Factors Affecting Yeast Digestibility and Immunostimulation in Aquatic Animals
Abstract
Simple Summary
Abstract
1. Introduction
2. Yeast Cell Wall Components and Their Nutritional Properties
3. Use of Yeast in Fish Feeding
4. Use of Yeast as the Primary Protein Source in Aquafeed
5. Use of Yeast as Supplement in Aquafeed
5.1. Use of Yeast as Probiotics in Aquafeed
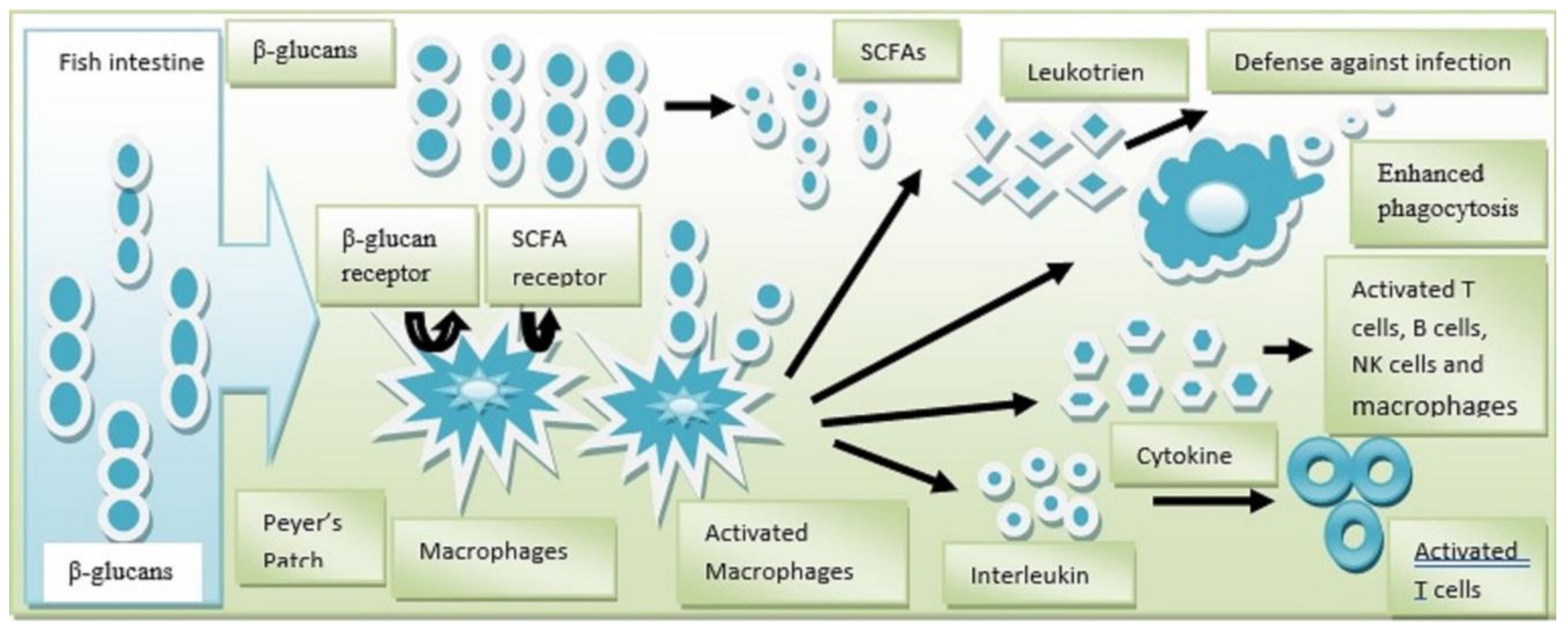
5.2. Use of Yeast as Immune-Stimulant for Fish
6. Digestibility of Yeast in Fish and Crustaceans
7. Use of Yeast in Live Aquafeed Culture
8. Conclusions and Aspects of Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2022; p. 266. [Google Scholar]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards more sustainable aquaculture practices: A meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquac. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Agboola, J.; Schiavone, M.; Øverland, M.; Morales-Lange, B.; Lagos, L.; Arntzen, M.Ø.; Lapeña, D.; Eijsink, V.G.H.; Horn, S.J.; Mydland, L.T.; et al. Impact of Down-Stream Processing on Functional Properties of Yeasts in Diets of Atlantic Salmon (Salmo salar): Implications for Gut Health. Sci. Rep. 2021, 11, 4496. [Google Scholar]
- Jia, S.; Li, X.; He, W.; Wu, G. Protein-Sourced Feedstuffs for Aquatic Animals in Nutrition Research and Aquaculture. In Advances in Experimental Medicine and Biology; Dong, H., Radeke, H.H., Rezaei, N., Steinlein, O., Xiao, J., Rosenhouse-Dantsker, A., Gerlai, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1354. [Google Scholar] [CrossRef]
- IFFO. Marine Ingredients Organization, Statistical Yearbook 2019. Marine Ingredients Organization. 2019. Available online: https://www.iffo.com/feeding-growing-population (accessed on 6 August 2024).
- Ekmay, R.D.; Plagnes-juan, E.; Aguirre, P.; Surget, A.; Terrier, F.; Frohn, L.; Skiba-Cassy, S. Partially replacing plant protein sources with torula yeast in rainbow trout (Oncorhynchus mykiss) feed increases growth and factors related to immune status. J. World Aquac. Soc. 2024, 55, 169–186. [Google Scholar] [CrossRef]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J.Ø. Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Hou, Y.; He, W.; Hu, S.; Wu, G. Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 2019, 51, 1153–1165. [Google Scholar] [CrossRef]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; Basuini, M.F.E.; Olivier, A.; Zaineldin, A.I. Growth performance, blood health, antioxidant status and immune response in red sea bream (Pagrus major) fed Aspergillus oryzae fermented rapeseed meal (RM-Koji). Fish Shellfish Immunol. 2018, 75, 253–262. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO Fisheries: Rome, Italy, 2018. [Google Scholar]
- Froehlich, H.E.; Jacobsen, N.S.; Essington, T.E.; Clavelle, T.; Halpern, B.S. Avoiding the ecological limits of forage fish for fed aquaculture. Nat. Sustain. 2018, 1, 298–303. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zheng, C.; Zenger, K.; et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Richard, N.; Costas, B.; Machado, M.; Fernández-Boo, S.; Girons, A.; Dias, J.; Corraze, G.; Terrier, F.; Marchand, Y.; Skiba-Cassy, S. Inclusion of a protein-rich yeast fraction in rainbow trout plant-based diet: Consequences on growth performances, flesh fatty acid profile and health-related parameters. Aquaculture 2021, 544, 737132. [Google Scholar] [CrossRef]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The application of single-cell ingredients in aquaculture feeds—a review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Øverland, M.; Skrede, A. Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture. J. Sci. Food Agric. 2017, 97, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Basuini, E.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; El-Sayed Ali, T.; Ragaa, N.M.; Ali, S.E.; Abd-Elsalam, R.M.; Younis, N.A.; Abdel-Moneam, D.A.; Hamdien, A.H.; Bonato, M.; Dawood, M.A.O. Analysis of the Productivity, Immunity, and Health Performance of Nile Tilapia (Oreochromis niloticus) Broodstock-fed Dietary Fermented Extracts Sourced from Saccharomyces cerevisiae (Hilyses): A Field Trial. Animals 2021, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.; Jamal, M.T.; Al-Harb, M.; Al-Mur, B.A.; Haque, M.F. Use of yeasts in aquaculture nutrition and immunostimulation: A review. J. Appl. Biol. Biotechnol. 2022, 10, 59–65. [Google Scholar] [CrossRef]
- Zhou, X. Notes from the Aquaculture Statistician. FAO Aquac. Newsl. 2018, 58, 6–8. [Google Scholar]
- Torrecillas, S.; Montero, D.; Izquierdo, M. Improved health and growth of fish fed mannan oligosaccharides: Potential mode of action. Fish Shellfish Immunol. 2014, 36, 525–544. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Moghadam, M.S.; Dawood, M.A.O.; Hoseinifar, S.H. Lactobacillus fermentum and/or ferulic acid improved the immune responses, antioxidative defence and resistance against Aeromonas hydrophila in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 2019, 94, 916–923. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Magouz, F.I.; Mansour, M.; Saleh, A.A.; Asely, A.M.E.; Fadl, S.E.; Ahmed, H.A.; Al-Ghanim, K.A.; Mahboob, S.; Al-Misned, F. Evaluation of yeast fermented poultry by-product meal in Nile Tilapia (Oreochromis niloticus) feed: Effects on growth performance, digestive enzymes activity, innate immunity, and antioxidant capacity. Front. Vet. Sci. 2020, 6, 516. [Google Scholar] [CrossRef]
- Navarrete, P.; Tovar-Ramírez, D. Use of yeasts as probiotics in fish aquaculture. Sustain. Aquac. Tech. 2014, 1, 135–172. [Google Scholar]
- Nathanailides, C.; Kolygas, M.; Choremi, K.; Mavraganis, T.; Gouva, E.; Vidalis, K.; Athanassopoulou, F. Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes 2021, 6, 76. [Google Scholar] [CrossRef]
- Schiavone, M.; Vax, A.; Formosa, C.; Martin-Yken, H.; Dague, E.; François, J.M. A combined chemical and enzymatic method to determine quantitatively the polysaccharide components in the cell wall of yeasts. FEMS Yeast Res. 2014, 14, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Rawling, M.; Leclercq, E.; Foey, A.; Castex, M.; Merrifield, D. A novel dietary multi-strain yeast fraction modulates intestinal toll-like-receptor signalling and mucosal responses of rainbow trout (Oncorhynchus mykiss). PLoS ONE 2021, 16, e0245021. [Google Scholar] [CrossRef] [PubMed]
- Rawling, M.; Schiavone, M.; Apper, E.; Merrifield, D.L.; Castex, M.; Leclercq, E.; Foey, A. Yeast cell wall extracts from Saccharomyces cerevisiae varying in structure and composition differentially shape the innate immunity and mucosal tissue responses of the intestine of zebrafish (Danio rerio). Front. Immunol. 2023, 14, 1158390. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Hicks, T.M.; Verbeek, C.J.R. Meat Industry Protein by-Products: Sources and Characteristics. In Protein Byproducts; Dhillon, G.D., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 37–61. [Google Scholar] [CrossRef]
- Cuppett, S.L.; Soares, J.H. The metabolizable energy values and digestibilities of menhaden fish meal, fish solubles and fish oils. Poult. Sci. 1972, 51, 2078–2083. [Google Scholar] [CrossRef]
- Li, P.; He, W.; Wu, G. Composition of amino acids in foodstuffs for humans and animals. In Amino Acids in Nutrition and Health; Wu, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 189–210. [Google Scholar]
- Deng, Y.; Borewicz, K.; van Loo, J.; Olabarrieta, M.Z.; Kokou, F.; Sipkema, D.; Verdegem, M.C.J. In-Situ Biofloc Affects the Core Prokaryotes Community Composition in Gut and Enhances Growth of Nile Tilapia (Oreochromis niloticus). Microb. Ecol. 2022, 84, 879–892. [Google Scholar] [CrossRef]
- Bardhan, P.; Gupta, K.; Kishor, S.; Chattopadhyay, P.; Chaliha, C.; Kalita, E.; Goud, V.V.; Mandal, M. Oleaginous yeasts isolated from traditional fermented foods and beverages of Manipur and Mizoram, India, as a potent source of microbial lipids for biodiesel production. Ann. Microbiol. 2020, 70, 1–14. [Google Scholar] [CrossRef]
- Parolini, G. Building Human and Industrial Capacity in European Biotechnology: The Yeast Genome. Biotechnology 1996, 16, 365–368. [Google Scholar]
- Shurson, G.C. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Øverland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae yeasts as protein sources in diets for Atlantic salmon (Salmo salar). Aquaculture 2013, 402, 1–7. [Google Scholar] [CrossRef]
- Lapeña, D.; Olsen, P.M.; Arntzen, M.Ø.; Kosa, G.; Eijsink, V.G.H.; Horn, S.J. Spruce sugars and poultry hydrolysate as growth medium in repeated fed-batch fermentation processes for production of yeast biomass. Bioprocess Biosyst. Eng. 2020, 43, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Adeshina, I.; Issa, Z.A. Antioxidants and immune responses, resistance to Aspergilus flavus infection, and growth performance of Nile tilapia, Oreochromis niloticus, fed diets supplemented with yeast, Saccharomyces cerevisiae. Anim. Feed Sci. Technol. 2020, 263, 114484. [Google Scholar] [CrossRef]
- Vidakovic, A.; Huyben, D.; Sundh, H.; Nyman, A.; Vielma, J.; Passoth, V.; Kiessling, A.; Lundh, T. Growth performance, nutrient digestibility and intestinal morphology of rainbow trout (Oncorhynchus mykiss) fed graded levels of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquac. Nutr. 2020, 26, 275–286. [Google Scholar] [CrossRef]
- Abass, D.A.; Obirikorang, K.A.; Campion, B.B.; Edziyie, R.E.; Skov, P.V. Dietary supplementation of yeast (Saccharomyces cerevisiae) improves growth, stress tolerance, and disease resistance in juvenile Nile tilapia (Oreochromis niloticus). Aquac. Int. 2018, 26, 843–855. [Google Scholar] [CrossRef]
- Vidakovic, A.; Langeland, M.; Sundh, H.; Sundell, K.; Olstorpe, M.; Vielma, J.; Kiessling, A.; Lundh, T. Evaluation of growth performance and intestinal barrier function in Arctic Charr (Salvelinus alpinus) fed yeast (Saccharomyces cerevisiae), fungi (Rhizopus oryzae) and blue mussel (Mytilus edulis). Aquac. Nutr. 2016, 22, 1348–1360. [Google Scholar] [CrossRef]
- Banu, M.R.; Akter, S.; Islam, M.R.; Mondol, M.N.; Hossain, M.A. Probiotic yeast enhanced growth performance and disease resistance in freshwater catfish gulsa tengra, Mystus cavasius. Aquac. Rep. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Ozório, R.O.A.; Portz, L.; Borghesi, R.; Cyrino, J.E.P. Effects of dietary yeast (Saccharomyces cerevisia) supplementation in practical diets of tilapia (Oreochromis niloticus). Animals 2012, 2, 16–24. [Google Scholar] [CrossRef]
- Yones, A.-M.M.; Hussein, M.S.; Ali, M.W.; Abdel-Azem, A.-A.M. Effect of dietary Lacto cel-con probiotic on growth performance and hematology indices of fingerlings mono-sex Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish. 2019, 23, 227–239. [Google Scholar] [CrossRef]
- El-Bab, A.F.F.; Saghir, S.A.M.; El-Naser, I.A.A.; El-Kheir, S.M.M.A.; Abdel-Kader, M.F.; Alruhaimi, R.S.; Alqhtani, H.A.; Mahmoud, A.M.; Naiel, M.A.E.; El-Raghi, A.A. The effect of dietary Saccharomyces cerevisiae on growth performance, oxidative status, and immune response of sea bream (Sparus aurata). Life 2022, 12, 1013. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Pradhan, D.; Swain, H.S.; Upadhyay, A.; Sahu, B.; Nanda, S.; Patra, S.K.; Kasturi, S.; Mohanta, K.N.; Giri, S.S. On Valorization of Brewer’s Yeast as an Environmentally Sustainable Fishmeal Replacement in Labeo rohita Nutrition: Insight to Growth Attributes, Digestive Enzyme Activities and Haemato-biochemical Indices. Waste Biomass Valorization 2024, 15, 3503–3517. [Google Scholar] [CrossRef]
- Pongpet, J.; Ponchunchoovong, S.; Payooha, K. Partial replacement of fishmeal by brewer’s yeast (Saccharomyces cerevisiae) in the diets of Thai Panga (Pangasianodon hypophthalmus× Pangasius bocourti). Aquac. Nutr. 2016, 22, 575–585. [Google Scholar] [CrossRef]
- Rosales, M.; Castillo, S.; Pohlenz, C.; Gatlin, D.M., III. Evaluation of dried yeast and threonine fermentation biomass as partial fish meal replacements in the diet of red drum Sciaenops ocellatus. Anim. Feed Sci. Technol. 2017, 232, 190–197. [Google Scholar] [CrossRef]
- Hao, Q.; Xia, R.; Zhang, Q.; Xie, Y.; Ran, C.; Yang, Y.; Zhou, W.; Chu, F.; Zhang, X.; Wang, Y.; et al. Partially replacing dietary fish meal by Saccharomyces cerevisiae culture improve growth performance, immunity, disease resistance, composition and function of intestinal microbiota in channel catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2022, 125, 220–229. [Google Scholar] [CrossRef]
- Andriamialinirina, H.J.T.; Irm, M.; Taj, S.; Lou, J.H.; Jin, M.; Zhou, Q. The effects of dietary yeast hydrolysate on growth, hematology, antioxidant enzyme activities and non-specific immunity of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2020, 101, 168–175. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.-Q.; Tian, L.-X.; Liu, Y.-J.; Niu, J. Enhanced intestinal health, immune responses and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac. Rep. 2020, 17, 100385. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gini, E.; Koch, J.F.A.; Iannini, F.; Brambilla, F.; Terova, G. Erratum: Effects of hydrolyzed fish protein and autolyzed yeast as substitutes of fishmeal in the gilthead sea bream (Sparus aurata) diet, on fish intestinal microbiome. BMC Vet. Res. 2020, 16, 118, Erratum in BMC Vet. Res. 2020, 16, 219. https://doi.org/10.1186/s12917-020-02416-1. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, F.; Hu, J.; Liu, C.; Liu, H.; Han, D.; Jin, J.; Yang, Y.; Zhu, X.; Yi, J.; et al. Effects of dietary yeast hydrolysate on the growth, antioxidant response, immune response and disease resistance of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2019, 94, 548–557. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.-Y.; Li, M.; Chen, W.; Bao, P.-Y.; Yu, Z.-C.; Chang, Y.-Q. Effects of dietary probiotic yeast on growth parameters in juvenile sea cucumber, Apostichopus japonicus. Aquaculture 2019, 499, 203–211. [Google Scholar] [CrossRef]
- Jarmołowicz, S.; Rożyński, M.; Kowalska, A.; Zakęś, Z. Growth in juvenile pikeperch (Sander lucioperca L.) stimulated with yeast, Saccharomyces cerevisiae, extract. Aquac. Res. 2018, 49, 614–620. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, S.; Zou, T.; Han, D.; Liu, H.; Jin, J.; Yang, Y.; Zou, X.; Xie, S.; Zhou, W. Effects of dietary yeast culture on growth performance, immune response and disease resistance of gibel carp (Carassius auratus gibelio CAS III). Fish Shellfish Immunol. 2018, 82, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.-Y.; Liu, W.-B.; Liang, C.; Sun, C.-X.; Xue, Y.-F.; Wan, Z.-D.; Jiang, G.-Z. Effects of partial replacement of fish meal by yeast hydrolysate on complement system and stress resistance in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2017, 67, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Gumus, E.; Aydin, B.; Kanyilmaz, M. Growth and feed utilization of goldfish (Carassius auratus) fed graded levels of brewer’s yeast (Saccharomyces cerevisiae). Iran. J. Fish. Sci. 2016, 15, 1124–1133. [Google Scholar]
- Yu, H.H.; Han, F.; Xue, M.; Wang, J.; Tacon, P.; Zheng, Y.H.; Wu, X.F.; Zhang, Y.J. Efficacy and tolerance of yeast cell wall as an immunostimulant in the diet of Japanese seabass (Lateolabrax japonicus). Aquaculture 2014, 432, 217–224. [Google Scholar] [CrossRef]
- Peterson, B.C.; Booth, N.J.; Manning, B.B. Replacement of fish meal in juvenile channel catfish, Ictalurus punctatus, diets using a yeast-derived protein source: The effects on weight gain, food conversion ratio, body composition and survival of catfish challenged with Edwardsiella ictaluri. Aquac. Nutr. 2012, 18, 132–137. [Google Scholar] [CrossRef]
- Goda, A.M.A.; Mabrouk, H.A.-H.H.; Wafa, M.A.E.-H.; El-Afifi, T.M. Effect of using baker’s yeast and exogenous digestive enzymes as growth promoters on growth, feed utilization and hematological indices of Nile tilapia, Oreochromis niloticus fingerlings. J. Agric. Sci. Technol. B 2012, 2, 15–28. [Google Scholar]
- Gause, B.; Trushenski, J. Replacement of fish meal with ethanol yeast in the diets of sunshine bass. N. Am. J. Aquac. 2011, 73, 97–103. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Dietary carbohydrate utilization by European sea bass (Dicentrarchus labrax L.) and gilthead sea bream (Sparus aurata L.) juveniles. Rev. Fish. Sci. 2011, 19, 201–215. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Mirvaghefi, A.; Merrifield, D.L. The effects of dietary inactive brewer’s yeast Saccharomyces cerevisiae var. ellipsoideus on the growth, physiological responses and gut microbiota of juvenile beluga (Huso huso). Aquaculture 2011, 318, 90–94. [Google Scholar] [CrossRef]
- Lunger, A.N.; Craig, S.R.; McLean, E. Replacement of fish meal in cobia (Rachycentron canadum) diets using an organically certified protein. Aquaculture 2006, 257, 393–399. [Google Scholar] [CrossRef]
- Li, P.; Gatlin, D.M., III. Evaluation of brewer’s yeast (Saccharomyces cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysops× M. saxatilis). Aquaculture 2003, 219, 681–692. [Google Scholar] [CrossRef]
- Long, M.; Lin, W.; Hou, J.; Gou, H.; Li, L.; Li, D.; Tang, R.; Yang, F. Dietary supplementation with selenium yeast and tea polyphenols improve growth performance and nitrite tolerance of Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2017, 68, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.A.; Craig, S.R.; McLean, E. Hyperaccumulation of selenium in hybrid striped bass: A functional food for aquaculture? Aquac. Nutr. 2008, 14, 215–222. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Wu, L.; Liu, Q.; Zhang, D.; Yin, J. Expression of selenoprotein genes in muscle is crucial for the growth of rainbow trout (Oncorhynchus mykiss) fed diets supplemented with selenium yeast. Aquaculture 2018, 492, 82–90. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Ren, X.; Huang, D.; Si, G.; Chen, J. Replacement of fish meal with gamma-ray irradiated soybean meal in the diets of largemouth bass Micropterus salmoides. Aquac. Nutr. 2021, 27, 977–985. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.Z.; Wang, F.; Wu, Y.B.; Qin, J.G.; Li, P. Supplementations of poultry by-product meal and selenium yeast increase fish meal replacement by soybean meal in golden pompano (Trachinotus ovatus) diet. Aquac. Res. 2017, 48, 1904–1914. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, H.; Ma, H.; Wang, X. Supplementation of Selenium-Yeast Enhances Fishmeal Replacement by Soy Protein Concentrate in Diets for Golden Pompano (Trachinotus ovatus). Aquac. Res. 2023, 2023, 8953076. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Magouz, F.I.; Essa, M.; Mansour, M. Impact of Yeast Fermented Poultry by-Product Meal on Growth, Digestive Enzyme Activities, Intestinal Morphometry and Immune Response Traits of Common Carp (Cyprinus carpio). Ann. Anim. Sci. 2020, 20, 939–959. [Google Scholar] [CrossRef]
- Bob-Manuel, F.G. A comparative study of the effect of yeast single cell protein on growth, feed utilization and condition factor of the African catfish Clarias gariepinus (Burchell) and tilapia, Oreochromis niloticus (Linnaeus) fingerlings. Afr. J. Agric. Res. 2014, 9, 2005–2011. [Google Scholar]
- Yousefi, S.; Shokri, M.M.; Navirian, H.A.; Hoseinifar, S.H. Effects of yeast cell membrane prebiotic (Immunowall®) on growth performance and hematological parameters in juvenile Persian sturgeon (Acipenser persicus). J. Anim. Environ. 2020, 12, 221–228. [Google Scholar]
- Khalil, H.S.; Mansour, A.T.; Goda, A.M.A.; Omar, E.A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquaculture 2019, 501, 135–143. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Mohammady, E.Y.; Elashry, M.A.; El-Haroun, E.R.; Davies, S.J. Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 2018, 495, 592–601. [Google Scholar] [CrossRef]
- Tavana, B.G.Z.; Banaee, M.; Jourdehi, A.Y.; Haghi, B.N.; Hassani, M.H.S. Effects of dietary Sel-Plex supplement on growth performance, hematological and immunological parameters in Siberian sturgeon (Acipenser baerii Brandt, 1869). Iran. J. Fish. Sci. 2019, 18, 830–846. [Google Scholar] [CrossRef]
- Ezenwaji, N.E.; Iluno, A.; Atama, C.; Nwaigwe, C.O.; Nwaigwe, C.U. Substitution of soyabean meal with bioactive yeast in the diet of Clarias gariepinus: Effect on growth rate, haematological and biochemical profile. Afr. J. Biotechnol. 2012, 11, 15802–15810. [Google Scholar]
- Hauptman, B.S.; Barrows, F.T.; Block, S.S.; Gaylord, T.G.; Paterson, J.A. Evaluation of grain distillers dried yeast as a fish meal substitute in practical-type diets of juvenile rainbow trout, Oncorhynchus mykiss. Aquaculture 2014, 432, 7–14. [Google Scholar] [CrossRef]
- Plaipetch, P.; Yakupitiyage, A. Effect of replacing soybean meal with yeast-fermented canola meal on growth and nutrient retention of Nile tilapia, Oreochromis niloticus (Linnaeus 1758). Aquac. Res. 2014, 45, 1744–1753. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Yang, Z.; Li, M.; Liu, J.; Song, J. Effects of dietary live yeast Hanseniaspora opuntiae C21 on the immune and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2013, 34, 66–73. [Google Scholar] [CrossRef]
- Plaipetch, P.; Yakupitiyage, A. Use of yeast-fermented canola meal to replace fishmeal in the diet of Asian sea bass Lates calcarifer (Bloch, 1790). J. Aquac. Res. Dev. 2012, 3, 1000125. [Google Scholar] [CrossRef]
- Trosvik, K.A.; Rawles, S.D.; Thompson, K.R.; Metts, L.A.; Gannam, A.; Twibell, R.; Webster, C.D. Growth and body composition of Nile tilapia, Oreochromis niloticus, fry fed organic diets containing yeast extract and soybean meal as replacements for fish meal, with and without supplemental lysine and methionine. J. World Aquac. Soc. 2012, 43, 635–647. [Google Scholar] [CrossRef]
- Buentello, J.A.; Neill, W.H.; Gatlin, D.M., III. Effects of dietary prebiotics on the growth, feed efficiency and non-specific immunity of juvenile red drum Sciaenops ocellatus fed soybean-based diets. Aquac. Res. 2010, 41, 411–418. [Google Scholar] [CrossRef]
- Ebrahim, M.S.M.; Abou-Seif, R.A. Fish Meal Replacement by Yeast Protein (Saccharomyces cerevisiae) Supplemented with Biogenic L-Carintine as a Source of Methionine Plus Lysine Mixture in Feed for Nile Tilapia (Oreochromis niloticus) Fingerlinges. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture 2008, Cairo, Egypt, 12–14 October 2008; pp. 999–1099. Available online: http://ag.arizona.edu/azaqua/ista/ISTA8/FinalPapers/11Nutrition/PDF/22Ramadan.pdf (accessed on 5 June 2024).
- Ghosh, K.; Sen, S.K.; Ray, A.K. Feed Utilization Efficiency and Growth Performance in Rohu, Supplemented Diets. Acta Ichthyol. Piscat. 2005, 35, 111–117. [Google Scholar] [CrossRef]
- Muzinic, L.A.; Thompson, K.R.; Morris, A.; Webster, C.D.; Rouse, D.B.; Manomaitis, L. Partial and total replacement of fish meal with soybean meal and brewer’s grains with yeast in practical diets for Australian red claw crayfish Cherax quadricarinatus. Aquaculture 2004, 230, 359–376. [Google Scholar] [CrossRef]
- Ziarati, M.; Zorriehzahra, M.J.; Hassantabar, F.; Mehrabi, Z.; Dhawan, M.; Shraun, K.; Emran, T.B.; Dhama, K.; Chaicumpa, W.; Shamsi, S. Zoonotic diseases of fish and their prevention and control. Vet. Q. 2022, 42, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, E.E.; Savin, V.; Popa, M.D.; Dima, F.M. The Effect of Probiotics on Growth Performance, Haematological and Biochemical Profiles in Siberian Sturgeon (Acipenser baerii Brandt, 1869). Fishes 2022, 7, 239. [Google Scholar] [CrossRef]
- Kwasek, K.; Thorne-Lyman, A.L.; Phillips, M. Can human nutrition be improved through better fish feeding practices? a review paper. Crit. Rev. Food Sci. Nutr. 2020, 60, 3822–3835. [Google Scholar] [CrossRef]
- Bunnoy, A.; Yanglang, A.; Tribamrung, N.; Keawthong, C.; Tumree, P.; Kumwan, B.; Meachasompop, P.; Saengrung, J.; Vanichvatin, K.; Muangrerk, C.; et al. Dietary administration of yeast (Saccharomyces cerevisiae) hydrolysate from sugar byproducts promotes the growth, survival, immunity, microbial community and disease resistance to VP (AHPND) in Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2024, 145, 109327. [Google Scholar] [CrossRef]
- Da Silva Berto, R.; Pereira, G.D.V.; Mouriño, J.L.P.; Martins, M.L.; Fracalossi, D.M. Yeast extract on growth, nutrient utilization and haemato-immunological responses of Nile tilapia. Aquac. Res. 2016, 47, 2650–2660. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Serna-Saldívar, S.O.; Rosales-Medina, M.F.; Tinoco-Alvear, M.; Briones-García, M. Application of Saccharomyces cerevisiae var. boulardii in food processing: A review. J. Appl. Microbiol. 2018, 125, 943–951. [Google Scholar] [CrossRef]
- Ansari, F.; Samakkhah, S.A.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 457–485. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Austin, B. Probiotics for disease control in aquaculture. In Diagnosis and Control of Diseases of Fish and Shellfish; Austin, B., Newaj-Fyzul, A., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 189–222. [Google Scholar]
- Darafsh, F.; Soltani, M.; Abdolhay, H.A.; Mehrejan, M.S. Improvement of growth performance, digestive enzymes and body composition of Persian sturgeon (Acipenser persicus) following feeding on probiotics: Bacillus licheniformis, Bacillus subtilis and Saccharomyces cerevisiae. Aquac. Res. 2020, 51, 957–964. [Google Scholar] [CrossRef]
- Saba, A.O.; Yasin, I.S.M.; Azmai, M.N.A. Meta-analyses indicate that dietary probiotics significantly improve growth, immune response, and disease resistance in tilapia. Aquac. Int. 2024, 32, 4841–4867. [Google Scholar] [CrossRef]
- Noman, M.; Kazmi, S.S.U.H.; Saqib, H.S.A.; Fiaz, U.; Pastorino, P.; Barcelò, D.; Tayyab, M.; Liu, W.; Wang, Z.; Yaseen, Z.M. Harnessing probiotics and prebiotics as eco-friendly solution for cleaner shrimp aquaculture production: A state of the art scientific consensus. Sci. Total Environ. 2024, 915, 169921. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, L.; Liu, J.; Wang, H.; Xiao, S. Effect of potential probiotic Rhodotorula benthica D30 on the growth performance, digestive enzyme activity and immunity in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015, 43, 330–336. [Google Scholar] [CrossRef]
- Pourgholam, M.A.; Khara, H.; Safari, R.; Sadati, M.A.Y.; Aramli, M.S. Influence of Lactobacillus plantarum inclusion in the diet of Siberian sturgeon (Acipenser baerii) on performance and hematological parameters. Turkish J. Fish. Aquat. Sci. 2017, 17, 1–5. [Google Scholar]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef]
- Guluarte, C.; Reyes-Becerril, M.; Gonzalez-Silvera, D.; Cuesta, A.; Angulo, C.; Esteban, M.Á. Probiotic properties and fatty acid composition of the yeast Kluyveromyces lactis M3. In vivo immunomodulatory activities in gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 2019, 94, 389–397. [Google Scholar] [CrossRef]
- Pooramini, M.; Kamali, A.; Hajimoradloo, A.; Alizadeh, M.; Ghorbani, R. Effect of using yeast (Saccharomyces cerevisiae) as probiotic on growth parameters, survival and carcass quality in rainbow trout Oncorhynchus mykiss fry. Int. Aquat. Res. 2009, 1, 39. [Google Scholar]
- Pratiwy, F.M.; Kharima, Z.P. The Use of Bread Yeast (Saccharomyces cerevisiae) in Increasing Growth, Disease Resistance, and as Immunostimulating for Various Kinds of Fish: A Review. Asian J. Fish. Aquat. Res. 2022, 20, 64–70. [Google Scholar] [CrossRef]
- Tukmechi, A.; Bandboni, M. Effects of Saccharomyces cerevisiae supplementation on immune response, hematological parameters, body composition and disease resistance in rainbow trout, Oncorhynchus mykiss (Walbaum, 1792). J. Appl. Ichthyol. 2014, 30, 55–61. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; Ahmed, M.; AbdelHamid, F.M.; Gadalla, H.A. Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 28, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, N.; Heidarieh, M.; Pashaki, A.K.; Nofouzi, K.; Farshbafi, M.A.; Akbari, M. Hilyses®, fermented Saccharomyces cerevisiae, enhances the growth performance and skin non-specific immune parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2012, 32, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, S.; Klinder, A.; Brigidi, P.; Cavina, P.; Costabile, A. Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl. Environ. Microbiol. 2012, 78, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Zanuzzo, F.S.; Koch, J.F.A.; de Oliveira, C.A.F.; Sima, P.; Vetvicka, V. Development of fish immunity and the role of β-glucan in immune responses. Molecules 2020, 25, 5378. [Google Scholar] [CrossRef]
- Voloski, A.P.D.S.; de Figueiredo Soveral, L.; Dazzi, C.C.; Sutili, F.; Frandoloso, R.; Kreutz, L.C. β-Glucan improves wound healing in silver catfish (Rhamdia quelen). Fish Shellfish Immunol. 2019, 93, 575–579. [Google Scholar] [CrossRef]
- Divya, M.; Gopi, N.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Vaseeharan, B. β-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: Dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb. Pathog. 2020, 139, 103917. [Google Scholar] [CrossRef]
- Guzmán-Villanueva, L.T.; Ascencio-Valle, F.; Macías-Rodríguez, M.E.; Tovar-Ramírez, D. Effects of dietary β-1, 3/1, 6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol. Biochem. 2014, 40, 827–837. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, M.; Sanchez, V.; Guluarte, C.; Angulo, C. β-D-glucan from marine yeast Debaryomyces hansenii BCS004 enhanced intestinal health and glucan-expressed receptor genes in Pacific red snapper Lutjanus peru. Microb. Pathog. 2020, 143, 104141. [Google Scholar] [CrossRef]
- Machuca, C.; Méndez-Martínez, Y.; Reyes-Becerril, M.; Angulo, C. Yeast β-Glucans as Fish Immunomodulators: A Review. Animals 2022, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Pionnier, N.; Falco, A.; Miest, J.J.; Shrive, A.K.; Hoole, D. Feeding common carp Cyprinus carpio with β-glucan supplemented diet stimulates C-reactive protein and complement immune acute phase responses following PAMPs injection. Fish Shellfish Immunol. 2014, 39, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Tungland, B. Human Microbiota in Health and Disease: From Pathogenesis to Therapy; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Rawling, M.D.; Pontefract, N.; Rodiles, A.; Anagnostara, I.; Leclercq, E.; Schiavone, M.; Castex, M.; Merrifield, D. The effect of feeding a novel multistrain yeast fraction on European seabass (Dicentrachus labrax) intestinal health and growth performance. J. World Aquac. Soc. 2019, 50, 1108–1122. [Google Scholar] [CrossRef]
- Yousuf, S.; Tyagi, A.; Singh, R. Probiotic supplementation as an emerging alternative to chemical therapeutics in finfish aquaculture: A Review. Probiotics Antimicrob. Proteins 2023, 15, 1151–1168. [Google Scholar] [CrossRef]
- Eissa, E.H.; Ahmed, N.H.; El-Badawi, A.A.; Munir, M.B.; Al-Kareem, O.M.A.; Eissa, M.E.H.; Hussien, E.H.M.; Sakr, S.E.-S. Assessing the influence of the inclusion of Bacillus subtilis AQUA-GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp, Litopenaeus vannamei. Aquac. Res. 2022, 53, 6606–6615. [Google Scholar] [CrossRef]
- Goda, A.M.; Omar, E.A.; Srour, T.M.; Kotiet, A.M.; El-Haroun, E.; Davies, S.J. Effect of diets supplemented with feed additives on growth, feed utilization, survival, body composition and intestinal bacterial load of early weaning European seabass, Dicentrarchus labrax post-larvae. Aquac. Int. 2018, 26, 169–183. [Google Scholar] [CrossRef]
- Öztürk, F.; Esendal, Ö.M. Usage of Lactobacillus rhamnosus as a Probiotic in Sea Bass (Dicentrarchus labrax). J. Anatol. Environ. Anim. Sci. 2020, 5, 93–99. [Google Scholar] [CrossRef][Green Version]
- Hansen, J.Ø.; Lagos, L.; Lei, P.; Reveco-Urzua, F.E.; Morales-Lange, B.; Hansen, L.D.; Schiavone, M.; Mydland, L.T.; Arntzen, M.Ø.; Mercado, L. Down-stream processing of baker’s yeast (Saccharomyces cerevisiae)—Effect on nutrient digestibility and immune response in Atlantic salmon (Salmo salar). Aquaculture 2021, 530, 735707. [Google Scholar] [CrossRef]
- Langeland, M.; Lindberg, J.E.; Lundh, T. Digestive enzyme activity in Eurasian perch (Perca fluviatilis) and Arctic charr (Salvelinus alpinus). J. Aquac. Res. Dev. 2013, 5, 2. [Google Scholar]
- Zhao, L.; Wang, W.; Huang, X.; Guo, T.; Wen, W.; Feng, L.; Wei, L. The effect of replacement of fish meal by yeast extract on the digestibility, growth and muscle composition of the shrimp Litopenaeus vannamei. Aquac. Res. 2017, 48, 311–320. [Google Scholar] [CrossRef]
- Vidakovic, A.; Huyben, D.; Nyman, A.; Kiessling, A.; Lundh, T. Evaluation of growth performance, nutrient retention and apparent digestibility in rainbow trout (O. mykiss) fed graded levels of yeasts Saccharomyces cerevisae and Wickerhamomyces anomalus. In Proceedings of the Aquaculture Europe 2015 Conference, Rotterdam, The Netherlands, 20–23 October 2015. [Google Scholar]
- Arup, T.; Patra, B.C. Oral administration of baker’s yeast (Saccharomyces cerevisiae) acts as a growth promoter and immunomodulator in Labeo rohita (Ham.). J. Aquac. Res. Dev. 2011, 2, 109. [Google Scholar]
- Das, J.; Hossain, M.S.; Hasan, J.; Siddique, M.A.M. Growth performance and egg ratio of a marine rotifer Brachionus rotundiformis fed different diets in captivity. Thalass. An. Int. J. Mar. Sci. 2021, 37, 113–118. [Google Scholar] [CrossRef]
- Khatun, B.; Rahman, R.; Rahman, M.S. Evaluation of yeast Saccharomyces cerevisiae and algae Chlorella vulgaris as diet for Rotifer Brachionus calyciflorus. Agriculturists 2014, 12, 1–9. [Google Scholar] [CrossRef]
- Talens-Perales, D.; Marín-Navarro, J.; Garrido, D.; Almansa, E.; Polaina, J. Fixation of bioactive compounds to the cuticle of Artemia. Aquaculture 2017, 474, 95–100. [Google Scholar] [CrossRef]
- Ajah, P.O. Mass culture of Rotifera (Brachionus quadridentatus [Hermann, 1783]) using three different algal species. Afr. J. Food Sci. 2010, 4, 80–85. [Google Scholar]
- Radhakrishnan, K.; Aanand, S.; Rameshkumar, S.; Divya, F. Effect of feeding rate and feeding frequency in mass culture of Brachionus plicatilis in semi-continuous method with a yeast-based diet. J. Fish. Life Sci. 2017, 2, 40–44. [Google Scholar]
- Hu, J.; Wang, G.; Huang, Y.; Sun, Y.; Zhao, H.; Li, N. Effects of Substitution of Fish Meal with Black Soldier Fly (Hermetia illucens) Larvae Meal, in Yellow Catfish (Pelteobagrus fulvidraco) Diets. Isr. J. Aquac. 2017, 69, 9. [Google Scholar]
- El-Khodary, G.M.; Mona, M.M.; El-Sayed, H.S.; Ghoneim, A.Z. Phylogenetic identification and assessment of the nutritional value of different diets for a copepod species isolated from Eastern Harbor coastal region. Egypt. J. Aquat. Res. 2020, 46, 173–180. [Google Scholar] [CrossRef]
| Composition | Saccharomyces cerevisiae from Beer Fermentation | Menhaden Fishmeal |
|---|---|---|
| Dry matter% | 93 | 91.2–92 |
| ME (kcal/kg) | 1990 | 3370 |
| Crude protein% | 44.4 | 59–68.5 |
| Crude fat% | 1 | 9.1–10.4 |
| Crude fiber% | 2.7 | 0.9 |
| Ca% | 0.12 | 4.87–5.34 |
| P% | 1.4 | 2.93–3.05 |
| Product Name | Fish Species | Inclusion Levels | Effects | Source |
|---|---|---|---|---|
| Brewer’s yeast | Largemouth bass (Micropterus salmoides) | 20, 40, 60 g/kg | The supplementation of S. cerevisiae at 10 g kg−1 did not significantly reduce fishmeal in the diet. | [50] |
| Dietary yeast polysaccharides | Channel catfish (Ictalurus punctatus) | 20 g/kg | The study found that fish fed experimental diets for 12 weeks significantly improved growth performance compared to the control. | [50] |
| Yeast, Saccharomyces cerevisiae (SC) | Gilthead sea bream (Sparus aurata) | 1, 2, and 4 g/kg | The study showed that at a dose of 4 g/kg, a meal high in SC dramatically improved growth performance, intestinal morphology, redox homeostasis, and immunological response. | [45] |
| Protein-rich yeast fraction | Rainbow trout (Oncorhynchus mykiss) | 0, 5, 10, or 15% | The use of yeasts in 60% of fishmeal protein can cause haemolytic anemia in rainbow trout, potentially restricting their inclusion in farmed fish diets. | [13] |
| Dietary yeast hydrolysate | Nile tilapia (Oreochromis niloticus) | 1 and 3% | 1% yeast hydrolysate supplementation enhances growth performance, feed utilization, and antioxidant status. | [51] |
| Baker’s yeast | Freshwater catfish gulsa tengra (Mystus cavasius) | 0.5, 1, and 1.5 g/kg | Yeast supplement is a potentially effective growth promoter that could replace antibiotics in the treatment of M. cavasius infections. | [42] |
| Dietary hydrolyzed yeast | Pacific white shrimp (Litopenaeus vannamei) | 1% | The study found no significant effect on growth performance or body composition, but it did affect intestinal health, immunological responses, and ammonia resistance. | [52] |
| Autolyzed dried yeast | Gilthead sea bream (Sparus aurata) | 0 and 5% | Following a 92-day feeding trial, there were no discernible variations between the dietary groups in terms of fish growth rate, mortality, or feed efficiency. | [53] |
| Dietary yeast hydrolysate | Largemouth bass (Micropterus salmoides) | 0, 1.5, 3.0, 4.5% | Dietary yeast hydrolysate has been found to enhance the antioxidant capacity and immune response of largemouth bass without any adverse growth effects. | [54] |
| Yeast (S. cerevisiae) | Sea cucumber, (Apostichopus japonicus) | 5% | The study aimed to enhance the growth, digestive enzyme activity, nutritional value, and immune system of sea cucumber juveniles. | [55] |
| Yeast, Saccharomyces cerevisiae, extract | Juvenile pikeperch (Sander lucioperca L.) | 2, 4, and 6% | The study found that pikeperch growth is stimulated by the lowest analyzed dose of yeast, which is 2% yeast extract. | [56] |
| Yeast culture (YC) | Gibel carp (Carassius auratus gibelio CAS Ⅲ) | 0, 20, 40, and 60% | The study suggests that yeast culture could be a suitable fishmeal alternative for Gibel carp diets, with a dietary inclusion of 4 g of yeast culture per 100 g diet. | [57] |
| Yeast hydrolysate (YH) | Juvenile Jian carp (Cyprinus carpio var. Jian) | 1, 3, 5, and 7% | The study found that 3% YH was the most effective substitution for fishmeal (FM) in enhancing innate immunity and growth performance. | [58] |
| Dried yeast | Red drum (Sciaenops ocellatus) | 20, 30, 40, 50% | Without impairing the red drum’s functionality, the material may replace between 30 and 50% of the protein supplied by fishmeal. | [49] |
| Brewer’s yeast (Saccharomyces cerevisiae) | Thai Panga (Pangasianodon hypophthalmus × Pangasius bocourti) | 30, 45, 60, 75% | By substituting brewer’s yeast for 45% of the fishmeal, one can improve the immune response and growth performance of the Thai Panga. | [48] |
| Brewer’s yeast | Goldfish (Carassius auratus) | 0, 15, 25, 35, and 45% | Fish fed a diet with yeast replacing 35% of their meal showed better weight gain, SGR, FCR, and protein efficiency ratio compared to other diets. | [59] |
| Dietary yeast cell wall (YCW) | Japanese seabass (Lateolabrax japonicus) | 0, 250, 500, 1000, 2000, and 20,000 mg/kg | The study found that the optimal dose of YCW is 2000 mg/kg, with a 10 × safety margin. | [60] |
| Yeast (Saccharomyces cerevisiae) | Juvenile channel catfish (Ictalurus punctatus) | 25, 50, 75, 100, and 125 g/kg | The study found that adding up to 100 g kg−1 of dried yeast without affecting growth performance is possible. | [61] |
| Baker yeast, Saccharomyces cerevisiae (SC) | Nile tilapia (Oreochromis niloticus) fingerlings | 1 and 2 g/100 g | Nile tilapia fingerlings fed a yeast mixture for 119 days showed improved growth performance, feed efficiency, and hematological indices. | [62] |
| Ethanol yeast (EY) | Sunshine bass (female white bass Morone chrysops × male striped bass M. saxatilis) | 0, 7.5, 15% | Reductions in carcass protein and increase in carcass lipid was the results of replacing fishmeal entirely with EY. It also caused changes in whole-body composition. | [63] |
| Brewer’s yeast | Sea bass (Dicentrachus labrax) | 0, 10, 20, 30, or 50% | Brewer’s yeast can replace 50% of fishmeal protein without negative effects on fish performance and improve feed efficiency by up to 30% in the diet. | [64] |
| Brewer’s yeast (Saccharomyces cerevisiae) | Beluga sturgeon (Huso huso) juveniles | 1 and 2% | Brewer’s yeast can enhance growth performance and modify intestinal microbiota in beluga sturgeon without negatively impacting basic hematological parameters. | [65] |
| Yeast-based, certified organic protein source | Cobia (Rachycentron canadum) | 25, 50, 75, and 100% | The data indicates that at least 25% of dietary protein can be provided by yeast-based protein in diets for cobia, despite negative effects on production characteristics. | [66] |
| Brewer’s yeast (Saccharomyces cerevisiae) | Hybrid striped bass (Morone chrysops × M. saxatilis) | 1, 2, and 4% | The growth performance, feed efficiency, and infection resistance of hybrid striped bass were found to be greatly improved by brewer’s yeast. | [67] |
| Product Name | Fish Species | Inclusion Levels | Effects/Outcome | Source |
|---|---|---|---|---|
| Torula Yeast (C. utilis) | Rainbow trout (Oncorhynchus mykiss) | 10 and 20% | The findings of this study suggested that torula yeast (C. utilis) may promote more robust rainbow trout growth when given a diet free of fishmeal. | [6] |
| Selenium yeast | Golden pompano (Trachinotus ovatus) | 1 g/kg | The study suggests that golden pompano can be reduced in dietary fishmeal to 1 g/kg Selenium-yeast. | [73] |
| Saccharomyces cerevisiae- fermented poultry byproduct meal (pbm) | Common carp (Cyprinus carpio) | 0, 5, 10, 15, 20% | The inclusion of 15–20% yeast and fermented poultry byproduct meal in the common carp’s diet has been proven to enhance digestive enzyme activity, immunological function, and growth. | [74] |
| Dietary inclusion of fermented poultry byproduct meal (FPBM) | Nile tilapia (Oreochromis niloticus) | 10, 20, 30, and 40% | To improve tilapia health and growth, 11.17–25.14% of FPBM can be added to their diets in an efficient manner. | [22] |
| Yeast cell wall | Juvenile Persian sturgeon (Acipenser persicus) | 0.5 and 1% | The growth metrics and feeding performance are not significantly affected by the administration of 0.5 and 1% Immunowall. | [76] |
| Selenium yeast (Se) | Meagre (Argyrosomus regius) fingerlings | 0.77, 1.51, 2.97, and 3.98 mg /kg | To improve the development performance, feed utilization, liver and kidney histology, and economic benefits of meagre juveniles, a meal intake of 3.98 mg Se kg−1 (3 mg Se-yeast) is advised. | [77] |
| Yeast-fermented sunflower meal (YFSFM) | Nile tilapia (Oreochromis niloticus) | 0, 25, 50, and 75% | The varying levels of YFSFM had no significant effect on the fish’s dry matter, lipid, crude protein, or ash content. | [78] |
| Selenium yeast | Siberian Sturgeon Acipenser baerii | 5, 10, 15 g/kg | A food supplement of Sel-Plex at levels ≤15 g kg−1 increased the growth performance of Siberian sturgeons even though the hematological indicators improved. | [79] |
| Substituting soybean meal with yeast (Sacharomyces cerevisae) meal | African catfish (Clarias gariepinus) | 0, 10, 20, 30, 40, 50, and 100 | The diet with a 50% yeast inclusion was deemed optimal due to its enhanced nutritional status, improved blood parameters, and enhanced fish health. | [80] |
| Grain distillers dried yeast (GDDY) | Juvenile rainbow trout (Oncorhynchus mykiss) | 0, 25, 37.5, 50, 62.5, 75, 87.5, and 100% | Rainbow trout development and feed conversion were highly affected by high GDDY incorporation rates, but feed intake was not affected. | [81] |
| Yeast-fermented canola meal | Nile tilapia (Oreochromis niloticus) | 0, 25, 50, 75, and 100% | The study found that replacing fish with 75 and 100% levels significantly reduced their protein efficiency ratio and nutrient digestibility compared to lower levels. | [82] |
| Yeast, Metschnikowia sp. in combination with Rhodotorula | Juvenile of sea cucumber (Apostichopus japonicus) | 104, 105, and 106 CFU g−1 | Boost the immune system, boost growth, increase the synthesis of digestive enzymes, and provide nourishment. | [83] |
| Yeast-fermented canola meal | Asian sea bass (Lates calcarifer) | 25, 50, 75, 100% | The study revealed that yeast-fermented canola meal can replace 50% of fishmeal in the Asian sea bass diet without affecting growth. | [84] |
| Fry Fed Organic Diets Containing Yeast Extract (YE) | Nile tilapia (Oreochromis niloticus) | 0, 15, 30, and 45% | Fry fed a control diet with 20% FM and a diet with 45% YE/36%SBM with amino acid supplementation showed no significant differences in final weight, weight gain, and specific growth rate. | [85] |
| Ethanol yeast | Sunshine bass (Morone chrysops × M. saxatilis) | 0, 7.5, 15, and 22% | According to the study, sunshine bass should be fed ethanol yeast-based diets with an FM level of between 7.5% and 15%. | [63] |
| Dietary supplementation with brewer’s yeast | Red drum (Sciaenops ocellatus) | 10 g/kg | The study suggests that a ten-dose dose of several prebiotics is sufficient to enhance the feed efficiency and disease resistance of red drums. | [86] |
| Yeast protein Saccharomyces cerevisiae supplemented with biogenic L-carintine | Nile tilapia (Oreochromis niloticus) fingerlings | 25, 50, 75, and 100% | Tilapia fed a diet containing 7.14 and 10.71% yeast, supplemented with 100 mg/100 g diet, showed optimal growth performance, feed and protein utilization. | [87] |
| Yeast extract powder (YEP) | Rohu (Labeo rohita) fingerlings | 0.1, 0.2, 0.3, 0.4, and 0.5% | Up to a 0.2% margin, the fish fed diets enriched with YEP grew more rapidly than the control group. | [88] |
| Brewer’s grains with yeast (BGY) | Australian red claw crayfish (Cherax quadricarinatus) | 10, 20, and 30% | The study suggests that soybean meal and BGY can be completely replaced with fish and shrimp meal in the diets of juvenile red claw crayfish. | [89] |
| Fish Species | Inclusion Levels | Effects | Source |
|---|---|---|---|
| Pacific white shrimp (Litopenaeus vannamei) | 10 g/kg | According to the study, adding yeast byproduct additions to sugar byproducts may enhance development, immunity, histological changes, and resistance to the AHPND-causing V. parahaemolyticus. | [94] |
| Siberian Sturgeon (Acipenser baerii) | 50% Lactic acid + 50% Saccharomyces boulardii | Growth indices, survival, and well-being of Acipenser baerii fish were dramatically improved when probiotics, such as lactic acid bacteria and yeasts, were introduced to their meals. | [92] |
| Persian sturgeon (Acipenser persicus) | 5 g | This study contributes to the growing literature on the effects of probiotics on the growth, body composition, digestive enzymes, and intestinal morphology of A. persicus fingerlings. | [99] |
| Gilthead sea bream (Sparus aurata) | 0.55 or 1.1% | The study found that yeast, when consumed at a 0.55 or 1% basal diet, exhibited immunostimulant activity in gilthead seabream. | [106] |
| Rainbow trout, (Oncorhynchus mykiss fry) | 1, 5, and 10% | Adding yeast to rainbow trout fry diets during early life stages is suitable, with a 5% concentration likely enhancing growth performance and feed efficiency ratio. | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, S.; Biró, J.; Kucska, B.; Hancz, C. Factors Affecting Yeast Digestibility and Immunostimulation in Aquatic Animals. Animals 2024, 14, 2851. https://doi.org/10.3390/ani14192851
Sultana S, Biró J, Kucska B, Hancz C. Factors Affecting Yeast Digestibility and Immunostimulation in Aquatic Animals. Animals. 2024; 14(19):2851. https://doi.org/10.3390/ani14192851
Chicago/Turabian StyleSultana, Sadia, Janka Biró, Balázs Kucska, and Csaba Hancz. 2024. "Factors Affecting Yeast Digestibility and Immunostimulation in Aquatic Animals" Animals 14, no. 19: 2851. https://doi.org/10.3390/ani14192851
APA StyleSultana, S., Biró, J., Kucska, B., & Hancz, C. (2024). Factors Affecting Yeast Digestibility and Immunostimulation in Aquatic Animals. Animals, 14(19), 2851. https://doi.org/10.3390/ani14192851







