Analysis of Fibropapillomatosis in Roe Deer (Capreolus capreolus) Confirms High Content of Heavy Metals
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
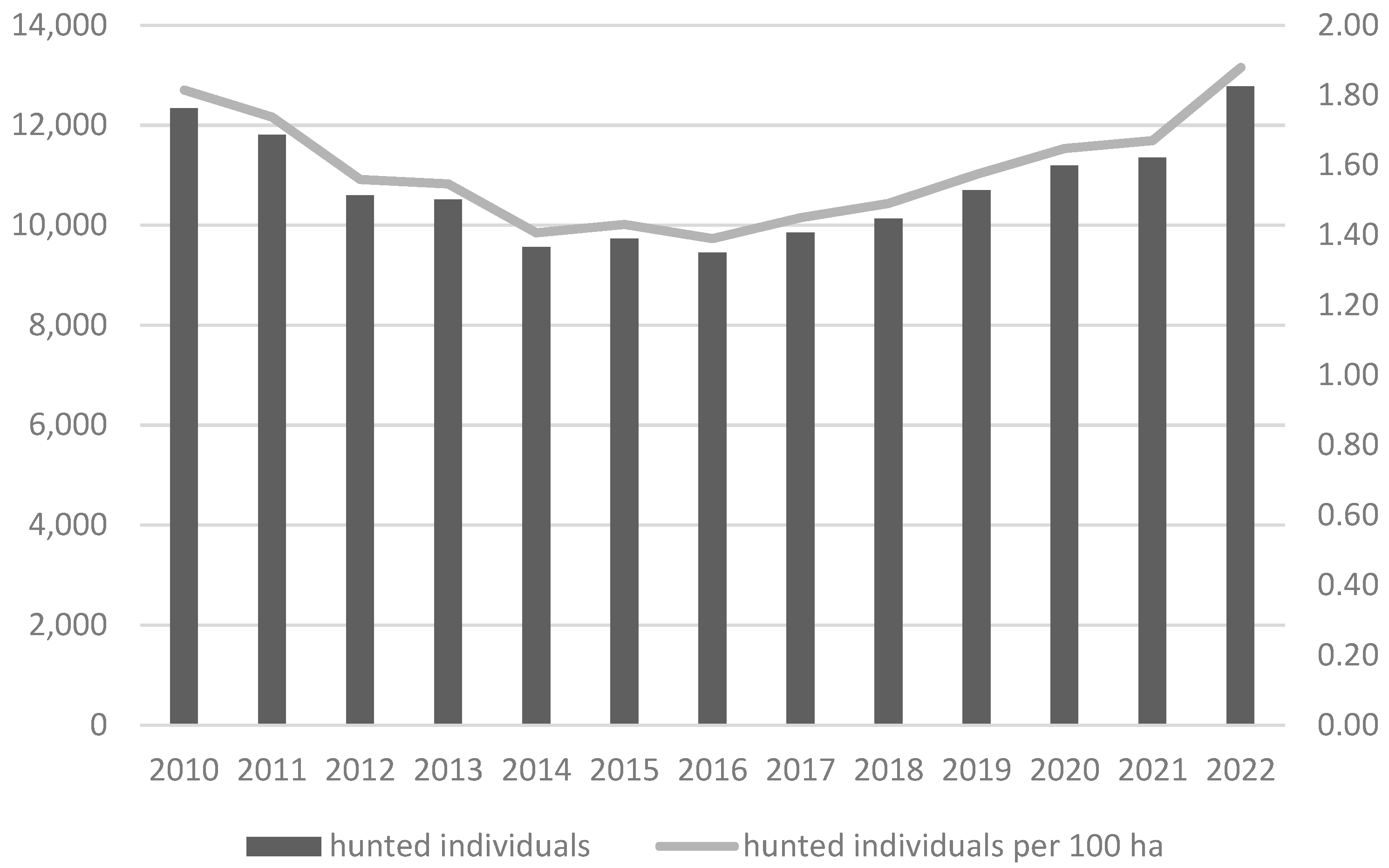
2.1. Study Area
2.2. Sample Collection
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Representation of Metals in Skin Tumours
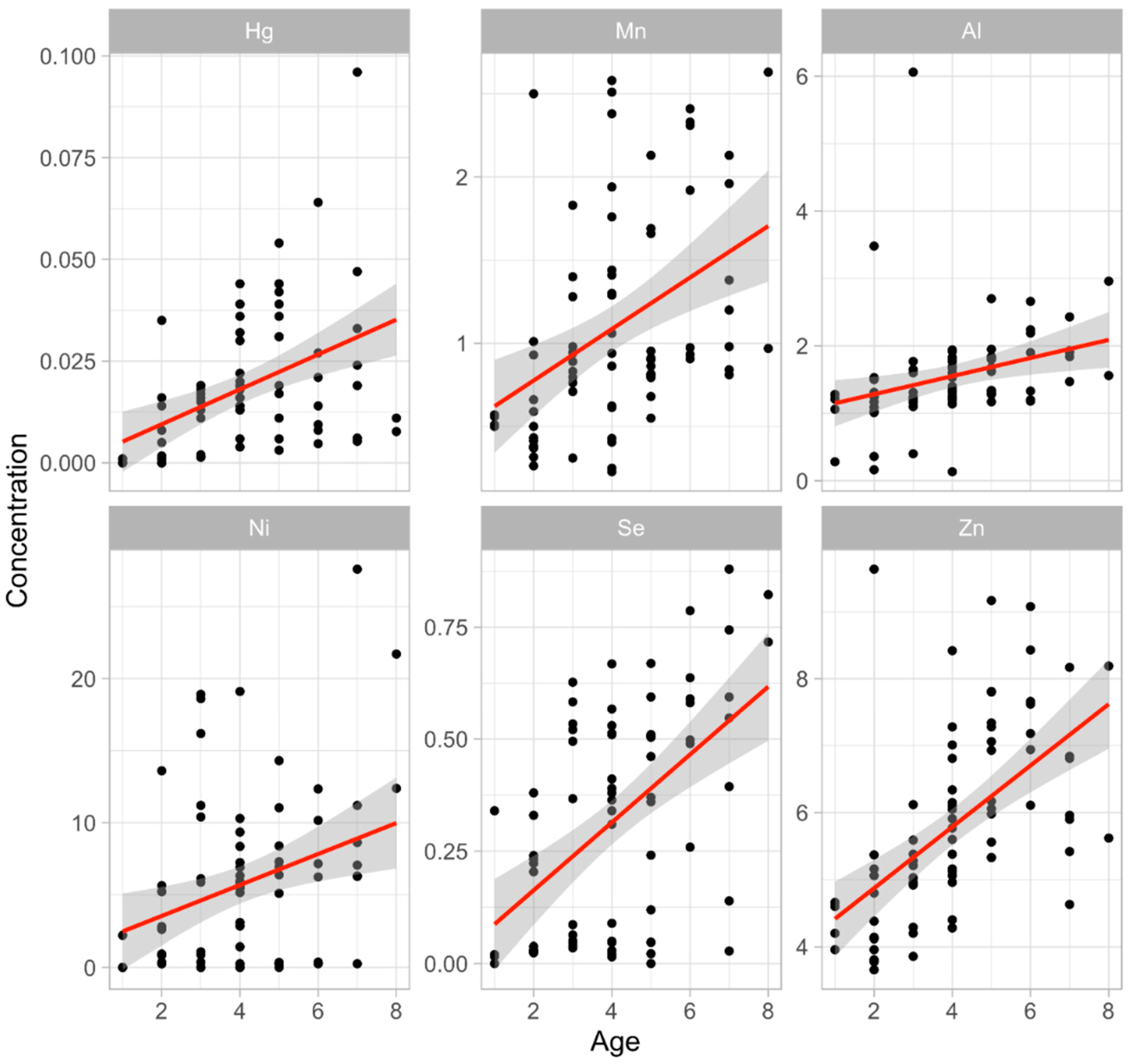
3.2. The Metal Concentration and Age of the Individual
3.3. Relationship of the Metal Concentration and Sex of the Individual
3.4. Relationship of the Metal Concentration and Papilloma Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaudry, W.; Gaillard, J.M.; Saïd, S.; Mårell, A.; Baltzinger, C.; Rocquencourt, A.; Bonenfant, C. Population Density and Plant Availability Interplay to Shape Browsing Intensity by Roe Deer in a Deciduous Forest. For. Ecol. Manag. 2022, 515, 120153. [Google Scholar] [CrossRef]
- Schwegmann, S.; Hendel, A.L.; Frey, J.; Bhardwaj, M.; Storch, I. Forage, Forest Structure or Landscape: What Drives Roe Deer Habitat Use in a Fragmented Multiple-Use Forest Ecosystem? For. Ecol. Manag. 2023, 532, 120830. [Google Scholar] [CrossRef]
- Linnell, J.D.C.; Cretois, B.; Nilsen, E.B.; Rolandsen, C.M.; Solberg, E.J.; Veiberg, V.; Kaczensky, P.; Van Moorter, B.; Panzacchi, M.; Rauset, G.R.; et al. The Challenges and Opportunities of Coexisting with Wild Ungulates in the Human-Dominated Landscapes of Europe’s Anthropocene. Biol. Conserv. 2020, 244, 108500. [Google Scholar] [CrossRef]
- Benjamin, C.S.; Uphus, L.; Lüpke, M.; Rojas-Botero, S.; Dhillon, M.S.; Englmeier, J.; Fricke, U.; Ganuza, C.; Haensel, M.; Redlich, S.; et al. Modelling the Relative Abundance of Roe Deer (Capreolus capreolus L.) along a Climate and Land-Use Gradient. Animals 2022, 12, 222. [Google Scholar] [CrossRef]
- Borkowski, J.; Banul, R.; Jurkiewicz-Azab, J.; Hołdyński, C.; Święczkowska, J.; Nasiadko, M.; Załuski, D. There Is Only One Winner: The Negative Impact of Red Deer Density on Roe Deer Numbers and Distribution in the Słowiński National Park and Its Vicinity. Ecol. Evol. 2021, 11, 6889–6899. [Google Scholar] [CrossRef]
- Illanas, S.; Croft, S.; Smith, G.C.; López-Padilla, S.; Vicente, J.; Blanco-Aguiar, J.A.; Scandura, M.; Apollonio, M.; Ferroglio, E.; Zanet, S.; et al. New Models for Wild Ungulates Occurrence and Hunting Yield Abundance at European Scale. EFSA Support. Publ. 2022, 19, 7631E. [Google Scholar] [CrossRef]
- Ayotte, P.; Le Corre, M.; Côté, S.D. Synergistic Population Density and Environmental Effects on Deer Body Condition. J. Wildl. Manag. 2020, 84, 938–947. [Google Scholar] [CrossRef]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild Ungulate Overabundance in Europe: Contexts, Causes, Monitoring and Management Recommendations. Mamm. Rev. 2021, 51, 95–108. [Google Scholar] [CrossRef]
- Valente, A.M.; Acevedo, P.; Figueiredo, A.M.; Fonseca, C.; Torres, R.T. Overabundant Wild Ungulate Populations in Europe: Management with Consideration of Socio-Ecological Consequences. Mamm. Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Linda, R.; Vacek, S.; Šimůnek, V.; Brichta, J.; Gallo, J.; Prokůpková, A. Bark Stripping, the Crucial Factor Affecting Stem Rot Development and Timber Production of Norway Spruce Forests in Central Europe. For. Ecol. Manag. 2020, 474, 118360. [Google Scholar] [CrossRef]
- Cukor, J.; Vacek, Z.; Linda, R.; Vacek, S.; Šimůnek, V.; Macháček, Z.; Brichta, J.; Prokůpková, A. Scots Pine (Pinus sylvestris L.) Demonstrates a High Resistance against Bark Stripping Damage. For. Ecol. Manag. 2022, 513, 120182. [Google Scholar] [CrossRef]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century; Cambridge University Press: New York, NY, USA, 2010; p. 604. [Google Scholar]
- Kahlert, J.; Fox, A.D.; Heldbjerg, H.; Asferg, T.; Sunde, P. Functional Responses of Human Hunters to Their Prey-Why Harvest Statistics May Not Always Reflect Changes in Prey Population Abundance. Wildlife Biol. 2015, 21, 294–302. [Google Scholar] [CrossRef]
- Gaudiano, L.; Pucciarelli, L.; Mori, E. Livestock Grazing Affects Movements and Activity Pattern of Italian Roe Deer in Southern Italy. Eur. J. Wildl. Res. 2021, 67, 66. [Google Scholar] [CrossRef]
- Franchini, M.; Peric, T.; Frangini, L.; Prandi, A.; Comin, A.; Rota, M.; Filacorda, S. You’re Stressing Me out! Effect of Interspecific Competition from Red Deer on Roe Deer Physiological Stress Response. J. Zool. 2023, 320, 63–74. [Google Scholar] [CrossRef]
- van Beest, F.M.; Petersen, H.H.; Krogh, A.K.H.; Frederiksen, M.L.; Schmidt, N.M.; Hansson, S.V. Estimating Parasite-Condition Relationships and Potential Health Effects for Fallow Deer (Dama dama) and Red Deer (Cervus elaphus) in Denmark. Int. J. Parasitol. Parasites Wildl. 2023, 21, 143–152. [Google Scholar] [CrossRef]
- Český statistický úřad Výsledky Mysliveckého Hospodaření. Available online: https://www.czso.cz/csu/czso/zakladni-udaje-o-honitbach-stavu-a-lovu-zvere-od-1-4-2021-do-31-3-2022 (accessed on 10 July 2024).
- Garcês, A.; Pires, I.; Savini, F.; Scagliarini, A.; Gallina, L. Cutaneous Fibropapilloma in a Red Deer (Cervus elaphus) Associated with Cervus Elaphus Papillomavirus in Portugal. J. Wildl. Dis. 2020, 56, 636–639. [Google Scholar] [CrossRef]
- Rajský, D.; Rajský, M.; Garaj, P.; Kropil, R.; Ivan, M.; Vodnansky, M.; Hanzal, V.; Erdélyi, K. Emergence and Expansion of Roe Deer (Capreolus capreolus) Fibropapillomatosis in Slovakia. Eur. J. Wildl. Res. 2016, 62, 43–49. [Google Scholar] [CrossRef]
- Erdélyi, K.; Bálint, Á.; Dencso, L.; Dán, Á.; Ursu, K. Characterisation of the First Complete Genome Sequence of the Roe Deer (Capreolus capreolus) Papillomavirus. Virus Res. 2008, 135, 307–311. [Google Scholar] [CrossRef]
- Kmetec, J.; Kuhar, U.; Fajfar, A.G.; Vengušt, D.Ž.; Vengušt, G. A Comprehensive Study of Cutaneous Fibropapillomatosis in Free-Ranging Roe Deer (Capreolus capreolus) and Red Deer (Cervus elaphus): From Clinical Manifestations to Whole-Genome Sequencing of Papillomaviruses. Viruses 2020, 12, 1001. [Google Scholar] [CrossRef]
- Ahola, H.; Bergman, P.; Ström, A.C.; Moreno-Lopéz, J.; Pettersson, U. Organization and Expression of the Transforming Region from the European Elk Papillomavirus (EEPV). Gene 1986, 50, 195–205. [Google Scholar] [CrossRef]
- Moar, M.H.; Jarret, W.F.H. A Cutaneous Fibropapilloma from a Red Deer (Cervus elaphus) Associated with a Papillomavirus. Intervirology 1985, 24, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P.; Wayne, L.D. Deer Papillomaviruses. Dev. Vet. Virol 1988, 6, 279–291. [Google Scholar]
- van Dyk, E.; Bosman, A.-M.; van Wilpe, E.; Williams, J.H.; Bengis, R.G.; van Heerden, J.; Venter, E.H. Detection and Characterisation of Papillomavirus in Skin Lesions of Giraffe and Sable Antelope in South Africa. J. S. Afr. Vet. Assoc. 2011, 82, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; van Dyk, E.; Nel, P.J.; Lane, E.; Van Wilpe, E.; Bengis, R.G.; de Klerk-Lorist, L.M.; van Heerden, J. Pathology and Immunohistochemistry of Papillomavirus-Associated Cutaneous Lesions in Cape Mountain Zebra, Giraffe, Sable Antelope and African Buffalo in South Africa. J. S. Afr. Vet. Assoc. 2011, 82, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, O.; Borzacchiello, G.; Nava, D.; Iovane, G.; Russo, V.; Vecchio, D.; D’Ausilio, F.; Gault, E.A.; Campo, M.S.; Paciello, O. Bovine Papillomavirus Type 1 DNA and E5 Oncoprotein Expression in Water Buffalo Fibropapillomas. Vet. Pathol. 2009, 46, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Elfadl, A.K.; Jäger, K.; Schoon, H.A.; Gameel, A.A. Frequency, Pathology and Electron Microscopy of Dromedary Camel Viral Fibro-Papilloma in Sudan. Brazilian J. Vet. Pathol. 2016, 9, 39–46. [Google Scholar]
- Cladel, N.M.; Peng, X.; Christensen, N.; Hu, J. The Rabbit Papillomavirus Model: A Valuable Tool to Study Viral-Host Interactions. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 4–11. [Google Scholar] [CrossRef]
- Schulman, F.Y.; Krafft, A.E.; Janczewski, T.; Mikaelian, I.; Irwin, J.; Hassinger, K. Cutaneous Fibropapilloma in a Mountain Lion (Felis concolor). J. Zoo Wildl. Med. 2003, 34, 179–183. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Dubovi, E.J. Papillomaviridae and Polyomaviridae. In Fenner’s Veterinary Virology., 4th ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 213–223. ISBN 9780123751584. [Google Scholar]
- Kràl, J.; Bandouchovà, H.; Brichta, J.; Kovàčovà, V.; Ondràček, K.; Osičkovà, J.; Hrubà, H.; Hutařovà, Z.; Kominkovà, M.; Cernei, N.; et al. Papillomavirus Infection of Roe Deer in the Czech Republic and Fibropapilloma-Associated Levels of Metallothionein, Zinc, and Oxidative Stress. Acta Vet. Brno 2015, 84, 105–111. [Google Scholar] [CrossRef]
- Erdélyi, K.; Dencso, L.; Lehoczki, R.; Heltai, M.; Sonkoly, K.; Csányi, S.; Solymosi, N. Endemic Papillomavirus Infection of Roe Deer (Capreolus capreolus). Vet. Microbiol. 2009, 138, 20–26. [Google Scholar] [CrossRef]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of Papillomaviruses (PVs) Based on 189 PV Types and Proposal of Taxonomic Amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Shope, R.E. An Infectious Fibroma of Deer. Exp. Biol. Med. 1955, 88, 533–535. [Google Scholar] [CrossRef]
- Bukovjan, K.; Kodet, R. Problematika Fibropapilomatózy Srnčí Zvěře; VULHM: Jíloviště, Česká republika, 2014. [Google Scholar]
- Kraus, M. Monitoring Fibropapilomatózy v České Republice. Myslivost 2018, 12, 50–51. [Google Scholar]
- Savini, F.; Molin, E.D.; Gallina, L.; Casà, G.; Scagliarini, A. Papillomavirus in Healthy Skin and Mucosa of Wild Ruminants in the Italian Alps. J. Wildl. Dis. 2016, 52, 82–87. [Google Scholar] [CrossRef]
- Farkaš, V.; Konjević, D.; Grabarević, Ž.; Janicki, Z.; Slavica, A.; Sabočanec, R. ROE DEER (Capreolus capreolus) WARTS—FIBROMAS, PAPILLOMAS OR FIBROPAPILLOMAS. In Proceedings of the Acta Clin, 22nd Ljudevit Jurak International Symposium on Comparative Pathology, Zagreb, Croatia, 3–4 June 2012; Volume 55, pp. 169–188. [Google Scholar]
- Žele Vengušt, D.; Kuhar, U.; Jerina, K.; Vengušt, G. Twenty Years of Passive Disease Surveillance of Roe Deer (Capreolus capreolus) in Slovenia. Animals 2021, 11, 407. [Google Scholar] [CrossRef]
- Duncan, K. Metallothioneins and Related Chelators. In Metal Ions in Life Sciences Vol. 5; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2009; Volume 48, ISBN 1847558992. [Google Scholar]
- Pokorny, B.; Ribarič-Lasnik, C. Seasonal Variability of Mercury and Heavy Metals in Roe Deer (Capreolus capreolus) Kidney. Environ. Pollut. 2002, 117, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Crête, M.; Nault, R.; Walsh, P.; Benedetti, J.L.; Lefebvre, M.A.; Weber, J.P.; Gagnon, J. Variation in Cadmium Content of Caribou Tissues from Northern Québec. Sci. Total Environ. 1989, 80, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Holm, J. Investigation of Roe Deer—Criteria for Use as a Bioindicator in Specimen Banking. Sci. Total Environ. 1993, 139–140, 237–249. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Khurana, U.; Dhagarra, N. TLC Separation of Transition Metal Ions and Their Quantitative Estimation by Atomic Absorption Spectroscopy. J. Liq. Chromatogr. 1995, 18, 1671–1681. [Google Scholar] [CrossRef]
- Azeh Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: London, UK, 2019. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinical and Environmental Toxicicology Volume 3: Environmental Toxicology, Springer: Basel, Switzerland, 2012; Volume 101, ISBN 978-3-7643-8339-8.
- Ahmed, S.; Khurshid, S.; Qureshi, F.; Hussain, A.; Bhattacharya, A. Heavy Metals and Geo-Accumulation Index Development for Groundwater of Mathura City, Uttar Pradesh. Desalin. Water Treat. 2019, 138, 291–300. [Google Scholar] [CrossRef]
- Doyi, I.N.Y.; Isley, C.F.; Soltani, N.S.; Taylor, M.P. Human Exposure and Risk Associated with Trace Element Concentrations in Indoor Dust from Australian Homes. Environ. Int. 2019, 133, 105125. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium Toxicity and Tolerance in Plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Hans Wedepohl, K. The Composition of the Continental Crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Joseph, P. Mechanisms of Cadmium Carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Beiglböck, C.; Steineck, T.; Tataruch, F.; Ruf, T. Environmental Cadmium Induces Histopathological Changes in Kidneys of Roe Deer. Environ. Toxicol. Chem. 2002, 21, 1811–1816. [Google Scholar] [CrossRef]
- Eltayeb Ehdaa Abdelsalam, E.; Banďouchová, H.; Heger, T.; Kaňová, M.; Kobelková, K.; Němcová, M.; Piaček, V.; Sedláčková, J.; Seidlová, V.; Vitula, F.; et al. Reproductive Toxicity of Heavy Metals in Fallow Deer in Vitro. Acta Vet. Brno 2021, 90, 277–286. [Google Scholar] [CrossRef]
- Krajský úřad kraje Vysočina Profil Kraje Vysočina, Krajský úřad kraje Vysočina, Jihlava, 2016, 167.
- Komínková, J.; Mestek, O. Atomová Absorpční Spektrometrie 1997, 21.
- Mestek, O. Hmotnostní Spektrometrie s Indukčně Vázaným Plazmatem; Vysoká škola chemicko-technologická, Praha, 2010; 34.
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Curi, N.H.D.A.; Brait, C.H.H.; Filho, N.R.A.; Talamoni, S.A. Heavy Metals in Hair of Wild Canids from the Brazilian Cerrado. Biol. Trace Elem. Res. 2012, 147, 97–102. [Google Scholar] [CrossRef]
- García, M.H.D.M.; Hernández Moreno, D.; Soler Rodríguez, F.; Beceiro, A.L.; Álvarez, L.E.F.; López, M.P. Sex- and Age-Dependent Accumulation of Heavy Metals (Cd, Pb and Zn) in Liver, Kidney and Muscle of Roe Deer (Capreolus capreolus) from NW Spain. J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2011, 46, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. Efflux-Mediated Heavy Metal Resistance in Prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Lehel, J.; Zwillinger, D.; Bartha, A.; Lányi, K.; Laczay, P. Food Safety Aspects of Primary Environmental Contaminants in the Edible Tissues of Roe Deer (Capreolus capreolus). Environ. Sci. Pollut. Res. 2017, 24, 25372–25382. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Report of the 32nd Session of the Codex Committee of the Food Additives and Contaminants; Food and Agriculture Organization of the United Nations: World Health Organization: Rome, Italy, 2006. [Google Scholar]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochemistry 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Jarzyń’ska, G.; Falandysz, J. Selenium and 17 Other Largely Essential and Toxic Metals in Muscle and Organ Meats of Red Deer (Cervus elaphus)—Consequences to Human Health. Environ. Int. 2011, 37, 882–888. [Google Scholar] [CrossRef]
- European Parliament and Council Regulation (EC) No 178/2002 Law the European Food Safety Authority; Official Journal of the European Communities: Brussel, Belgium, 2002; Volume L31.
- Bąkowska, M.; Pilarczyk, B.; Tomza-Marciniak, A.; Udała, J.; Pilarczyk, R. The Bioaccumulation of Lead in the Organs of Roe Deer (Capreolus capreolus L.), Red Deer (Cervus elaphus L.), and Wild Boar (Sus scrofa L.) from Poland. Environ. Sci. Pollut. Res. 2016, 23, 14373–14382. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Mihaljev, Ž.; Jakšić, S.; Prica, N.; Lazić, G.; Kapetanov, M.; Prodanov Radulović, J. Incidence of heavy metals and other toxic elements in roe deer (Capreolus capreolus) tissues. Arch. Vet. Med. 2016, 8, 3–10. [Google Scholar] [CrossRef]
- FAO. Heavy Metals Regulations. Aquaculture 2003, 66, 34–41. [Google Scholar]
- Lehel, J.; Laczay, P.; Gyurcsó, A.; Jánoska, F.; Majoros, S.; Lányi, K.; Marosán, M. Toxic Heavy Metals in the Muscle of Roe Deer (Capreolus capreolus)—Food Toxicological Significance. Environ. Sci. Pollut. Res. 2016, 23, 4465–4472. [Google Scholar] [CrossRef]
- Malmsten, A.; Dalin, A.M.; Pettersson, J.; Persson, S. Concentrations of Cadmium, Lead, Arsenic, and Some Essential Metals in Wild Boar from Sweden. Eur. J. Wildl. Res. 2021, 67, 18. [Google Scholar] [CrossRef]
- Cawthorn, D.-M.; Fitzhenry, L.B.; Kotrba, R.; Bureš, D.; Hoffman, L.C. Chemical Composition of Wild Fallow Deer (Dama Dama) Meat from South Africa: A Preliminary Evaluation. Foods 2020, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Długaszek, M.; Kopczyński, K. Correlations between Elements in the Fur of Wild Animals. Bull. Environ. Contam. Toxicol. 2014, 93, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Srebočan, E.; Pompe-Gotal, J.; Konjević, D.; Prevendar-Crnić, A.; Popović, N.; Kolić, E. Cadmium in Fallow Deer Tissue. Vet. Arh. 2006, 76, 143–150. [Google Scholar]
- Gizejewska, A.; Spodniewska, A.; Barski, D. Concentration of Lead, Cadmium, and Mercury in Tissues of European Beaver (Castor Fiber) from the North-Eastern Poland. Bull. Vet. Inst. Pulawy 2014, 58, 77–80. [Google Scholar] [CrossRef]
- Gašparík, J.; Binkowski, Ł.J.; Jahnátek, A.; Šmehýl, P.; Dobiaš, M.; Lukáč, N.; Błaszczyk, M.; Semla, M.; Massanyi, P. Levels of Metals in Kidney, Liver, and Muscle Tissue and Their Influence on the Fitness for the Consumption of Wild Boar from Western Slovakia. Biol. Trace Elem. Res. 2017, 177, 258–266. [Google Scholar] [CrossRef]
- Lénárt, Z.; Bartha, A.; Abonyi-Tóth, Z.; Lehel, J. Monitoring of Metal Content in the Tissues of Wild Boar (Sus Scrofa) and Its Food Safety Aspect. Environ. Sci. Pollut. Res. 2023, 30, 15899–15910. [Google Scholar] [CrossRef]
- Nawrocka, A.; Durkalec, M.; Szkoda, J.; Filipek, A.; Kmiecik, M.; Żmudzki, J.; Posyniak, A. Total Mercury Levels in the Muscle and Liver of Livestock and Game Animals in Poland, 2009–2018. Chemosphere 2020, 258, 127311. [Google Scholar] [CrossRef] [PubMed]
- Gasparik, J.; Dobias, M.; Capcarova, M.; Smehyl, P.; Slamecka, J.; Bujko, J. Concentration of Cadmium, Mercury, Zinc, Copper and Cobalt in the Tissues of Wild Boar (Sus scrofa) Hunted in the Western Slovakia. J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2012, 47, 1212–1216. [Google Scholar] [CrossRef]
- Jota Baptista, C.; Seixas, F.; Gonzalo-Orden, J.M.; Patinha, C.; Pato, P.; Ferreira da Silva, E.; Fernandes, G.; Oliveira, P.A. Heavy Metal and Metalloid Concentrations in Red Deer (Cervus elaphus) and Their Human Health Implications from One Health Perspective. Environ. Geochem. Health 2024, 46, 226. [Google Scholar] [CrossRef]
- Desideri, D.; Meli, M.A.; Cantaluppi, C.; Ceccotto, F.; Roselli, C.; Feduzi, L. Toxicological & Environmental Chemistry Essential and Toxic Elements in Meat of Wild and Bred Animals. Toxicol. Environ. Chem. 2012, 94, 1995–2005. [Google Scholar] [CrossRef]
- Jamaludin, M.H.; El, A.; Ahmed, D.; Clucas, L.; Cochrane, G.; Bremer, P. MACRO AND MICRO MINERAL CONTENT OF VENISON AND BEEF FARMED IN NEW ZEALAND. 2010; pp. 2–3. Available online: https://digicomst.ie/wp-content/uploads/2020/05/2010_04_16.pdf (accessed on 20 August 2024).
- Adei, E.; Forson-Adaboh, K. Toxic (Pb, Cd, Hg) and Essential (Fe, Cu, Zn, Mn) Metal Content of Liver Tissue of Some Domestic and Bush Animals in Ghana. Food Addit. Contam. Part B Surveill. 2008, 1, 100–105. [Google Scholar] [CrossRef]
- Strazdiòa, V.; Jemeïjanovs, A.; Ðterna, V. Nutrition Value of Wild Animal Meat. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact Appl. Sci. 2013, 67, 373–377. [Google Scholar] [CrossRef]
- Cygan-Szczegielniak, D.; Stasiak, K. Effects of Age and Sex on the Content of Heavy Metals in the Hair, Liver and the Longissimus Lumborum Muscle of Roe Deer Capreolus capreolus L. Environ. Sci. Pollut. Res. 2022, 29, 10782–10790. [Google Scholar] [CrossRef]
- Benson, K. Zinc Toxicosis in Animals; 2021. Available online: https://www.msdvetmanual.com/toxicology/zinc-toxicosis/zinc-toxicosis-in-animals (accessed on 22 September 2024).
- Cebulska, K.; Sobiech, P.; Tobolski, D.; Wysocka, D.; Janiszewski, P.; Zalewski, D.; Gugołek, A.; Illek, J. Comparison of the Content of Selected Heavy Metals in the Liver Tissue of the Wild Boar (Sus scrofa), Red Fox (Vulpes vulpes) and Red Deer (Cervus elaphus), Living in North-Eastern Poland. Pol. J. Vet. Sci. 2021, 24, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gasparik, J.; Massányi, P.; Slamecka, J.; Fabis, M.; Jurcik, R. Concentration of Selected Metals in Liver, Kidney, and Muscle of the Red Deer (Cervus elaphus). J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2004, 39, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Demesko, J.; Markowski, J.; Słaba, M.; Hejduk, J.; Minias, P. Age-Related Patterns in Trace Element Content Vary Between Bone and Teeth of the European Roe Deer (Capreolus capreolus). Arch. Environ. Contam. Toxicol. 2018, 74, 330–338. [Google Scholar] [CrossRef]
- Kostial, K.; Rabar, I.; Blanuša, M.; Landeka, M. Effect of Age on Heavy Metal Absorption. Proc. Nutr. Soc. 1979, 38, 251–256. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Vratarić, D.; Perić, T.; Šimić, B. Lead and Cadmium in Red Deer and Wild Boar from Different Hunting Grounds in Croatia. Sci. Total Environ. 2009, 407, 4243–4247. [Google Scholar] [CrossRef]
- Reglero, M.M.; Monsalve-González, L.; Taggart, M.A.; Mateo, R. Transfer of Metals to Plants and Red Deer in an Old Lead Mining Area in Spain. Sci. Total Environ. 2008, 406, 287–297. [Google Scholar] [CrossRef]
- Lazarus, M.; Orct, T.; Blanuša, M.; Vicković, I.; Šoštarić, B. Toxic and Essential Metal Concentrations in Four Tissues of Red Deer (Cervus elaphus) from Baranja, Croatia. Food Addit. Contam.—Part A 2008, 25, 270–283. [Google Scholar] [CrossRef]
| Hg | Pb | Cd | As | Cr | Mn | Al | |
|---|---|---|---|---|---|---|---|
| LOD | 0.001 | 0.01 | 0.001 | 0.005 | 0.001 | 0.01 | 0.01 |
| Co | Cu | Ni | Se | Zn | Fe | ||
| LOD | 0.05 | 0.05 | 0.05 | 0.01 | 0.001 | 0.0001 |
| Metal | Mean | Minimum | Maximum | SD |
|---|---|---|---|---|
| Hg | 0.02 | 0.001 | 0.1 | 0.02 |
| Pb | 0.01 | 0.01 | 0.07 | 0.01 |
| Cd | 0.03 | 0 | 0.2 | 0.03 |
| As | 0.01 | 0.01 | 0.01 | 0 |
| Cr | 0.99 | 0.18 | 19.6 | 2.23 |
| Mn | 1.1 | 0.23 | 2.63 | 0.65 |
| Al | 1.57 | 0.13 | 6.06 | 0.77 |
| Co | 0.33 | 0.05 | 2.4 | 0.51 |
| Cu | 1.13 | 0.06 | 4.36 | 0.78 |
| Ni | 5.8 | 0 | 27.6 | 5.91 |
| Se | 0.32 | 0 | 0.88 | 0.25 |
| Zn | 5.84 | 3.66 | 9.64 | 1.43 |
| Fe | 18.58 | 6.38 | 36.81 | 8.04 |
| Hg | Cd | Cr | Mn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | |
| ♀ | 0.000 | 0.017 | 0.044 | 0.000 | 0.027 | 0.160 | 0.175 | 1.145 | 19.600 | 0.249 | 0.993 | 2.500 |
| ♂ | 0.000 | 0.020 | 0.096 | 0.000 | 0.029 | 0.200 | 0.200 | 0.835 | 2.740 | 0.228 | 1.205 | 2.630 |
| Al | Co | Cu | Ni | |||||||||
| Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | |
| ♀ | 0.134 | 1.493 | 6.060 | 0.050 | 0.173 | 2.400 | 0.056 | 1.100 | 4.360 | 0.000 | 4.862 | 18.900 |
| ♂ | 0.281 | 1.635 | 2.960 | 0.000 | 0.178 | 2.250 | 0.184 | 1.167 | 2.830 | 0.000 | 6.690 | 27.600 |
| Se | Zn | Fe | Pb | |||||||||
| Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | |
| ♀ | 0.015 | 0.257 | 0.787 | 3.660 | 5.570 | 9.640 | 6.380 | 26.665 | 29.710 | 0.006 | 0.001 | 0.050 |
| ♂ | 0.000 | 0.383 | 0.880 | 4.120 | 6.090 | 9.080 | 7.650 | 20.775 | 36.810 | 0.005 | 0.019 | 0.070 |
| As | ||||||||||||
| Min | Mean | Max | ||||||||||
| ♀ | 0.005 | 0.005 | 0.006 | |||||||||
| ♂ | 0.005 | 0.005 | 0.008 | |||||||||
| Papiloma Type | Hg | Cd | Cr | Mn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | |
| S | 0.000 | 0.015 | 0.064 | 0.000 | 0.029 | 0.200 | 0.175 | 1.218 | 19.600 | 0.228 | 1.095 | 2.630 |
| P | 0.000 | 0.023 | 0.096 | 0.000 | 0.027 | 0.099 | 0.183 | 0.719 | 1.630 | 0.316 | 1.109 | 2.510 |
| Al | Co | Cu | Ni | |||||||||
| Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | |
| S | 0.134 | 1.549 | 6.060 | 0.000 | 0.439 | 2.400 | 0.179 | 1.165 | 4.360 | 0.000 | 5.976 | 19.100 |
| P | 0.163 | 1.585 | 2.700 | 0.055 | 0.194 | 0.930 | 0.056 | 1.099 | 2.830 | 0.000 | 5.595 | 27.600 |
| Se | Zn | Fe | Pb | |||||||||
| Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | |
| S | 0.000 | 0.312 | 0.823 | 3.660 | 5.808 | 9.640 | 6.380 | 26.415 | 263.100 | 0.006 | 0.014 | 0.070 |
| P | 0.000 | 0.333 | 0.880 | 3.780 | 5.869 | 9.170 | 7.280 | 20.394 | 36.810 | 0.005 | 0.016 | 0.060 |
| As | ||||||||||||
| Min | Mean | Max | ||||||||||
| S | 0.005 | 0.005 | 0.008 | |||||||||
| P | 0.005 | 0.005 | 0.008 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matějka Košinová, K.; Cukor, J.; Skoták, V.; Linda, R.; Vacek, Z.; Bukovjan, K.; Kušta, T. Analysis of Fibropapillomatosis in Roe Deer (Capreolus capreolus) Confirms High Content of Heavy Metals. Animals 2024, 14, 2847. https://doi.org/10.3390/ani14192847
Matějka Košinová K, Cukor J, Skoták V, Linda R, Vacek Z, Bukovjan K, Kušta T. Analysis of Fibropapillomatosis in Roe Deer (Capreolus capreolus) Confirms High Content of Heavy Metals. Animals. 2024; 14(19):2847. https://doi.org/10.3390/ani14192847
Chicago/Turabian StyleMatějka Košinová, Klára, Jan Cukor, Vlastimil Skoták, Rostislav Linda, Zdeněk Vacek, Karel Bukovjan, and Tomáš Kušta. 2024. "Analysis of Fibropapillomatosis in Roe Deer (Capreolus capreolus) Confirms High Content of Heavy Metals" Animals 14, no. 19: 2847. https://doi.org/10.3390/ani14192847
APA StyleMatějka Košinová, K., Cukor, J., Skoták, V., Linda, R., Vacek, Z., Bukovjan, K., & Kušta, T. (2024). Analysis of Fibropapillomatosis in Roe Deer (Capreolus capreolus) Confirms High Content of Heavy Metals. Animals, 14(19), 2847. https://doi.org/10.3390/ani14192847








