Acclimation during Embryogenesis Remodulates Telomerase Activity and Gene Expression in Baikal Whitefish Larvae, Mitigating the Effects of Acute Temperature Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
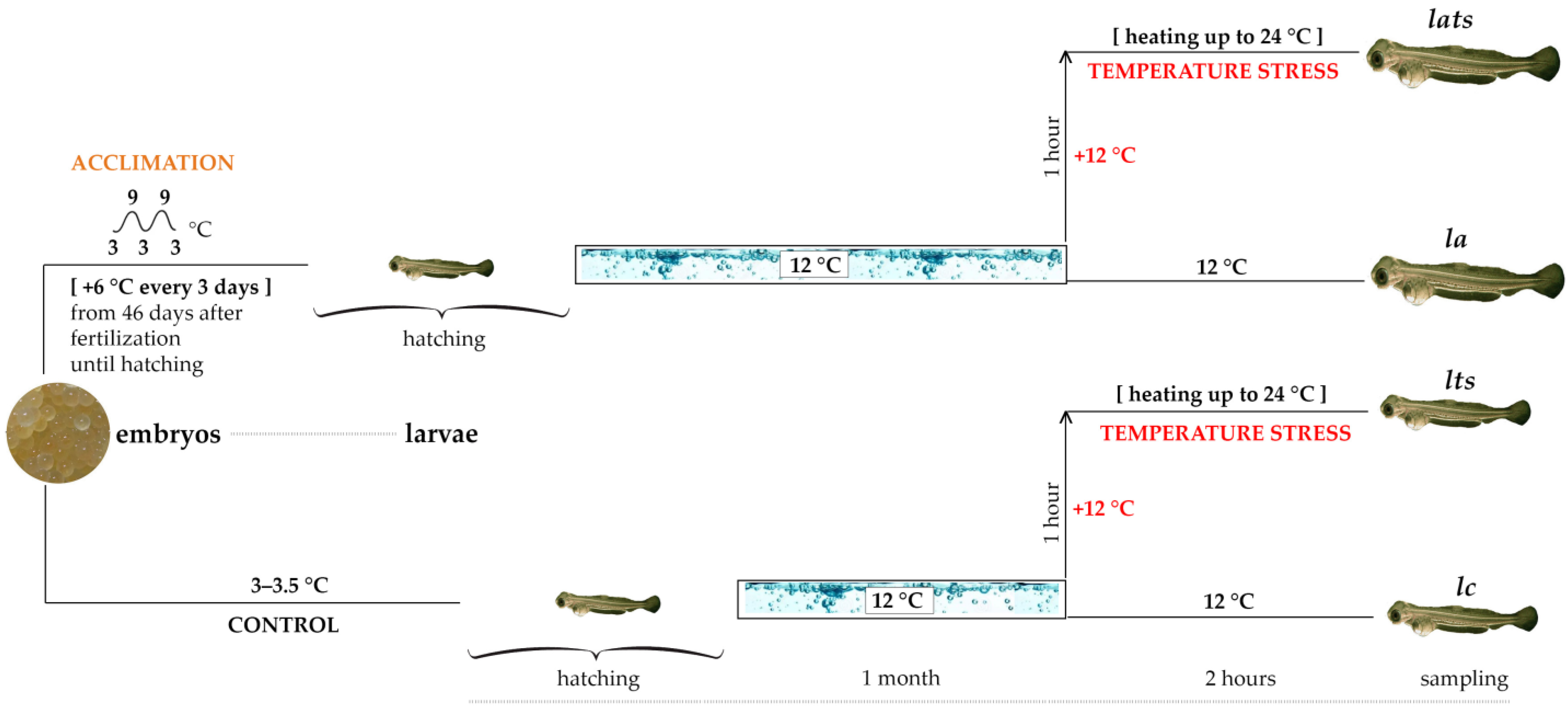
2.1. Rearing of the Baikal Whitefish Larvae and Experimental Setup
2.2. Sequencing and Transcriptome Analysis
2.3. Determination of the Telomere Length and Telomerase Activity
2.4. Statistical Analysis
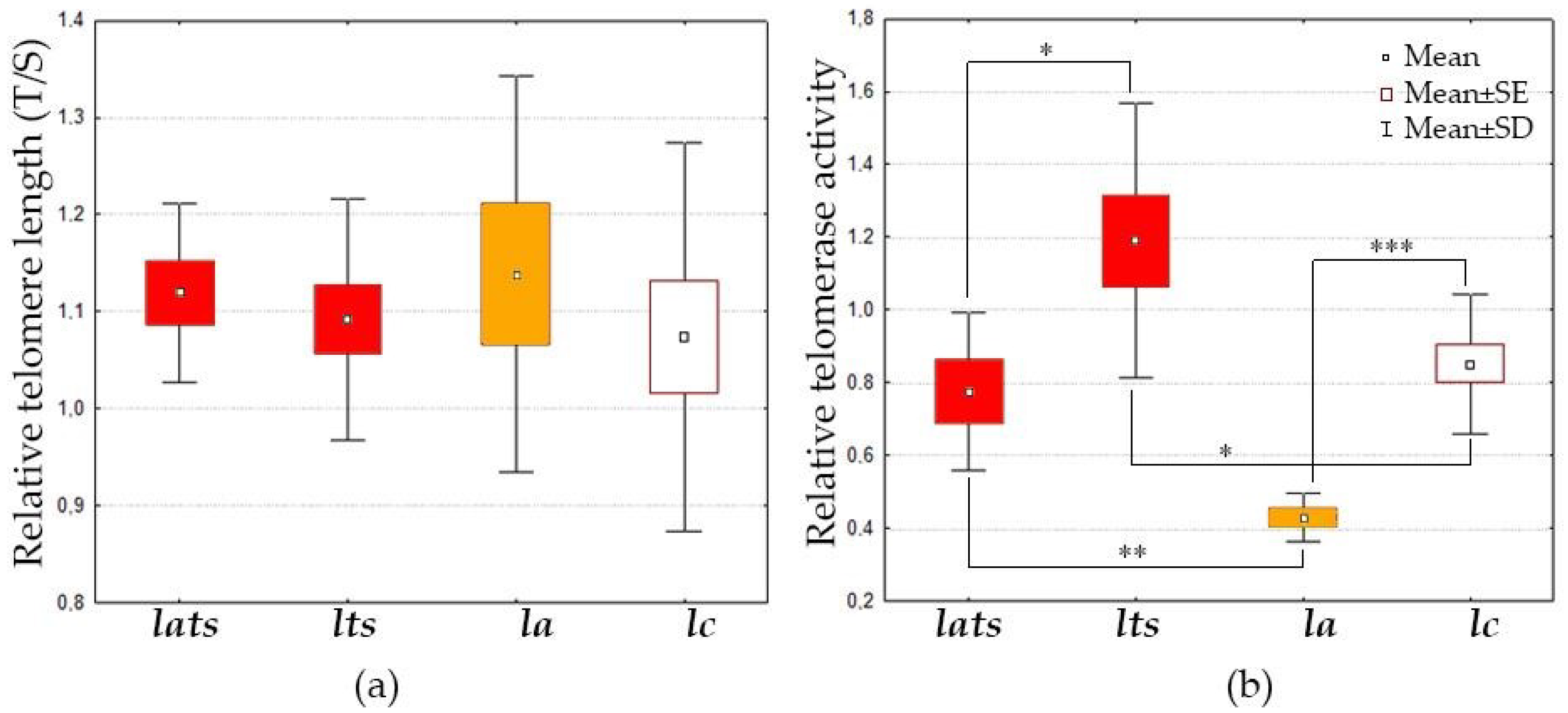
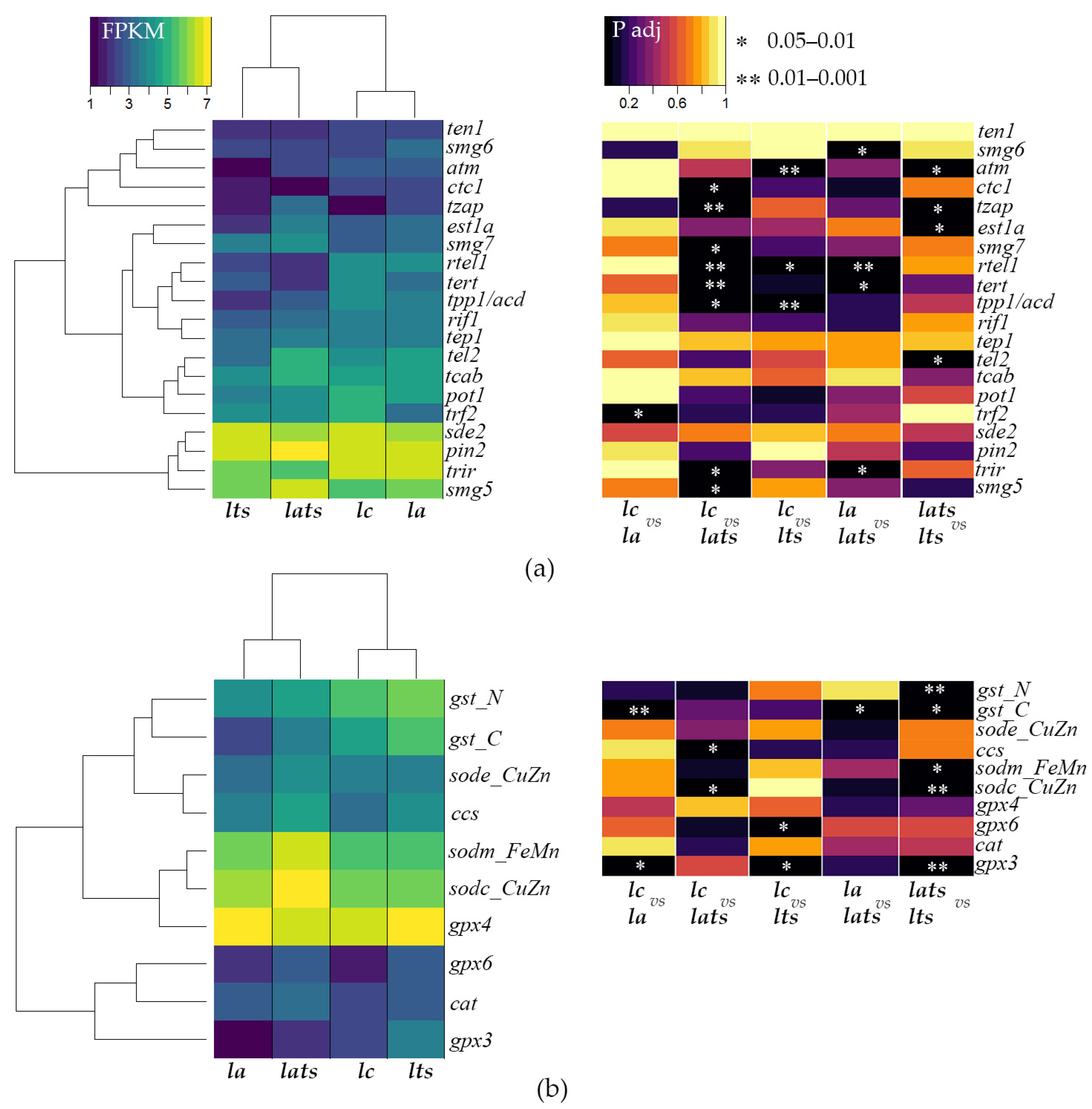
3. Results
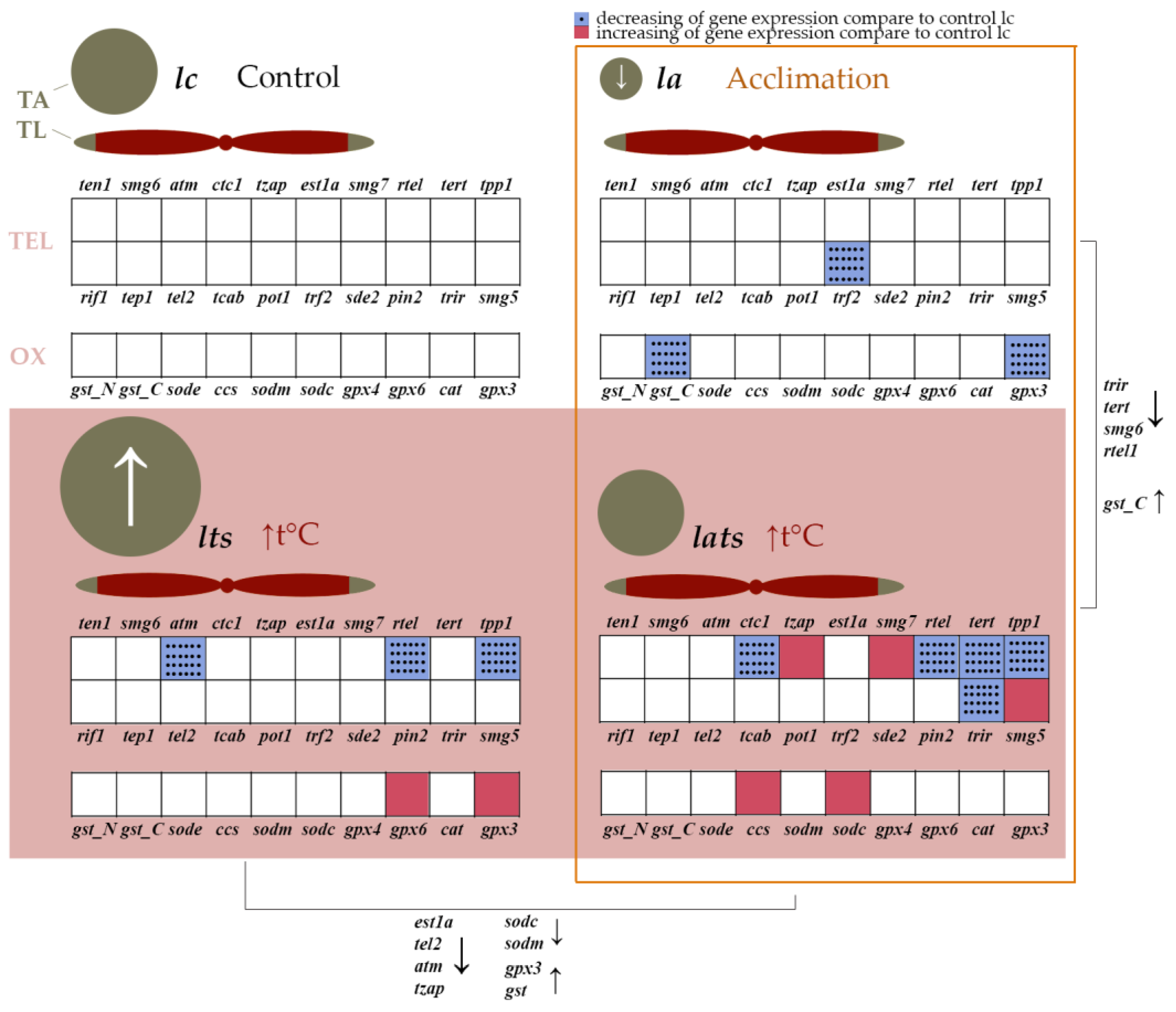
4. Discussion
4.1. Stability of Telomere Length Is Ensured by Different Telomerase Activity in Acclimated and Non-Acclimated Individuals under Acute Temperature Stress
4.2. Effect of Acclimation and Temperature Stress on the Expression of Genes Involved in Telomere Length Maintenance
4.3. Different Activity Profiles of Antioxidant Enzyme Genes in Acclimated and Non-Acclimated Individuals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulte, P. What is environmental stress? Insights from fish living in a variable environment. J. Exp. Biol. 2014, 217, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; Fisher, S., Reason, H., Eds.; John Wiley and Sons: New York, NY, USA, 1988; pp. 629–649. [Google Scholar]
- McEwen, B.S.; Wingfield, J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010, 57, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Habib, K.E.; Gold, P.W.; Chrousos, G.P. Neuroendocrinology of stress. Endocrinol. Metab. Clin. N. Am. 2001, 30, 695–728. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Collier, R.J.; Gebremedhin, K.G. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Asseng, S.; Spänkuch, D.; Hernandez-Ochoa, I.M.; Laporta, J. The upper temperature thresholds of life. Lancet Planet Health 2021, 5, e378–e385. [Google Scholar] [CrossRef]
- Healy, T.M.; Schulte, P.M. Factors affecting plasticity in whole-organism thermal tolerance in common killifish (Fundulus heteroclitus). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 49–62. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.; Vinagre, C.; Diniz, M. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Diniz, M.S. Are fish in hot water? Effects of warming on oxidative stress metabolism in the commercial species Sparus aurata. Ecol. Indic. 2016, 63, 324–331. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van, J.M. Raamsdonk beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Metcalfe, N.B.; Monaghan, P. Ecological processes in a hormetic framework. Ecol. Lett. 2010, 13, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.J.; Friesen, C.R. Invited review: Thermal effects on oxidative stress in vertebrate ectotherms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 263, 111082. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Haussmann, M. Telomeres: Linking stress and survival, ecology and evolution. Curr. Zool. 2010, 56, 714–727. [Google Scholar] [CrossRef]
- Monaghan, P.; Olsson, M.; Richardson, D.S.; Verhulst, S.; Rogers, S.M. Integrating telomere biology into the ecology and evolution of natural populations: Progress and prospects. Mol. Ecol. 2022, 31, 5909–5916. [Google Scholar] [CrossRef]
- Salmón, P.; Burraco, P. Telomeres and anthropogenic disturbances in wildlife: A systematic review and meta-analysis. Mol. Ecol. 2022, 31, 6018–6039. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Greider, C.W.; Szostak, J. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef]
- Rollings, N.; Miller, E.; Olsson, M. Telomeric attrition with age and temperature in Eastern mosquitofish (Gambusia holbrooki). Naturwissenschaften 2014, 101, 241–244. [Google Scholar] [CrossRef]
- Simide, R.; Angelier, F.; Gaillard, S.; Stier, A. Age and heat stress as determinants of telomere length in a long-lived fish, the Siberian sturgeon. Physiol. Biochem. Zool. 2016, 89, 441–447. [Google Scholar] [CrossRef]
- Debes, P.V.; Visse, M.; Panda, B.; Ilmonen, P.; Vasemägi, A. Is telomere length a molecular marker of past thermal stress in wild fish? Mol. Ecol. 2016, 25, 5412–5424. [Google Scholar] [CrossRef] [PubMed]
- McLennan, D.; Armstrong, J.D.; Stewart, D.C.; Mckelvey, S.; Boner, W.; Monaghan, P.; Metcalfe, N.B. Interactions between parental traits, environmental harshness and growth rate in determining telomere length in wild juvenile salmon. Mol. Ecol. 2016, 25, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Noreikiene, K.; Kuparinen, A.; Merilä, J. Age at maturation has sex- and temperature-specific effects on telomere length in a fish. Oecologia 2017, 184, 767–777. [Google Scholar] [CrossRef]
- Yang, S.; Li, D.; Feng, L.; Zhang, C.; Xi, D.; Liu, H.; Yan, C.; Xu, Z.; Zhang, Y.; Li, Y.; et al. Transcriptome analysis reveals the high temperature induced damage is a significant factor affecting the osmotic function of gill tissue in Siberian sturgeon (Acipenser baerii). BMC Genom. 2023, 24, 2. [Google Scholar] [CrossRef]
- Wang, Y.; Su, C.; Liu, Q.; Hao, X.; Han, S.; Doretto, L.B.; Rosa, I.F.; Yang, Y.; Shao, C.; Wang, Q. Transcriptome analysis revealed the early heat stress response in the brain of Chinese tongue sole (Cynoglossus semilaevis). Animals 2024, 14, 84. [Google Scholar] [CrossRef]
- McCormick, J.H.; Joncs, B.R.; Syrctlc, R.F. Temperature requircments for growth and survival of larval ciscos (Coregonus urtedii). J. Fish Res. Board Can. 1971, 28, 924–927. [Google Scholar] [CrossRef]
- Golovanov, V.K. Temperature Criteria of the Life Activity of Freshwater Fish; Golovanova, I.L., Ed.; Poligraf-plyus: Moscow, Russia, 2013; 300p. [Google Scholar]
- Rebl, A.; Verleih, M.; Nipkow, M.; Altmann, S.; Bochert, R.; Goldammer, T. Gradual and acute temperature rise induces crossing endocrine, metabolic, and immunological pathways in Maraena whitefish (Coregonus maraena). Front. Genet. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Manzon, L.A.; Zak, M.A.; Agee, M.; Boreham, D.R.; Wilson, J.Y.; Somers, C.M.; Manzon, R.G. Thermal acclimation alters both basal heat shock protein gene expression and the heat shock response in juvenile lake whitefish (Coregonus clupeaformis). J. Therm. Biol. 2022, 104, 103185. [Google Scholar] [CrossRef] [PubMed]
- Semenchenko, S.M.; Smeshlivaya, N.V. Resistance of larves and fry of Coregonus tugun and Stenodus leucichthys nelma to effects of high temperatures. Artif. Reprod. Aquat. Biol. Resour. 2022, 2, 235–239. [Google Scholar]
- Edsall, T.; Rottiers, D.V. Temperature tolerance of young-of-the-year lake whitefish, Coregonus clupeaformis. Wsq Women’s Stud. Q. 1976, 33, 177–180. [Google Scholar] [CrossRef]
- Stewart, T.R.; Vinson, M.R.; Stockwell, J.D. Effects of warming winter embryo incubation temperatures on larval cisco (Coregonus artedi) survival, growth, and critical thermal maximum. J. Great Lakes Res. 2022, 48, 1042–1049. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review. Aquac. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Sessions, K.J.; Whitehouse, L.M.; Manzon, L.A.; Boreham, D.R.; Somers, C.M.; Wilson, J.Y.; Manzon, R.G. The heat shock response shows plasticity in embryonic lake whitefish (Coregonus clupeaformis) exposed to repeated thermal stress. J. Therm. Biol. 2021, 100, 103036. [Google Scholar] [CrossRef]
- Chernyaev, Z.A. Reproduction of whitefish. In Ecological and Physiological Features of Reproduction and Development; Posuvalyuk, S.N., Ed.; Partnership of scientific publications KMK: Moskow, Russia, 2017; 329p. [Google Scholar]
- Kostuynichev, V.V.; Bogdanova, V.A.; Shumilina, A.K.; Ostroumova, I.N. Artificial reproduction of fishes in the Northwest of Russia. Tr. VNIRO 2015, 153, 26–41. [Google Scholar]
- Wanzenböck, J. Rearing and stocking of coregonids: A comparison of aquaculture practices in Eurasia and North America. Adv. Limnol. 2021, 66, 311–327. [Google Scholar] [CrossRef]
- Brooke, L.T. Effect of different constant incubation temperatures on egg survival and embryonic development in lake whitefish (Coregonus clupeaformis). Trans. Am. Fish. Soc. 1975, 104, 555–559. [Google Scholar] [CrossRef]
- Golovanov, V.K. Ecological and physiological optimum temperature and upper temperature limits of coregonid fish life. In Biology, Biotechnology of Breeding and the State of Stocks of Whitefish; Litvinenko, A.I., Reshetnikov, Y.S., Eds.; State scientific and production center of fisheries: Tyumen, Russia, 2013; pp. 51–55. [Google Scholar]
- Sidorova, T.V.; Smirnov, V.V.; Kirilchik, S.V.; Sukhanova, L.V. Study of population structure of Baikal whitefish based on the polymorphism of microsatellite loci. Russ. J. Genet. 2022, 58, 1311–1324. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Shen, S.; Park, J.W.; Lu, Z.X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Voropaeva, E.; Maksimov, V.; Malyutina, S.; Bobak, M.; Voevoda, M. Effects of DNA quality on the measurement of telomere length. Mol. Biol. 2015, 49, 508–512. [Google Scholar] [CrossRef]
- Cawthon, R. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Yip, B.W.; Mok, H.O.; Peterson, D.R.; Wan, M.T.; Taniguchi, Y.; Ge, W.; Au, D.W. Sex-dependent telomere shortening, telomerase activity and oxidative damage in marine medaka Oryzias melastigma during aging. Mar. Pollut. Bull. 2017, 124, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikova, Y.P.; Koroleva, A.G.; Yakhnenko, V.M.; Volkova, A.A.; Avezova, T.N.; Glyzina, O.Y.; Tolstikova, L.I.; Sakirko, M.V.; Sukhanova, L.V. Thermal preconditioning alters the stability of hump-snout whitefish (Coregonus fluviatilis) and its hybrid form, showing potential for aquaculture. Biology 2023, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.P.; Koroleva, A.G.; Sidorova, T.V.; Potapov, S.A.; Epifantsev, A.A.; Vakhteeva, E.A.; Tolstikova, L.I.; Glyzina, O.Y.; Yakhnenko, V.M.; Cherezova, V.M.; et al. Transcriptional rearrangements associated with thermal stress and preadaptation in Baikal whitefish (Coregonus baicalensis). Animals 2024. [Google Scholar]
- Seebacher, F.; White, C.R.; Franklin, C.E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Chang. 2015, 5, 61–66. [Google Scholar] [CrossRef]
- Coughlin, D.J.; Hittle, K.A.; Kitchin, M.; Kwon, E.S.; McCann, E.; Sheerer, A.; Wilcock, E.B. Thermal acclimation in brook trout myotomal muscle varies with fiber type and age. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 276, 111354. [Google Scholar] [CrossRef]
- Burbano, M.; Gilson, E. The power of stress: The telo-hormesis hypothesis. Cells 2021, 10, 1156. [Google Scholar] [CrossRef]
- Peterson, D.R.; Mok, H.O.L.; Au, D.W.T. Modulation of telomerase activity in fish muscle by biological and environmental factors. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 178, 51–59. [Google Scholar] [CrossRef]
- Pfennig, F.; Kind, B.; Zieschang, F.; Busch, M.; Gutzeit, H.O. Tert expression and telomerase activity in gonads and somatic cells of the Japanese medaka (Oryzias latipes). Dev. Growth Differ. 2008, 50, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Klapper, W.; Shin, T.; Mattson, M.P. Differential regulation of telomerase activity and TERT expression during brain development in mice. J. Neurosci. Res. 2001, 64, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.H.; Wong, J.M.Y. Non-canonical functions of telomerase reverse transcriptase: Emerging roles and biological relevance. Curr. Top. Med. Chem. 2020, 20, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G. Telomerase and neurons: An unusual relationship. Neural Regen. Res. 2022, 17, 2364–2367. [Google Scholar] [CrossRef]
- Seluanov, A.; Chen, Z.; Hine, C.; Sasahara, T.H.; Ribeiro, A.A.; Catania, K.C.; Presgraves, D.C.; Gorbunova, V. Telomerase activity coevolves with body mass not lifespan. Aging Cell 2007, 6, 45–52. [Google Scholar] [CrossRef]
- Seluanov, A.; Hine, C.; Bozzella, M.; Hall, A.; Sasahara, T.H.; Ribeiro, A.A.; Catania, K.C.; Presgraves, D.C.; Gorbunova, V. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell 2008, 7, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.M.; Ryder, O.A.; Houck, M.L.; Charter, S.J.; Walker, W.; Forsyth, N.R.; Austad, S.N.; Venditti, C.; Pagel, M.; Shay, J.W.; et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 2011, 10, 761–768. [Google Scholar] [CrossRef]
- Pepke, M.L.; Eisenberg, D.T.A. On the comparative biology of mammalian telomeres: Telomere length co-evolves with body mass, lifespan and cancer risk. Mol. Ecol. 2022, 31, 6286–6296. [Google Scholar] [CrossRef]
- Olsson, M.; Wapstra, E.; Friesen, C. Ectothermic telomeres: It’s time they came in from the cold. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018, 373, 20160449. [Google Scholar] [CrossRef]
- Campos, D.F.; Amanajás, R.D.; Almeida-Val, V.M.F.; Val, A.L. Climate vulnerability of South American freshwater fish: Thermal tolerance and acclimation. J. Exp. Zool. A Ecol. Integr. Physiol. 2021, 335, 723–734. [Google Scholar] [CrossRef]
- Val, L.A.; Wood, C.M. Global change and physiological challenges for fish of the Amazon today and in the near future. J. Exp. Biol. 2022, 225, jeb216440. [Google Scholar]
- Laverty, G.; Skadhauge, E. Adaptation of teleosts to very high salinity. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Rolshausen, G.; Uren Webster, T.M.; Tyler, C.R. Adaptive capabilities and fitness consequences associated with pollution exposure in fish. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372, 20160042. [Google Scholar] [CrossRef] [PubMed]
- Näslund, J.; Pauliny, A.; Blomqvist, D.; Johnsson, J.I. Telomere dynamics in wild brown trout: Effects of compensatory growth and early growth investment. Oecologia 2015, 177, 1221–1230. [Google Scholar] [CrossRef]
- McLennan, D.; Auer, S.K.; McKelvey, S.; McKelvey, L.; Anderson, G.; Boner, W.; Duprez, J.S.; Metcalfe, N.B. Habitat restoration weakens negative environmental effects on telomere dynamics. Mol. Ecol. 2022, 31, 6100–6113. [Google Scholar] [CrossRef]
- Panasiak, L.; Szubert, K.; Polonis, M.; Ocalewicz, K. Telomere length variation does not correspond with the growth disturbances in the rainbow trout (Oncorhynchus mykiss). J. Appl. Genet. 2022, 63, 133–139. [Google Scholar] [CrossRef]
- de Abechuco, E.L.; Bilbao, E.; Soto, M.; Diez, G. Molecular cloning and measurement of telomerase reverse transcriptase (TERT) transcription patterns in tissues of European hake (Merluccius merluccius) and Atlantic cod (Gadus morhua) during aging. Gene 2014, 541, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Friesen, S.R.; Wapstra, E.; Olsson, M. Of telomeres and temperature: Measuring thermal effects on telomeres in ectothermic animals. Mol. Ecol. 2021, 31, 6069–6086. [Google Scholar] [CrossRef]
- Logan, C.A.; Buckley, B.A. Transcriptomic responses to environmental temperature in eurythermal and stenothermal fishes. J. Exp. Biol. 2015, 218, 1915–1924. [Google Scholar] [CrossRef]
- Soyano, K.; Mushirobira, Y. The mechanism of low-temperature tolerance in fish. Adv. Exp. Med. Biol. 2018, 1081, 149–164. [Google Scholar]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Sodeinde, T.; Boston, A.; Chang, S. Telomeres cooperate with the nuclear envelope to maintain genome stability. Bioessays 2024, 46, e2300184. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Miralles Fusté, J.; Simavorian, T.; Bartocci, C.; Tsai, J.; Karlseder, J.; Lazzerini Denchi, E. TZAP: A telomere-associated protein involved in telomere length control. Science 2017, 355, 638–641. [Google Scholar] [CrossRef]
- Jahn, A.; Rane, G.; Paszkowski-Rogacz, M.; Sayols, S.; Bluhm, A.; Han, C.T.; Draškovič, I.; Londoño-Vallejo, J.A.; Kumar, A.P.; Buchholz, F.; et al. ZBTB48 is both a vertebrate telomere-binding protein and a transcriptional activator. EMBO Rep. 2017, 18, 929–946. [Google Scholar] [CrossRef]
- Chawla, R.; Azzalin, S.M. The telomeric transcriptome and SMG proteins at the crossroads. Cytogenet. Genome Res. 2008, 122, 194–201. [Google Scholar] [CrossRef]
- Yamashita, A. Role of SMG-1-mediated Upf1 phosphorylation in mammalian nonsense-mediated mRNA decay. Genes Cells 2013, 18, 161–175. [Google Scholar] [CrossRef]
- Snow, B.E.; Erdmann, N.; Cruickshank, J.; Goldman, H.; Gil, R.M.; Robinson, M.O.; Harrington, L. Functional conservation of the telomerase protein Est1p in humans. Curr. Biol. 2003, 13, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Rouan, A.; Pousse, M.; Djerbi, N.; Porro, B.; Bourdin, G.; Carradec, Q.; Hume, B.C.; Poulain, J.; Lê-Hoang, J.; Armstrong, E.; et al. Telomere DNA length regulation is influenced by seasonal temperature differences in short-lived but not in long-lived reef-building corals. Nat. Commun. 2023, 14, 3038. [Google Scholar] [CrossRef]
- Chen, L.Y.; Majerska, J.; Lingner, J. Molecular basis of telomere syndrome caused by CTC1 mutations. Genes Dev. 2013, 27, 2099–2108. [Google Scholar] [CrossRef]
- Rajavel, M.; Mullins, M.R.; Taylor, D.J. Multiple facets of TPP1 in telomere maintenance. Biochim. Biophys. Acta 2014, 1844, 1550–1559. [Google Scholar] [CrossRef]
- Rocha-Santos, C.; Bastos, F.F.; Dantas, R.F.; Hauser-Davis, R.A.; Rodrigues, L.C.; Cunha Bastos, V.L.F.; Cunha Bastos, J. Glutathione peroxidase and glutathione S-transferase in blood and liver from a hypoxia-tolerant fish under oxygen deprivation. Ecotoxicol. Environ. Saf. 2018, 163, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.S.; Kim, B.M.; Kim, R.O.; Seo, J.S.; Kim, I.C.; Lee, Y.M.; Lee, J.S. Co-expression of antioxidant enzymes with expression of p53, DNA repair, and heat shock protein genes in the gamma ray-irradiated hermaphroditic fish Kryptolebias marmoratus larvae. Aquat. Toxicol. 2013, 140–141, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Dahms, H.-U.; Rhee, J.-S.; Lee, Y.-M.; Lee, J.; Han, K.-N.; Lee, J.-S. Expression profiles of seven glutathione S-transferase (GST) genes in cadmium-exposed river pufferfish (Takifugu obscurus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 99–106. [Google Scholar] [CrossRef]
- Kumar, S.; Moniruzzaman, M.; Chakraborty, A.; Sarbajna, A.; Chakraborty, S.B. Crosstalk between heat shock proteins, NRF2, NF-kappaB and different endogenous antioxidants during lead-induced hepatotoxicity in Puntius ticto. Aquat. Toxicol. 2021, 233, 105771. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Srivastava, P.P.; Varghese, T.; Nazir, M.I.; Gupta, S.; Krishna, G. Temporal changes in superoxide dismutase, catalase, and heat shock protein 70 gene expression, cortisol and antioxidant enzymes activity of Labeo rohita fingerlings subjected to starvation and refeeding. Gene 2019, 692, 94–101. [Google Scholar] [CrossRef]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress-induced oxidative damages in the liver and associated death in fish, Labeo rohita. Fish Physiol. Biochem. 2021, 47, 21–32. [Google Scholar] [CrossRef]
- Petitjean, Q.; Jean, S.; Gandar, A.; Côte, J.; Laffaille, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Chaudière, J. Biological and catalytic properties of selenoproteins. Int. J. Mol. Sci. 2023, 24, 10109. [Google Scholar] [CrossRef]
- Nadarajapillai, K.; Liyanage, D.S.; Sellaththurai, S.; Jeong, T.; Lee, S.; Lee, J. Glutathione-S-transferase alpha-4 in Hippocampus abdominalis (big-belly seahorse): Molecular characterization, antioxidant properties, and its potent immune response. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 240, 108917. [Google Scholar] [CrossRef]
- Li, Z.H.; Li, P.; Wu, Y. Regulation of glutathione-dependent antioxidant defense system of grass carp Ctenopharyngodon idella under the combined stress of mercury and temperature. Environ. Sci. Pollut. Res. Int. 2021, 28, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Sukhovskaya, I.V.; Borvinskaya, E.V.; Smirnov, L.P.; Kochneva, A.A. The role of glutathione in functioning of the system of antioxidant protection in fish (Review). Inland Water Biol. 2017, 1, 93–99. [Google Scholar] [CrossRef]
- Coughlin, D.J.; Wilson, L.T.; Kwon, E.S.; Travitz, L.S. Thermal acclimation of rainbow trout myotomal muscle, can trout acclimate to a warming environment? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 245, 110702. [Google Scholar] [CrossRef]
- Ern, R.; Andreassen, A.H.; Jutfelt, F. Physiological mechanisms of acute upper thermal tolerance in fish. Physiology 2023, 38, 141–158. [Google Scholar] [CrossRef]
- Grim, J.M.; Simonik, E.A.; Semones, M.C.; Kuhn, D.E.; Crockett, E.L. The glutathione-dependent system of antioxidant defense is not modulated by temperature acclimation in muscle tissues from striped bass, Morone saxatilis. Comp Biochem. Phys. A 2023, 164, 383–390. [Google Scholar] [CrossRef]
- Yu, H.; Deng, W.; Zhang, D.; Gao, Y.; Yang, Z.; Shi, X.; Sun, J.; Zhou, J.; Ji, H. Antioxidant defenses of Onychostoma macrolepis in response to thermal stress: Insight from mRNA expression and activity of superoxide dismutase and catalase. Fish Shellfish Immunol. 2017, 66, 50–61. [Google Scholar] [CrossRef]
- An, K.W.; Kim, N.N.; Shin, H.S.; Kil, G.S.; Choi, C.Y. Profiles of antioxidant gene expression and physiological changes by thermal and hypoosmotic stresses in black porgy (Acanthopagrus schlegeli). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.Y.; Lorin-Nebel, C.; Lee, T.H. Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos chanos) marine euryhaline fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef]
- Almeida, J.R.; Gravato, C.; Guilhermino, L. Effects of temperature in juvenile seabass (Dicentrarchus labrax L.) biomarker responses and behavior: Implications for environment monitoring. Estuar. Coast 2015, 38, 45–55. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
| Name of the Gene | Description |
|---|---|
| Genes involved in the regulation of telomerase activity and telomere length | |
| Tert | Telomerase reverse transcriptase: catalytic subunit of telomerase; maintenance of telomeric DNA. |
| Tep1 | Telomerase-associated protein 1: required for the amplification and localization of the telomerase complex. |
| Ten1 | Telomeric pathways with STn1: protein binding to single-stranded telomeric DNA; involved in the negative regulation of the telomere length and capping, a component of the CST complex. |
| Tpp1/Acd | TINT1; PTOP; PIP1/Adrenocortical dysplasia phenotype: component of shelterin, bound to POT1 and TIN2, recruits telomerase and stimulates its processivity. |
| Tcab1 | Telomerase Cajal’s body protein 1: part of the telomerase complex, bound to telomerase RNA, in S phase of the cell cycle assists in the amplification of the telomerase complex in Cajal’s bodies. |
| Trf2 | Telomeric repeat-binding factor 2: a component of the Shelterin complex binding to double-stranded telomeric DNA; a negative regulator of the telomere length. |
| Pot1 | Protection of telomeres protein 1: a component of the Shelterin complex, regulates the telomere length and telomerase activity at telomeres. |
| Ctc1 | CST telomere replication complex component 1: regulation of the telomere length. |
| Atm | Ataxia-telangiectasia mutant phenotype: regulation of the telomere length and response to double-stranded DNA damage. |
| Pin2/Trf1 | Protein involved in G2/M regulation/Telomeric repeat-binding factor 1: a component of the Shelterin complex, regulates the telomere length. |
| Rif1 | Rap1p-interacting factor: negatively regulates the telomere length and is involved in DNA damage response, chromatin organization, and replication timing. |
| Rtel1 | Regulator of the Telomere Length 1: helicase, involved in telomere maintenance and DNA reparation, is recruited to telomeres by the TRF1 protein to unravel G-quadruplexes, facilitating telomeric DNA replication. |
| Tel2 | Telomere length regulation 2: involved in the response to DNA damage, stabilizes the TORC complex that regulates cell growth and survival. |
| Trir | Telomerase RNA Component Interacting Rnase: involved in the maturation of telomerase and other RNAs. |
| Est1a/Smg6 Smg5 Smg7 | EST1 telomerase component homolog A/Suppressors with morphogenetic defects in genitalia proteins, effectors of nonsense-mediated messenger RNA decay: component of the telomerase complex, binding to single-stranded telomeric DNA, maintains the telomere length; has three isoforms: 5, 6, and 7. |
| Sde2 | SDE2 telomere maintenance homolog: telomere silencing, genome stability, stress response, and cell cycle regulation. |
| Tzap | Telomeric zinc finger-associated protein: telomere trimming, prevents excessive telomere elongation. |
| Genes involved in the defense against ROS | |
| Sodc_CuZn | Copper, zinc superoxide dismutase cytosolic: converts superoxide radical to hydrogen peroxide in the cytoplasm. |
| Sodm_FeMn | Iron, manganese superoxide dismutase mitochondrial: converts superoxide radical to hydrogen peroxide in mitochondria. |
| Ccs | Copper chaperone for superoxide dismutase: activates cytoplasmic superoxide dismutase. |
| Sode_CuZn | Copper, zinc superoxide dismutase extracellular: reduces the amount of superoxide in the intercellular space. |
| Cat | Catalase: converts hydrogen peroxide into water. |
| Gpx4 Gpx3 Gpx6 | Glutathione peroxidase: catalyzes the reduction in lipid hydroperoxides to the corresponding alcohols and the reduction of hydrogen peroxide to water. |
| Gst_N Gst_C | Glutathione S-transferase: has peroxidase activity, binds and neutralizes various ligands, including xenobiotics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroleva, A.G.; Vakhteeva, E.A.; Epifantsev, A.A.; Sukhanova, L.V.; Yakhnenko, V.M.; Glyzina, O.Y.; Tolstikova, L.I.; Cherezova, V.M.; Sidorova, T.V.; Potapov, S.A.; et al. Acclimation during Embryogenesis Remodulates Telomerase Activity and Gene Expression in Baikal Whitefish Larvae, Mitigating the Effects of Acute Temperature Stress. Animals 2024, 14, 2839. https://doi.org/10.3390/ani14192839
Koroleva AG, Vakhteeva EA, Epifantsev AA, Sukhanova LV, Yakhnenko VM, Glyzina OY, Tolstikova LI, Cherezova VM, Sidorova TV, Potapov SA, et al. Acclimation during Embryogenesis Remodulates Telomerase Activity and Gene Expression in Baikal Whitefish Larvae, Mitigating the Effects of Acute Temperature Stress. Animals. 2024; 14(19):2839. https://doi.org/10.3390/ani14192839
Chicago/Turabian StyleKoroleva, Anastasiya G., Eugenia A. Vakhteeva, Alexander A. Epifantsev, Lyubov V. Sukhanova, Vera M. Yakhnenko, Olga Yu. Glyzina, Lyubov I. Tolstikova, Valeria M. Cherezova, Tuyana V. Sidorova, Sergey A. Potapov, and et al. 2024. "Acclimation during Embryogenesis Remodulates Telomerase Activity and Gene Expression in Baikal Whitefish Larvae, Mitigating the Effects of Acute Temperature Stress" Animals 14, no. 19: 2839. https://doi.org/10.3390/ani14192839
APA StyleKoroleva, A. G., Vakhteeva, E. A., Epifantsev, A. A., Sukhanova, L. V., Yakhnenko, V. M., Glyzina, O. Y., Tolstikova, L. I., Cherezova, V. M., Sidorova, T. V., Potapov, S. A., Kirilchik, S. V., & Sapozhnikova, Y. P. (2024). Acclimation during Embryogenesis Remodulates Telomerase Activity and Gene Expression in Baikal Whitefish Larvae, Mitigating the Effects of Acute Temperature Stress. Animals, 14(19), 2839. https://doi.org/10.3390/ani14192839









