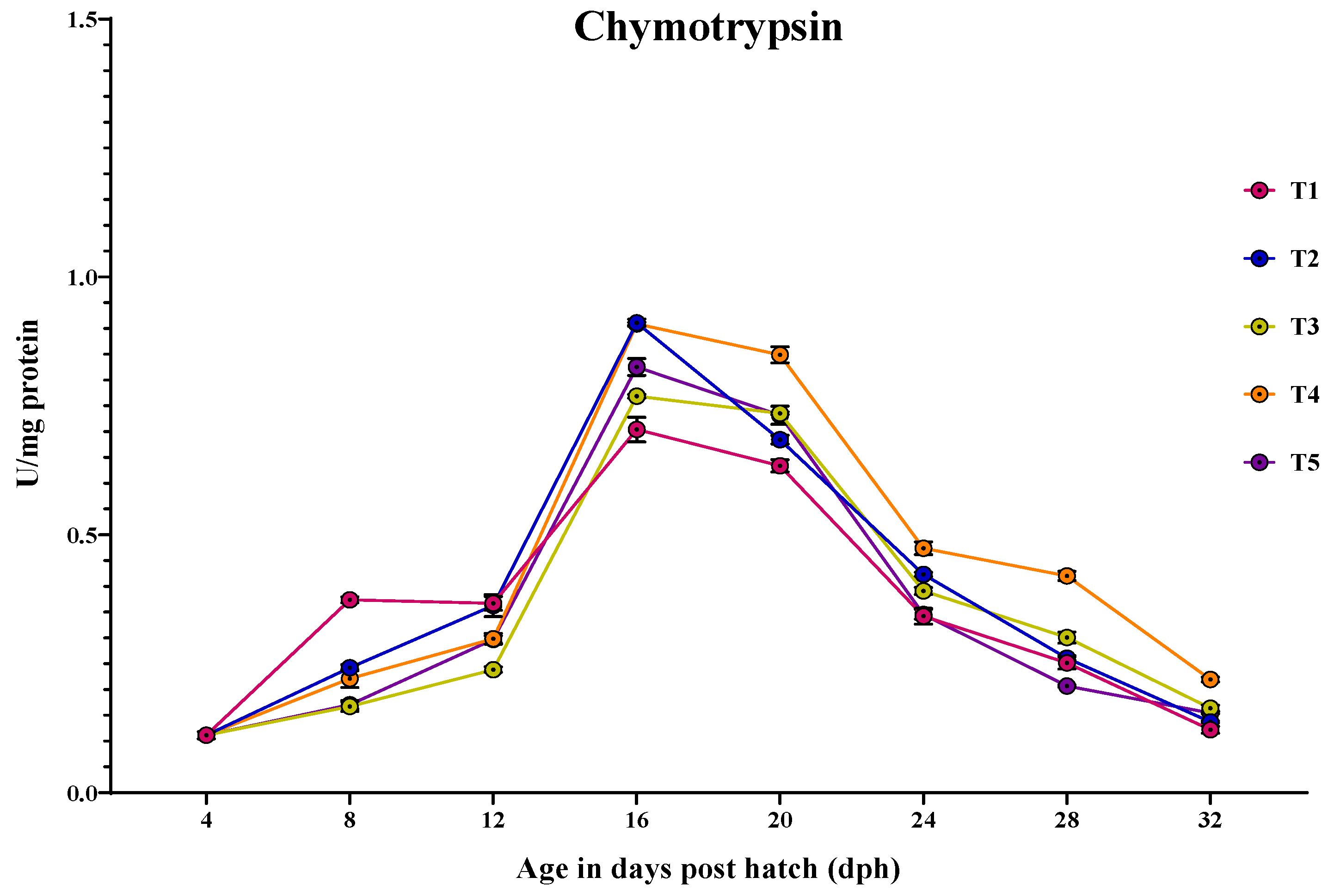
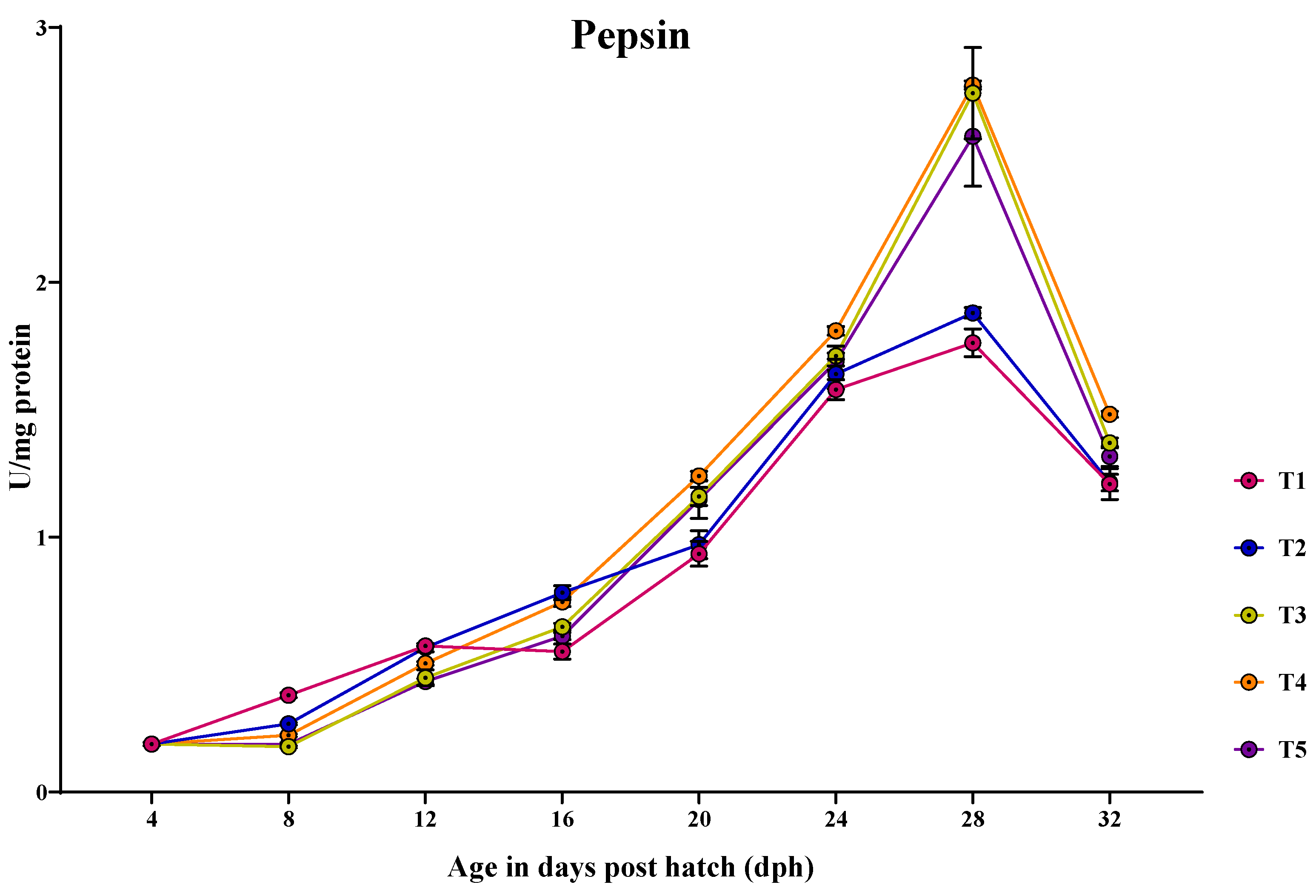
3.3. Ontogeny of Digestive Enzymes in Channa striatus Larvae Fed Different Experimental Diets
3.3.1. Trypsin
3.3.2. Chymotrypsin
3.3.3. Pepsin
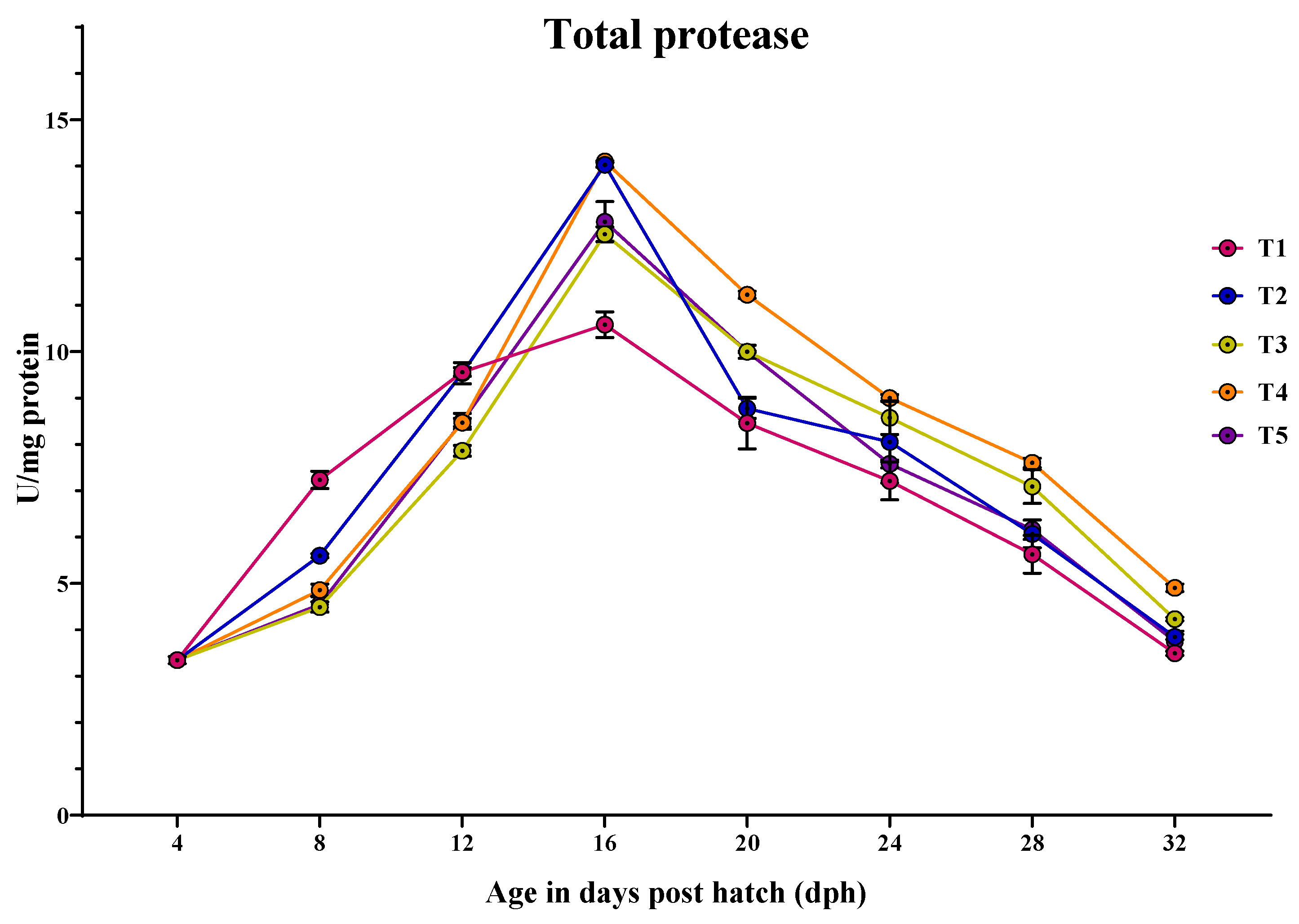
3.3.4. Total Protease
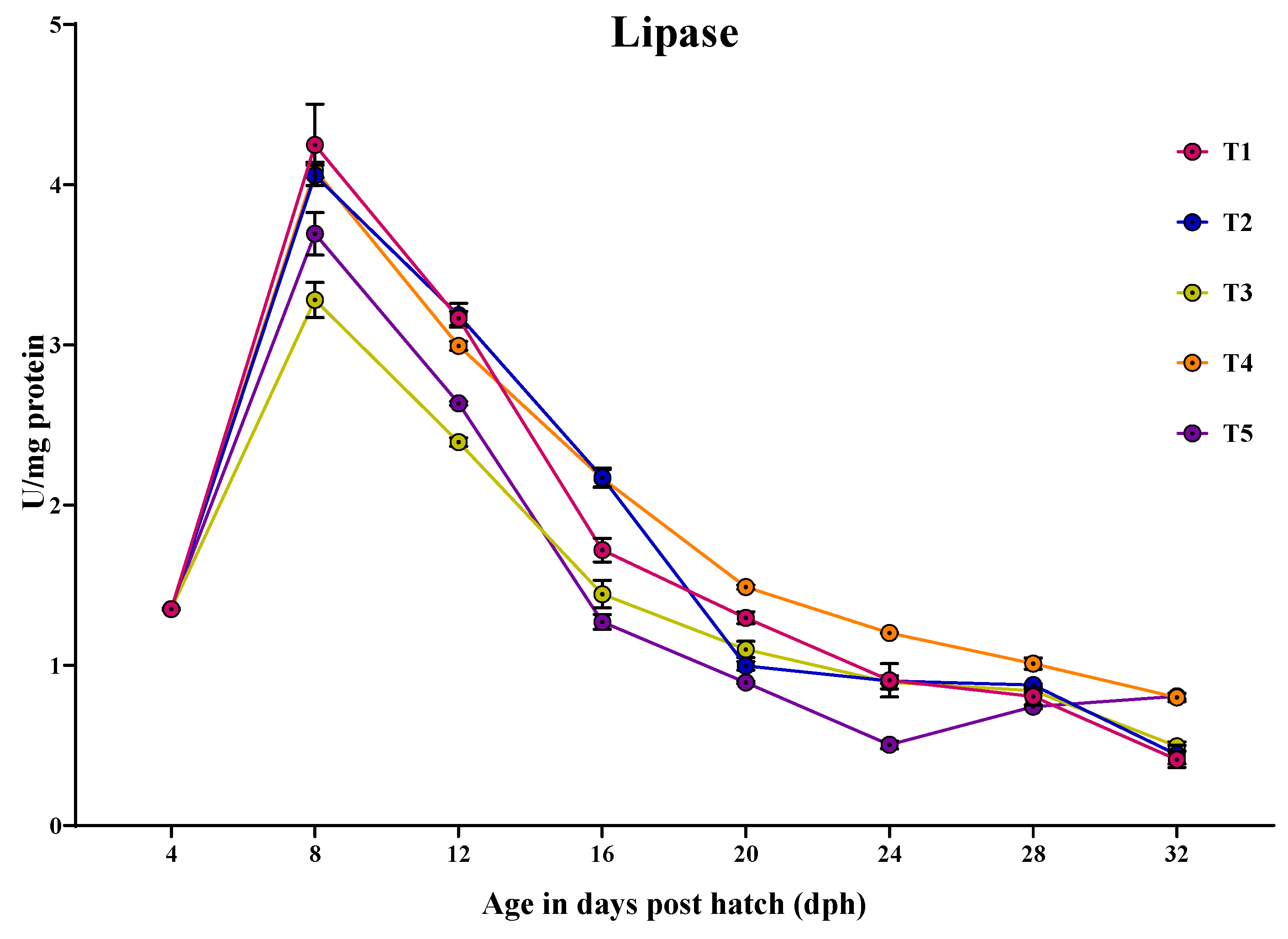
3.3.5. Lipase
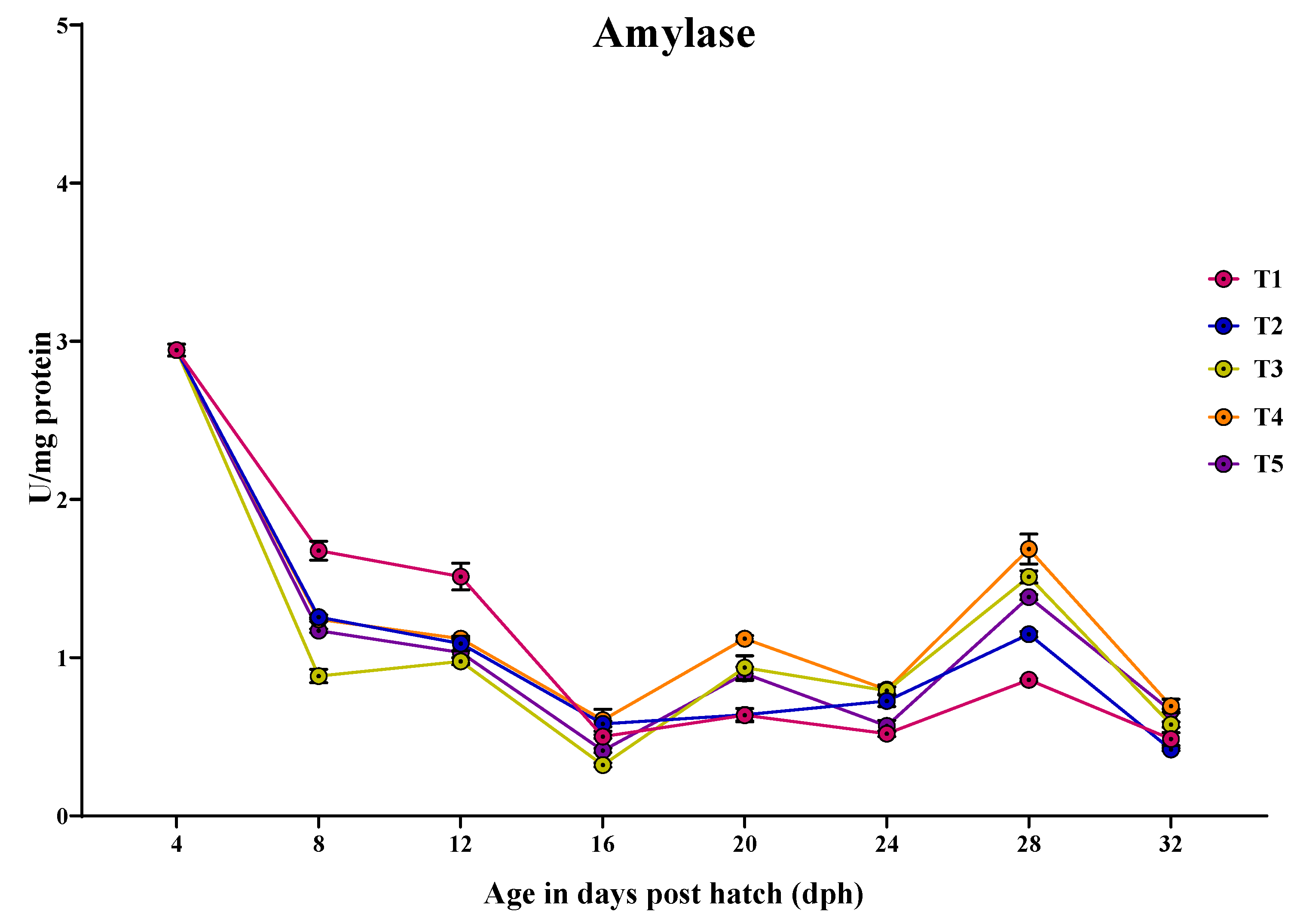
3.3.6. Amylase
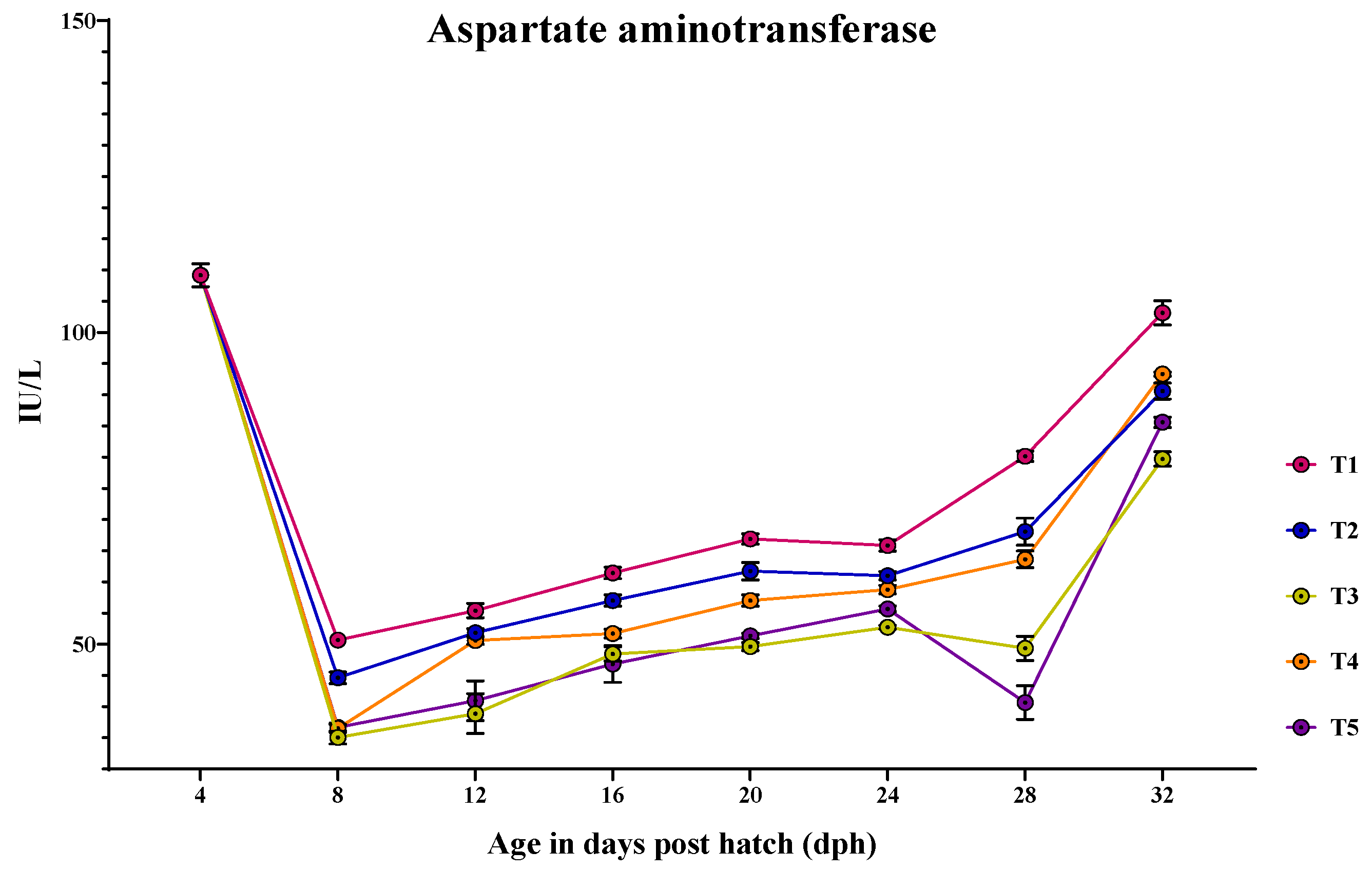
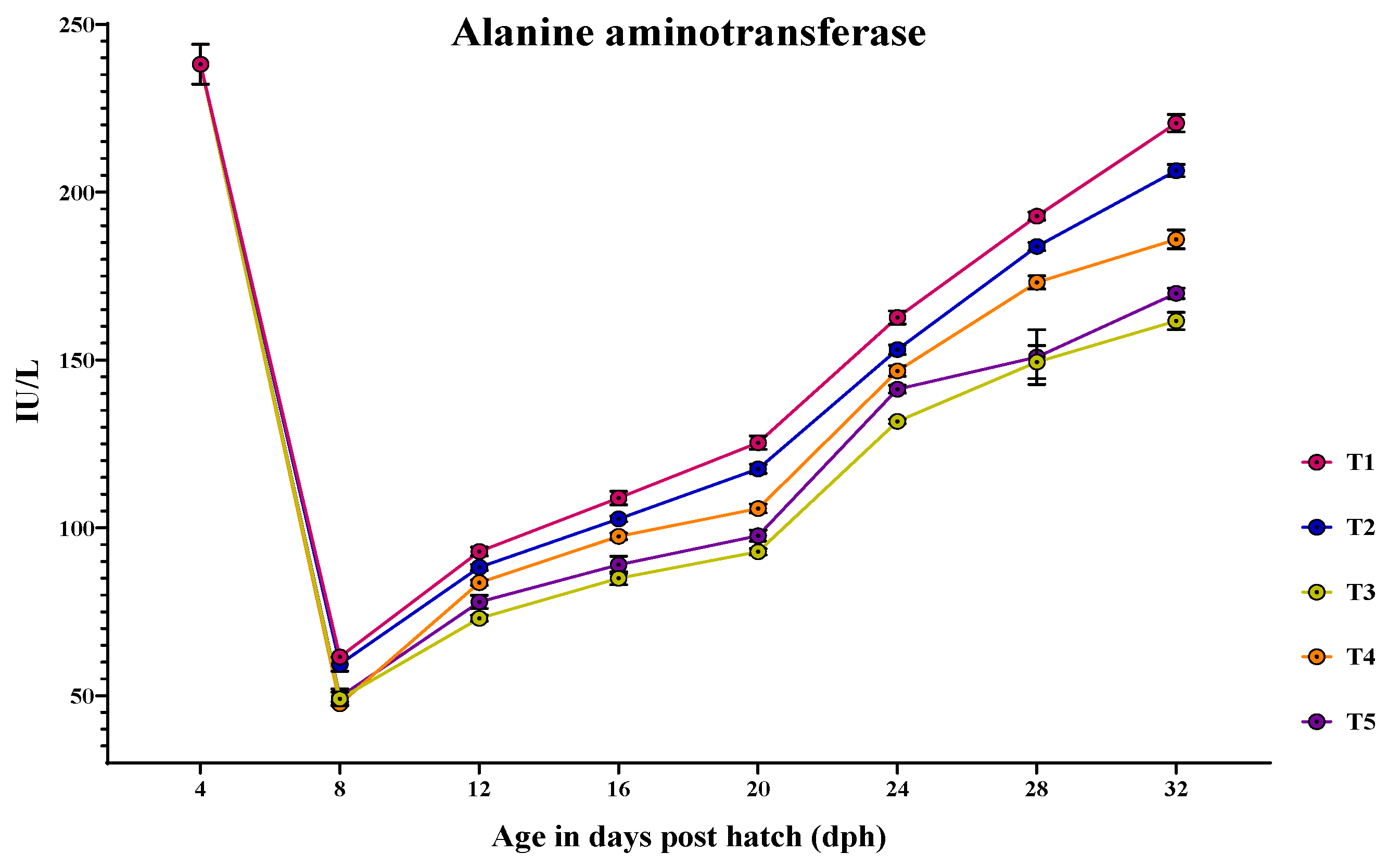
3.4. Ontogeny of Protein Metabolic Enzymes in Channa striatus Larvae Fed Different Experimental Diets
3.5. Histomorphological Development of Digestive System in Channa striatus Larvae Fed Different Experimental Diets
3.5.1. Intestine
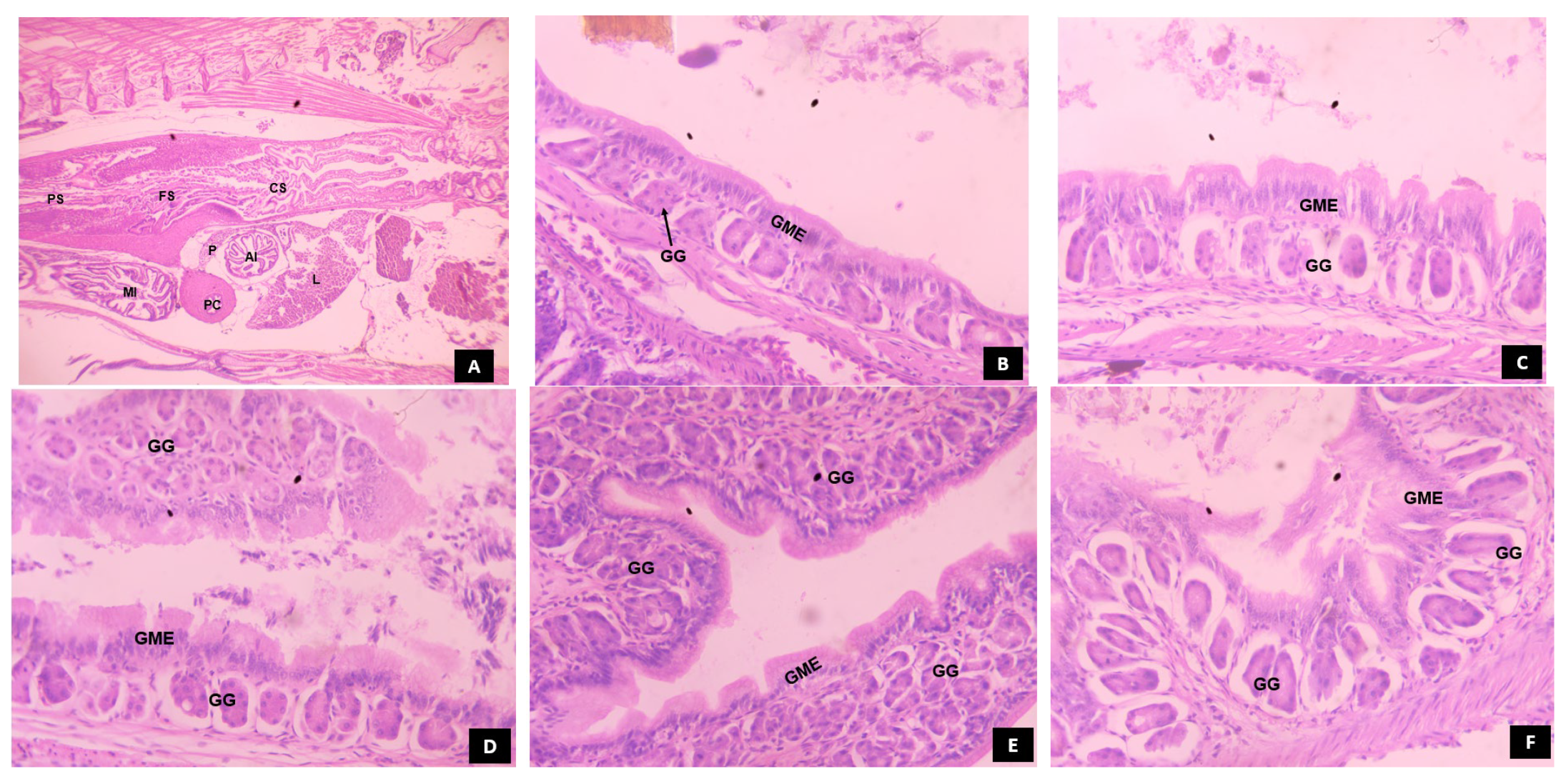
3.5.2. Stomach
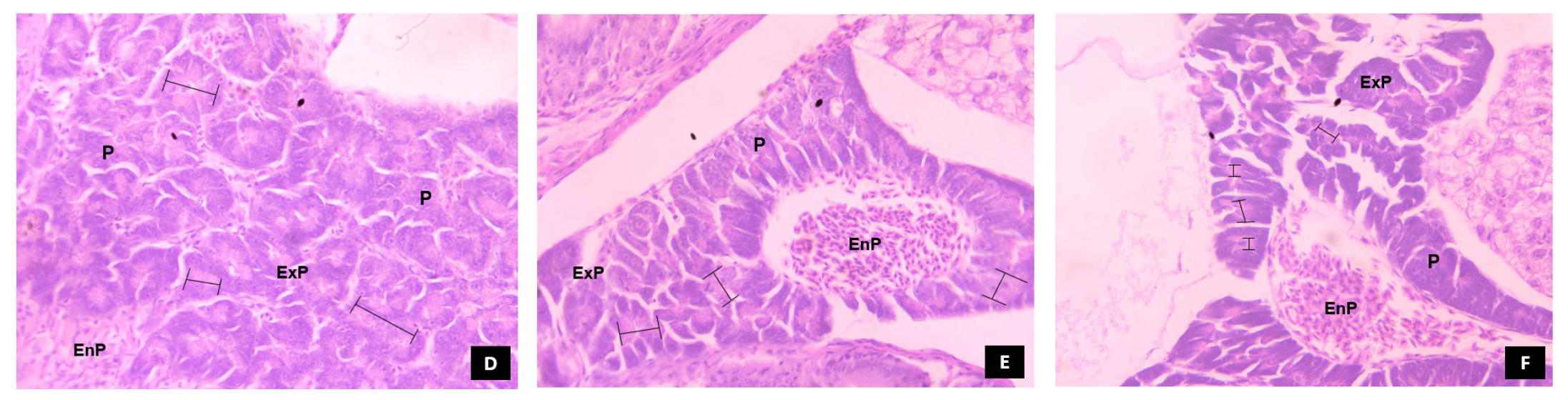
3.5.3. Pancreas
3.5.4. Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raizada, S.; Rawat, A.; Srivastava, P.P.; Lal, K.K. Cannibalism mitigation in striped murrel, Channa striata, with hatchery seed weaned on pellet diet: A review. Rev. Aquacult. 2022, 14, 1213–1233. [Google Scholar] [CrossRef]
- Siddaiah, G.M.; Kumar, R.; Kumari, R.; Chandan, N.K.; Debbarma, J.; Damle, D.K.; Das, A.; Giri, S.S. Dietary fishmeal replacement with Hermetia illucens (Black soldier fly, BSF) larvae meal affected production performance, whole body composition, antioxidant status, and health of snakehead (Channa striata) juveniles. Anim. Feed Sci. Technol. 2023, 297, 115597. [Google Scholar] [CrossRef]
- Kalaiselvan, P.; Ranjan, A.; Nazir, M.I.; Suresh, E. Exploring ontogenic development and larval rearing of striped murrel (Channa striatus). Aquacult. Int. 2024, 1–44. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nation: Rome, Italy, 2022. [Google Scholar]
- FAO. FAO FishStat: Global Aquaculture Production 2021 (Version 2.0), 2022. Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity (accessed on 29 March 2024).
- Lee, C.S. Biotechnological advances in finfish hatchery production: A review. Aquaculture 2003, 227, 439–458. [Google Scholar] [CrossRef]
- Kumari, S.; Tiwari, V.K.; Rani, A.M.; Kumar, R.; Prakash, S. Effect of feeding rate on growth, survival and cannibalism in striped snakehead, Channa striata (Bloch, 1793) fingerlings. J. Exper. Zool. India. 2018, 21, 205–210. [Google Scholar]
- Kumar, R.; Gokulakrishnan, M.; Debbarma, J.; Damle, D.K. Advances in captive breeding and seed rearing of striped murrel Channa striata, a high value food fish of Asia. Anim. Reprod. Sci. 2022, 238, 106957. [Google Scholar] [CrossRef]
- Sinha, M.; Mahapatra, B.K.; Saha, D.; Maitra, N.J. Mass scale seed production of Magur, Clarias batrachus at farm level through improvised modifications. Int. J. Fish. Aquat. Stud. 2014, 2, 210–214. [Google Scholar]
- Kumari, R.; Sharma, P.; Sarma, D.; Siddaiah, G.M.; Dubey, M.K.; Mir, I.N.; Srivastava, P.P. Ontogeny and development of the gastrointestinal system in Indian walking catfish (Clarias magur) during its early development. Fish Physiol. Biochem. 2021, 47, 1033–1052. [Google Scholar] [CrossRef]
- Saputra, A.; Suryaningrum, L.H.; Sunarno, M.T.D.; Samsudin, R.; Kholidin, E.B.; Prihadi, T.H.; Taukhid, T. Enhancing early weaning strategies through artificial feeding regimes for Channa striata larvae. Egypt. J. Aquat. Res. 2024, 50, 293–300. [Google Scholar] [CrossRef]
- Rosenlund, G.; Stoss, J.; Talbot, C. Co-feeding marine fish larvae with inert and live diets. Aquaculture 1997, 155, 183–191. [Google Scholar] [CrossRef]
- Hamre, K.; Yufera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquacult. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z. Substitution of live food by formulated diets in marine fish larvae. Aquaculture 2001, 200, 161–180. [Google Scholar] [CrossRef]
- Lazo, J.P.; Darias, M.J.; Gisbert, E. Ontogeny of the Digestive Tract. In Larval Fish Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 3–46. [Google Scholar]
- Gawlicka, A.; Parent, B.; Horn, M.H.; Ross, N.; Opstad, I.; Torrissen, O.J. Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): Indication of readiness for first feeding. Aquaculture 2000, 184, 303–314. [Google Scholar] [CrossRef]
- Kumari, R.; Srivastava, P.P.; Mohanta, K.N.; Das, P.; Kumar, R.; Sahoo, L.; Sharma, P.; Krishna, G.; Paul, A.; Siddaiah, G.M. Optimization of weaning age for striped murrel (Channa striata) based on expression and activity of proteases. Aquaculture 2024, 579, 740277. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Lei, W.; Yang, Y.; Han, D.; Jin, J.; Xie, S. Effects of different weaning strategies on survival and growth in Chinese longsnout catfish (Leiocassis longirostris Günther) larvae. Aquaculture 2012, 364, 13–18. [Google Scholar] [CrossRef]
- Mehraj-Ud-Din, W.; Altaf, K.; Hanifa, M.A. Comparative study on growth of Channa striatus fry and fingerlings using different feeding regimes. J. Aquat. Biol. 2009, 24, 173–176. [Google Scholar]
- Mehrajuddin, W.A.R.; Altaf, K.; Hanifa, M.A. Growth and survival of larval snakehead Channa striatus (Bloch, 1793) fed different live feed organisms. Turk. J. Fish. Aquat. Sci. 2011, 11, 523–528. [Google Scholar]
- Kemigabo, C.; Jere, L.W.; Sikawa, D.; Masembe, C.; Kang’ombe, J.; Abdel-Tawwab, M. Growth response of African catfish, Clarias gariepinus (B.), larvae and fingerlings fed protease-incorporated diets. J. App. Ichthyol. 2019, 35, 480–487. [Google Scholar] [CrossRef]
- Kandathil Radhakrishnan, D.; AkbarAli, I.; Schmidt, B.V.; John, E.M.; Sivanpillai, S.; Thazhakot Vasunambesan, S. Improvement of nutritional quality of live feed for aquaculture: An overview. Aquac. Res. 2020, 51, 1–17. [Google Scholar] [CrossRef]
- Pradhan, P.K.; Jena, J.; Mitra, G.; Sood, N.; Gisbert, E. Effects of different weaning strategies on survival, growth and digestive system development in butter catfsh Ompok bimaculatus (Bloch) larvae. Aquaculture 2014, 424, 120–130. [Google Scholar] [CrossRef]
- Wang, J.; Wan, A.; Fan, H.; Yin, X.; Qian, X.; Xie, S. Impacts of microdiet manufacturing technologies on the growth performance of large yellow croaker (Pseudosciaena crocea) larvae. Aquacult. Rep. 2020, 17, 100362. [Google Scholar] [CrossRef]
- Mozanzadeh, M.T.; Bahabadi, M.N.; Morshedi, V.; Azodi, M.; Agh, N.; Gisbert, E. Weaning strategies affect larval performance in yellowfin seabream (Acanthopagrus latus). Aquaculture 2021, 539, 736673. [Google Scholar] [CrossRef]
- Holt, G.J.; Webb, K.A.; Rust, M.B. Microparticulate diets: Testing and evaluating success. In Larval Fish Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 353–372. [Google Scholar]
- Djellata, A.; Sarih, S.; Hernández-Cruz, C.M.; Martínez-Rodríguez, G.; Gilannejad, N.; Roo, J. The effect of different co-feeding protocols on greater amberjack (Seriola dumerili, Risso 1810) larvae. Aquac. Nutr. 2021, 27, 1761–1776. [Google Scholar] [CrossRef]
- Koven, W.; Kolkovski, S.; Hadas, E.; Gamsiz, K.; Tandler, A. Advances in the development of microdiets for gilthead seabream, Sparus aurata: A review. Aquaculture 2001, 194, 107–121. [Google Scholar] [CrossRef]
- Gopalraaj, J.; Velayudhannair, K.; Arockiasamy, J.P.; Radhakrishnan, D.K. The effect of dietary supplementation of proteases on growth, digestive enzymes, oxidative stress, and intestinal morphology in fishes–A review. Aquacult. Int. 2024, 32, 745–765. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 18th ed.; AOAC Inc.: Washington, DC, USA, 2010. [Google Scholar]
- Kunitz, M. Crystalline soybean trypsin inhibitor: II. General properties. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef]
- Worthington, C.C. Worthington Enzyme Manual Related Biochemical; Freehold: New Jersey, NJ, USA, 1991; Volume 34, pp. 212–215. [Google Scholar]
- Drapeau, G. Protease from Staphylococcus aureus. In Methods in Enzymology; Lorand, B.L., Ed.; Academic Press: Cambirdge, MA, USA, 1974; p. 469. [Google Scholar]
- Rick, W.; Stegbauer, H.P. α-Amylase measurement of reducing groups. In Methods of Enzymatic Analysis; Academic Press: Cambirdge, MA, USA, 1974; pp. 885–890. [Google Scholar]
- Cherry, I.S.; Crandall, L.A., Jr. The specificity of pancreatic lipase: Its appearance in the blood after pancreatic injury. Am. J. Physiol. Leg. Content 1932, 100, 266–273. [Google Scholar] [CrossRef]
- Paray, B.A.; Hanifa, M.A.; War, M.U.D.; Al-Sadoon, M.K.; Park, Y.H.; Rather, I.A. Histological changes in the digestive tract of striped murrel larvae during ontogeny. Indian J. Geo-Mar. Sci. 2015, 44, 984–992. [Google Scholar]
- Abol-Munafi, A.B.; Tarn, B.M.; Ambak, M.A.; Ismail, P. Effect of different diets on growth and survival rates of snakehead (Channa striata, Bloch, 1797) larvae. Korean J. Biol. Sci. 2004, 8, 313–317. [Google Scholar] [CrossRef]
- Akbary, P.; Imanpoor, M.; Sudagar, M.; Makhdomi, N.M. Comparison between live food and artificial diet on survival rate, growth and body chemical composition of Oncorhynchus mykiss larvae. Iran. J. Fish. Sci. 2010, 9, 19–32. [Google Scholar]
- Hansen, Ø.J.; Puvanendran, V.; Jøstensen, J.P.; Falk-Petersen, I.B. Early introduction of an inert diet and unenriched Artemia enhances growth and quality of Atlantic cod (Gadus morhua) larvae. Aquac. Nutr. 2018, 24, 102–111. [Google Scholar] [CrossRef]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Civera-Cerecedo, R.; Alvarez-González, C.A.; García-Gómez, R.E.; Carrasco-Chávez, V.; Ortiz-Galindo, J.L.; RosalesVelázquez, M.O.; Grayeb-Del Álamo, T.; Moyano-López, F.J. Effect of microparticulate diets on growth and survival of spotted sand bass larvae, Paralabrax maculatofasciatus, at two early weaning times. J. World Aquac. Soc. 2008, 39, 22–36. [Google Scholar] [CrossRef]
- Engrola, S.; Conceição, L.E.; Dias, L.; Pereira, R.; Ribeiro, L.; Dinis, M.T. Improving weaning strategies for Senegalese sole: Effects of body weight and digestive capacity. Aquac. Res. 2007, 38, 696–707. [Google Scholar] [CrossRef]
- Onura, C.N.; den Broeck, W.; Nevejan, N.; Muendo, P.; Van Stappen, G. Growth performance and intestinal morphology of African catfish (Clarias gariepinus, Burchell, 1822) larvae fed on live and dry feeds. Aquaculture 2018, 489, 70–79. [Google Scholar] [CrossRef]
- Prakash, P.; Kumar, S.; Sardar, P.; Munilkumar, S.; Sahoo, S.; Satheesh, M.; Reena, H.; Mannur, V.; Patel, A. Optimization of weaning strategy in the climbing perch (Anabas testudineus, Bloch 1792) larvae on growth, survival, digestive, metabolic and stress responses. Fish Physiol. Biochem. 2023, 49, 1151–1169. [Google Scholar] [CrossRef]
- Mejri, S.C.; Tremblay, R.; Audet, C.; Wills, P.S.; Riche, M. Essential fatty acid requirements in tropical and coldwater marine fish larvae and juveniles. Front. Mar. Sci. 2021, 8, 680003. [Google Scholar] [CrossRef]
- Grayson, J.D.; Dabrowski, K. Utilization of live-food enrichment with polyunsaturated fatty acids (PUFA) for the intensive culture of Yellow perch Larvae. North. Am. J. Aquac. 2022, 84, 131–148. [Google Scholar] [CrossRef]
- Chepkirui-Boit, V.; Ngugi, C.C.; Bowman, J.; Oyoo-Okoth, E.; Rasowo, J.; Mugo-Bundi, J.; Cherop, L. Growth performance, survival, feed utilization and nutrient utilization of African catfish (Clarias gariepinus) larvae co-fed Artemia and a micro-diet containing freshwater atyid shrimp (Caridina nilotica) during weaning. Aquacult. Nutr. 2011, 17, 82–89. [Google Scholar] [CrossRef]
- Phan, T.C.T.; Nguyen, T.K.L.; Truong, T.P.T.; Pham, T.T.N.; Huynh, T.G.; Doan, X.D. Effects of noni fruit extract on the growth performance, digestive enzymes, and stress tolerance of juvenile whiteleg shrimp (Litopenaeus vannamei). Egypt. J. Aquat. Res. 2023, 49, 549–554. [Google Scholar] [CrossRef]
- Gisbert, E.; Piedrahita, R.H.; Conklin, D.E. Ontogenetic development of the digestive system in California halibut (Paralichthys californicus) with notes on feeding practices. Aquaculture 2004, 232, 455–470. [Google Scholar] [CrossRef]
- Pradhan, P.K.; Jena, J.K.; Mitra, G.; Sood, N.; Gisbert, E. Ontogeny of the digestive tract in butter catfish Ompok bimaculatus (Bloch) larvae. Fish Physiol. Biochem. 2012, 38, 1601–1617. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.N.; Srivastava, P.P.; Bhat, I.A.; Muralidhar, A.P.; Varghese, T.; Gireesh-Babu, P.; Jain, K.K. Expression and activity of trypsin and pepsin during larval development of Indian walking catfish (Clarias Magur). Aquaculture 2018, 491, 266–272. [Google Scholar] [CrossRef]
- Martínez-Lagos, R.; Tovar-Ramírez, D.; Gracia-Lopez, V.; Lazo, J.P. Changes in digestive enzyme activities during larval development of leopard grouper (Mycteroperca rosacea). Fish Physiol. Biochem. 2014, 40, 773–785. [Google Scholar] [CrossRef]
- Yúfera, M.; Moyano, F.J.; Martínez-Rodríguez, G. The digestive function in developing fish larvae and fry. From molecular gene expression to enzymatic activity. In Emerging Issues in Fish Larvae Research; Springer: Cham, Switzerland, 2018; pp. 51–86. [Google Scholar]
- Khoa, T.N.; Waqalevu, V.; Honda, A.; Shiozaki, K.; Kotani, T. An integrative description of the digestive system morphology and function of Japanese flounder (Paralichthys olivaceus) during early ontogenetic development. Aquaculture 2021, 531, 735855. [Google Scholar] [CrossRef]
- Tong, X.H.; Xu, S.H.; Liu, Q.H.; Li, J.; Xiao, Z.Z.; Ma, D.Y. Digestive enzyme activities of turbot (Scophthalmus maximus L.) during early developmental stages under culture condition. Fish Physiol. Biochem. 2012, 38, 715–724. [Google Scholar] [CrossRef]
- Gisbert, E.; Giménez, G.; Fernández, I.; Kotzamanis, Y.; Estévez, A. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 2009, 287, 381–387. [Google Scholar] [CrossRef]
- Darias, M.J.; Murray, H.M.; Martínez-Rodríguez, G.; Cardenas, S.; Yúfera, M. Gene expression of pepsinogen during the larval development of red porgy (Pagrus pagrus). Aquaculture 2005, 248, 245–252. [Google Scholar] [CrossRef]
- Srichanun, M.; Tantikitti, C.; Utarabhand, P.; Kortner, T.M. Gene expression and activity of digestive enzymes during the larval development of Asian seabass (Lates calcarifer). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 165, 1–9. [Google Scholar] [CrossRef]
- Murashita, K.; Fukasa, H.; Takahashi, N.; Hosomi, N.; Matsunari, H.; Furuita, H.; Oku, H.; Yamamoto, T. Effect of feed ingredients on digestive enzyme secretion in fish. Bull. Fish. Res. Agency 2015, 40, 69–74. [Google Scholar]
- Munilla-Moran, R.; Stark, J.R.; Barbour, A. The role of exogenous enzymes in digestion in cultured turbot larvae (Scophthalmus maximus L.). Aquaculture 1990, 88, 337–350. [Google Scholar] [CrossRef]
- Martinez, I.; Moyano, F.J.; Fernández-Díaz, C.; Yúfera, M. Digestive enzyme activity during larval development of the Senegal sole (Solea senegalensis). Fish Physiol. Biochem. 1999, 21, 317–323. [Google Scholar] [CrossRef]
- Alvarez-González, C.A.; Cervantes-Trujano, M.; Tovar-Ramírez, D.; Conklin, D.E.; Nolasco, H.; Gisbert, E.; Piedrahita, R. Development of digestive enzymes in California halibut Paralichthys californicus larvae. Fish Physiol. Biochem. 2005, 31, 83–93. [Google Scholar]
- Walford, J.; Lam, T.J. Development of digestive tract and proteolytic enzyme activity in seabass (Lates calcarifer) larvae and juveniles. Aquaculture 1993, 109, 187–205. [Google Scholar] [CrossRef]
- Morais, S.; Cahu, C.; Zambonino-Infante, J.L.; Robin, J.; Rønnestad, I.; Dinis, M.T.; Conceição, L.E.C. Dietary TAG source and level affect performance and lipase expression in larval sea bass (Dicentrarchus labrax). Lipids 2004, 39, 449–458. [Google Scholar] [CrossRef]
- Kolkovski, S.; Tandler, A.; Izquierdo, M.S. Effects of live food and dietary digestive enzymes on the efficiency of microdiets for seabass (Dicentrarchus labrax) larvae. Aquaculture 1997, 148, 313–322. [Google Scholar] [CrossRef]
- Kamaszewski, M.; Ostaszewska, T.; Prusińska, M.; Kolman, R.; Chojnacki, M.; Zabytyvskij, J.; Jankowska, B.; Kasprzak, R. Effects of Artemia sp. enrichment with essential fatty acids on functional and morphological aspects of the digestive system in Acipenser gueldenstaedtii larvae. Turk. J. Fish. Aquat. Sci. 2014, 14, 929–938. [Google Scholar] [CrossRef]
- Wiszniewski, G.; Jarmołowicz, S.; Hassaan, M.S.; Kamaszewski, M.; Szudrowicz, H.; Terech-Majewska, E.; Kawalski, K.; Martynow, J.; Szczepański, A.; Siwicki, A.K. Dietary effect of actinidin enzyme on growth, digestive enzymes activity, immunity, liver and intestine histology of juvenile sterlet sturgeon (Acipenser ruthenus). Aquacult. Rep. 2022, 25, 101196. [Google Scholar] [CrossRef]
- Wiszniewski, G.; Jarmołowicz, S.; Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Łuczyńska, J.; Tońska, E.; Terech-Majewska, E.; Ostaszewska, T.; Kamaszewski, M.; et al. The use of bromelain as a feed additive in fish diets: Growth performance, intestinal morphology, digestive enzyme and immune response of juvenile Sterlet (Acipenser ruthenus). Aquacult. Nutr. 2019, 25, 1289–1299. [Google Scholar] [CrossRef]
- Hamza, N.; Mhetli, M.; Kestemont, P. Effects of weaning age and diets on ontogeny of digestive activities and structures of pikeperch (Sander lucioperca) larvae. Fish Physiol. Biochem. 2007, 33, 121–133. [Google Scholar] [CrossRef]
- Lin, S.; Mai, K.; Tan, B. Effects of exogenous enzyme supplementation in diets on growth and feed utilization in tilapia, Oreochromis niloticus × O. aureus. Aquac. Res. 2007, 38, 1645–1653. [Google Scholar] [CrossRef]
- Haridas, H.; Verma, A.K.; Rathore, G.; Prakash, C.; Sawant, P.B.; Babitha Rani, A.M. Enhanced growth and immuno-physiological response of Genetically Improved Farmed Tilapia in indoor biofloc units at different stocking densities. Aquac. Res. 2017, 48, 4346–4355. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Socorro, J.; Arantzamendi, L.; Hernández-Cruz, C.M. Recent advances in lipid nutrition in fish larvae. Fish Physiol. Biochem. 2000, 22, 97–107. [Google Scholar] [CrossRef]
- Tan, P.; Zhu, W.; Zhang, P.; Wang, L.; Chen, R.; Xu, D. Dietary soybean lecithin inclusion promotes growth, development, and intestinal morphology of yellow drum (Nibea albiflora) larvae. Aquaculture 2022, 559, 738446. [Google Scholar] [CrossRef]
 , denotes the thickness of microvilli in the intestinal mucosal epithelium.
, denotes the thickness of microvilli in the intestinal mucosal epithelium.
 , denotes the thickness of microvilli in the intestinal mucosal epithelium.
, denotes the thickness of microvilli in the intestinal mucosal epithelium.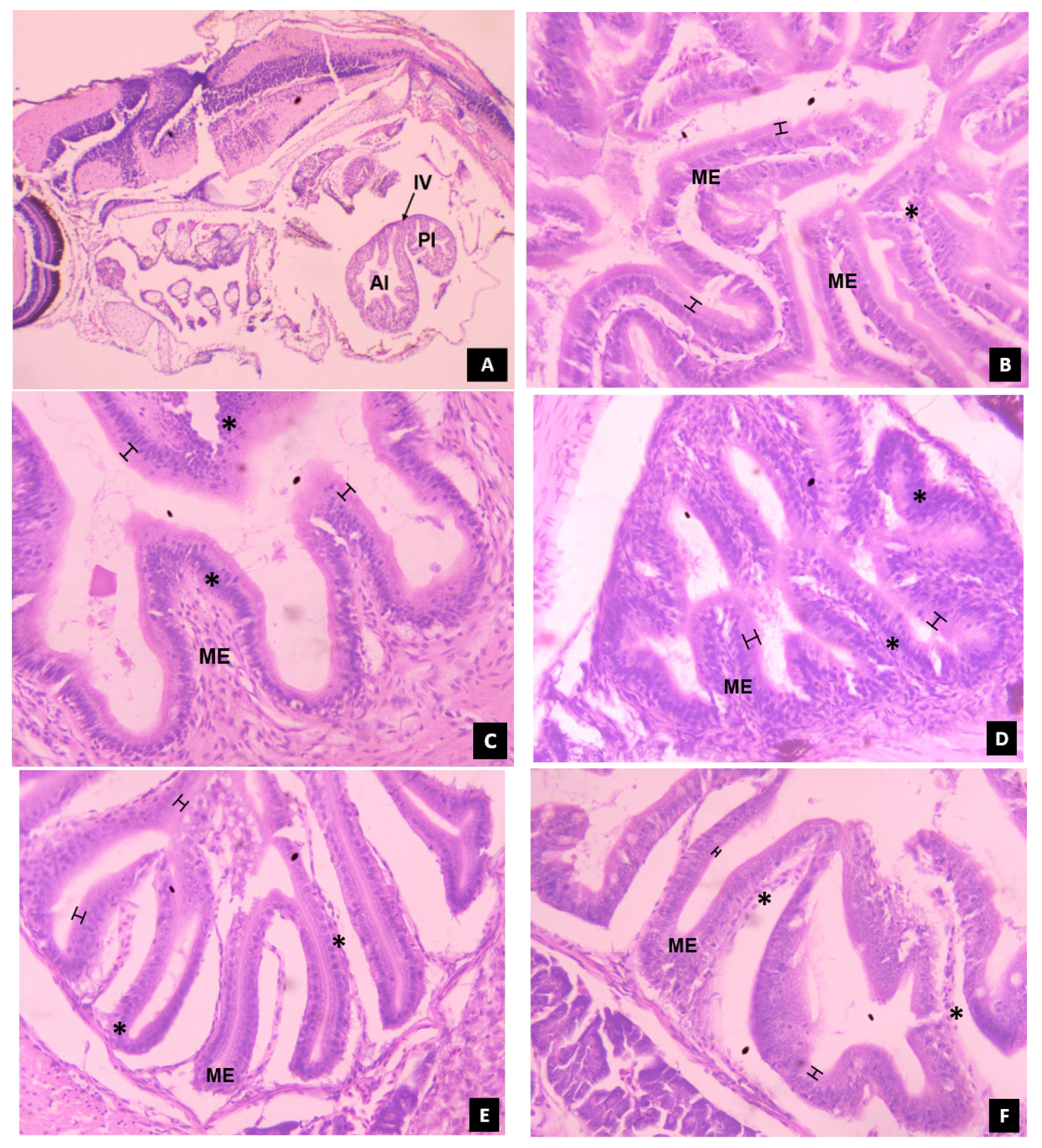
 , denotes the prevalence of zymogen granules in the pancreocytes arranged in acinus.
, denotes the prevalence of zymogen granules in the pancreocytes arranged in acinus.
 , denotes the prevalence of zymogen granules in the pancreocytes arranged in acinus.
, denotes the prevalence of zymogen granules in the pancreocytes arranged in acinus.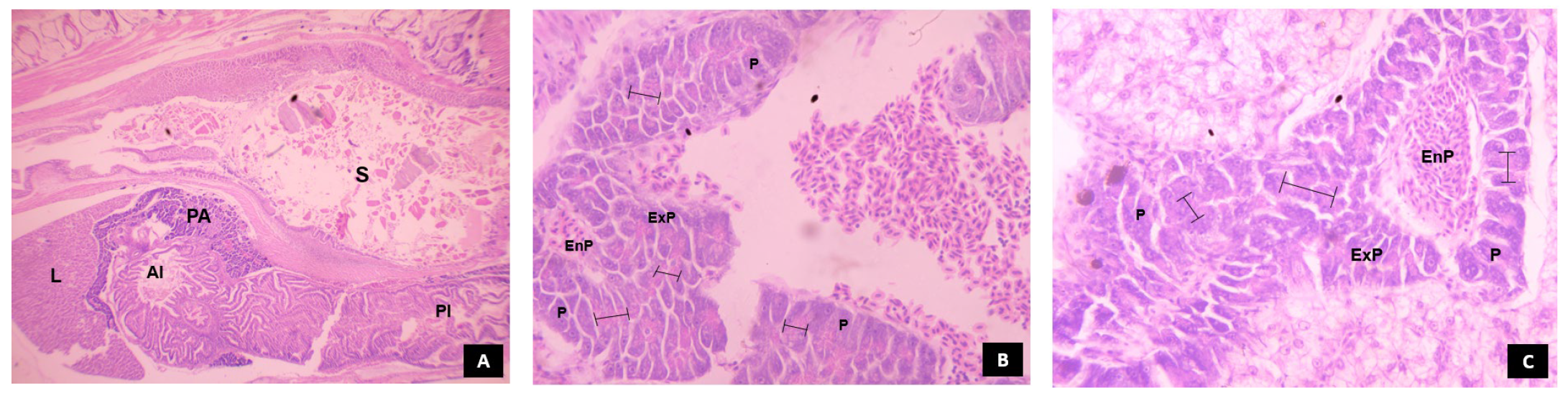

| Test Diet | Weaning Method |
|---|---|
| T1 | Live feed (Artemia nauplii instar stage I) |
| T2 | Live feed + Formulated micro diet |
| T3 | Formulated Micro diet |
| T4 | Formulated Micro diet with protease enzyme supplementation |
| T5 | Commercial larval diet |
| Ingredients (% of Dry Weight) | Formulated Micro Diet | Formulated Diet with Protease Enzyme |
|---|---|---|
| Tuna Fish Meal | 44 | 44 |
| Squid meal | 11 | 11 |
| Fish protein hydrolysate | 10 | 10 |
| Shrimp heal meal | 5 | 5 |
| Soy protein concentrate | 8.3 | 8.3 |
| Wheat flour | 8 | 7.9 |
| Sunflower oil | 2.5 | 2.5 |
| Fish oil | 2.5 | 2.5 |
| Soy lecithin | 2 | 2 |
| Vitamin premix 1 | 1.5 | 1.5 |
| Mineral premix 2 | 1.5 | 1.5 |
| Vitamin C | 1.5 | 1.5 |
| Vitamin E | 0.2 | 0.2 |
| L-Tryptophan | 0.5 | 0.5 |
| Carboxy methyl cellulose | 1.5 | 1.5 |
| Protease enzyme 3 | - | 0.1 |
| Proximate Composition (%) 4 | ||
| Moisture | 10.34 | 10.41 |
| Crude protein | 52.43 | 52.26 |
| Crude lipid | 12.44 | 12.21 |
| Total Ash | 15.34 | 15.54 |
| Parameters (%) 1 | Artemia nauplii Instar I | Commercial Diet |
|---|---|---|
| Moisture | 84.14 | 12.3 |
| Crude protein | 53.29 | 52.67 |
| Crude lipid | 15.23 | 12.23 |
| Total ash | 8.89 | 12.8 |
| Parameter | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | ||
| Survival rate (%) | ||||||
| 8-days post hatch | 80.479 ± 1.71 a | 61.979 ± 0.62 c | 65.688 ± 1.65 bc | 62.854 ± 1.38 c | 68.562 ± 0.53 b | <0.001 |
| 12-days post hatch | 93.113 ± 0.35 a | 90.665 ± 1.19 a | 83.280 ± 1.49 b | 90.627 ± 2.67 a | 83.896 ± 0.38 b | 0.003 |
| 16-days post hatch | 87.924 ± 0.24 bc | 92.414 ± 1.89 ab | 94.206 ± 2.47 a | 96.973 ± 0.33 a | 86.328 ± 1.88 c | 0.005 |
| 20-days post hatch | 98.858 ± 0.25 a | 99.858 ± 0.14 a | 99.329 ± 0.26 a | 99.580 ± 0.34 a | 94.326 ± 1.77 b | 0.004 |
| 24-days post hatch | 99.256 ± 0.38 a | 99.812 ± 0.12 a | 99.770 ± 0.164 a | 99.899 ± 0.05 a | 98.042 ± 1.17 a | 0.183 |
| 28-days post hatch | 99.964 ± 0.035 a | 99.558 ± 0.15 ab | 99.858 ± 0.08 a | 99.362 ± 0.56 ab | 98.432 ± 0.54 b | 0.081 |
| 32-days post hatch | 100.00 ± 0.00 a | 99.797 ± 0.20 a | 99.858 ± 0.08 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 0.461 |
| CM (%) | 34.875 ± 1.65 c | 47.292 ± 0.47 ab | 48.313 ± 1.04 a | 43.208 ± 1.63 b | 51.708 ± 2.01 a | <0.001 |
| Parameter | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | ||
| 1 IMW (mg) | 0.646 ± 0.01 | 0.646 ± 0.01 | 0.646 ± 0.01 | 0.646 ± 0.01 | 0.646 ± 0.01 | 1.000 |
| 8-day old weight (mg) | 24.896 ± 0.14 a | 23.020 ± 0.17 b | 17.863 ± 0.29 d | 21.016 ± 0.20 c | 17.896 ± 0.11 d | <0.001 |
| 12-day old weight (mg) | 47.136 ± 0.20 a | 47.110 ± 0.28 a | 32.813 ± 0.42 c | 42.066 ± 0.145 b | 32.833 ± 0.18 c | <0.001 |
| 16-day old weight (mg) | 68.533 ± 0.38 b | 71.620 ± 0.40 a | 65.023 ± 0.15 c | 71.600 ± 0.40 a | 64.556 ± 0.37 c | <0.001 |
| 20-day old weight (mg) | 115.890 ± 0.16 d | 118.966 ± 0.17 c | 122.023 ± 0.15 b | 128.156 ± 0.18 a | 121.890 ± 0.15 b | <0.001 |
| 24-day old weight (mg) | 134.856 ± 0.14 d | 142.103 ± 0.41 c | 149.566 ± 0.50 b | 152.356 ± 0.36 a | 149.086 ± 0.50 b | <0.001 |
| 28-day old weight (mg) | 160.240 ± 1.35 d | 171.066 ± 1.82 c | 177.526 ± 0.40 b | 182.426 ± 0.51 a | 177.626 ± 0.43 b | <0.001 |
| 32-day old weight (mg) | 194.606 ± 0.51 d | 216.123 ± 0.23 c | 237.047 ± 0.17 b | 245.870 ± 0.20 a | 236.240 ± 0.52 b | <0.001 |
| 2 ADG (mg) | 6.688 ± 0.01 d | 7.430 ± 0.008 c | 8.151 ± 0.006 b | 8.456 ± 0.007 a | 8.124 ± 0.17 b | <0.001 |
| 3 MWG (mg) | 193.966 ± 0.51 d | 215.483 ± 0.23 c | 236.407 ± 0.17 b | 245.230 ± 0.20 a | 235.607 ± 0.52 b | <0.001 |
| 4 FCR | 1.889 ± 0.17 a | 3.529 ± 0.03 b | 5.347 ± 0.20 d | 4.53 ± 0.03 c | 5.658 ± 0.27 d | <0.001 |
| 5 PER | 1.015 ± 0.08 a | 0.534 ± 0.004 b | 0.353 ± 0.01 c | 0.416 ± 0.002 bc | 0.341 ± 0.01 c | <0.001 |
| 6 SGR (%/day) | 19.609 ± 0.05 d | 19.918 ± 0.003 c | 20.236 ± 0.002 b | 20.363 ± 0.002 a | 20.225 ± 0.007 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaiselvan, P.; Ranjan, A.; Nazir, M.I.; Suresh, E.; Thangarani, A.J.; Malarvizhi, K. Effect of Different Early Weaning Diets on Survival, Growth, and Digestive Ontogeny of Channa striatus (Bloch, 1793) Larvae. Animals 2024, 14, 2838. https://doi.org/10.3390/ani14192838
Kalaiselvan P, Ranjan A, Nazir MI, Suresh E, Thangarani AJ, Malarvizhi K. Effect of Different Early Weaning Diets on Survival, Growth, and Digestive Ontogeny of Channa striatus (Bloch, 1793) Larvae. Animals. 2024; 14(19):2838. https://doi.org/10.3390/ani14192838
Chicago/Turabian StyleKalaiselvan, Pandi, Amit Ranjan, Mir Ishfaq Nazir, Eswaran Suresh, Albin Jemila Thangarani, and Kavitha Malarvizhi. 2024. "Effect of Different Early Weaning Diets on Survival, Growth, and Digestive Ontogeny of Channa striatus (Bloch, 1793) Larvae" Animals 14, no. 19: 2838. https://doi.org/10.3390/ani14192838
APA StyleKalaiselvan, P., Ranjan, A., Nazir, M. I., Suresh, E., Thangarani, A. J., & Malarvizhi, K. (2024). Effect of Different Early Weaning Diets on Survival, Growth, and Digestive Ontogeny of Channa striatus (Bloch, 1793) Larvae. Animals, 14(19), 2838. https://doi.org/10.3390/ani14192838






