The Immune Defense Response and Immune-Related Genes Expression in Macrobrachium nipponense Infected with Decapod Iridescent Virus 1 (DIV1)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Prawns Culture and DIV1 Infection Experiment
2.2. cDNA Library Construction, and Transcriptome Sequencing
2.3. Transcriptome Assembly and Functional Annotation
2.4. Screening and Enrichment Analysis of Differentially Expressed Genes (DEGs)
2.5. RNA-Seq Data Validation
2.6. Detection of Immune-Related Gene Expression after Different Hours Post-Infection
2.7. Statistical Analysis
3. Results
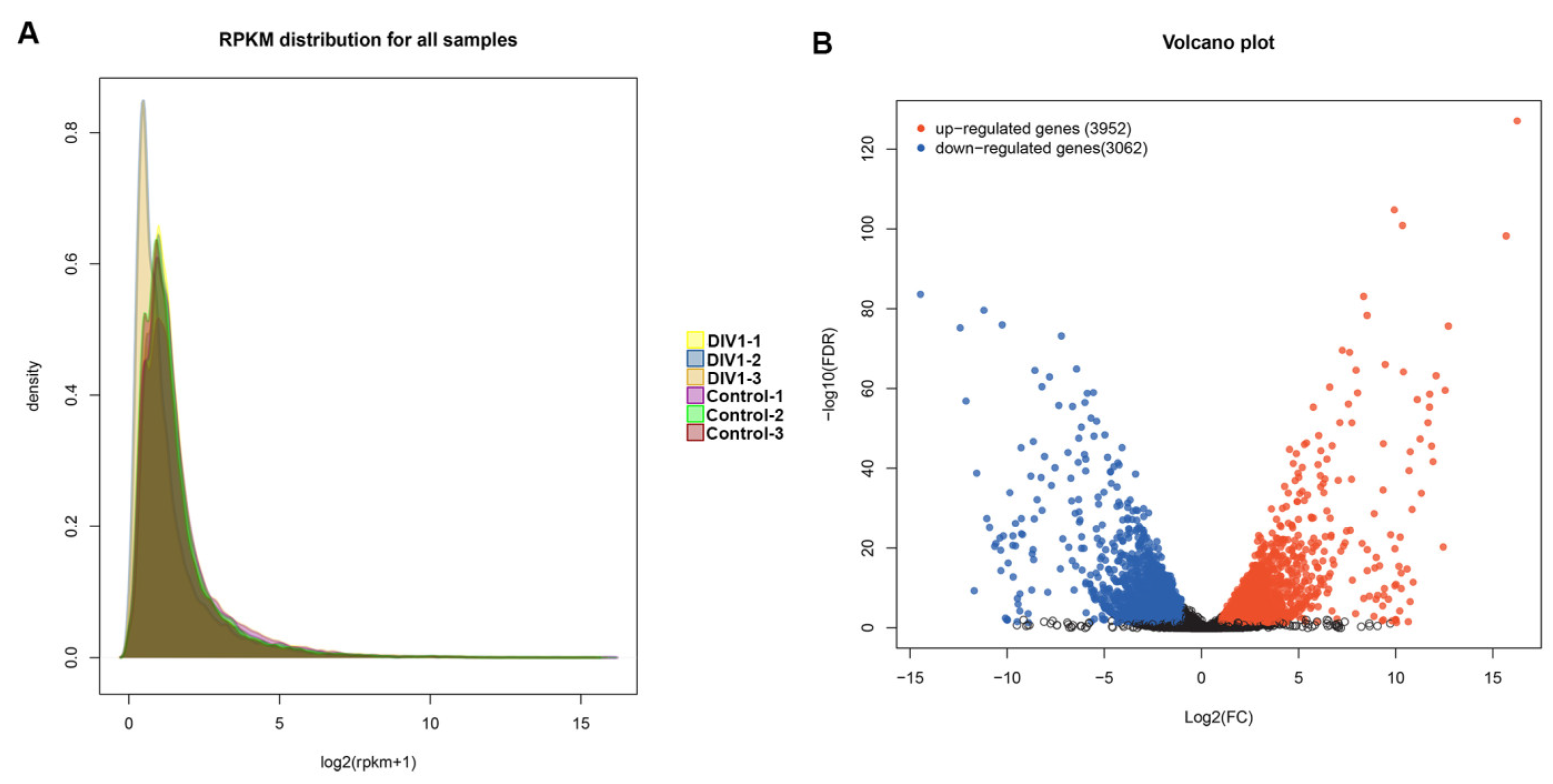
3.1. Transcriptome Sequencing and De Novo Assembly
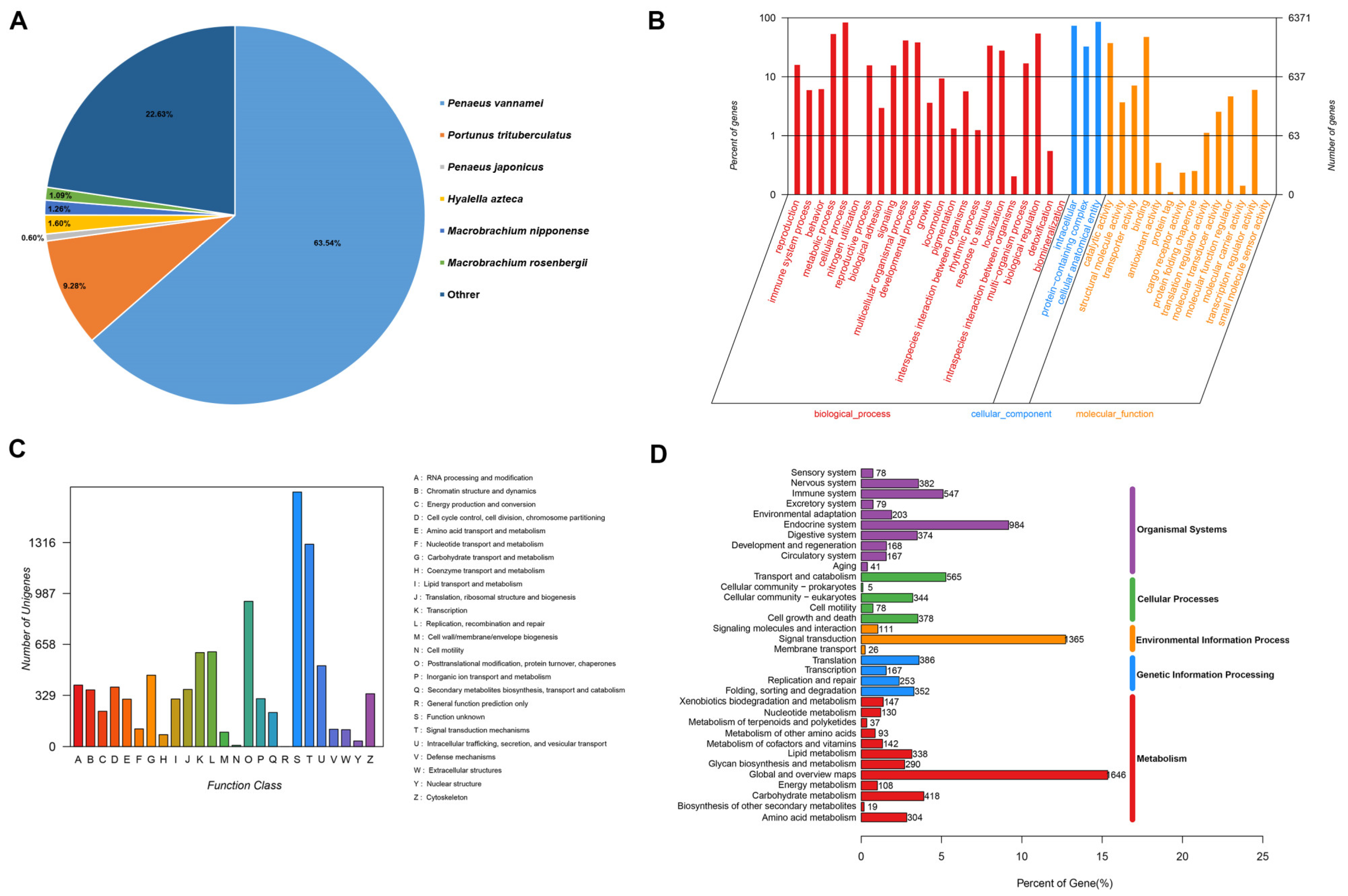
3.2. Functional Annotation and Classification of M. nipponense Transcriptome Sequences
3.3. Identification of DEGs Related to DIV1 Infection
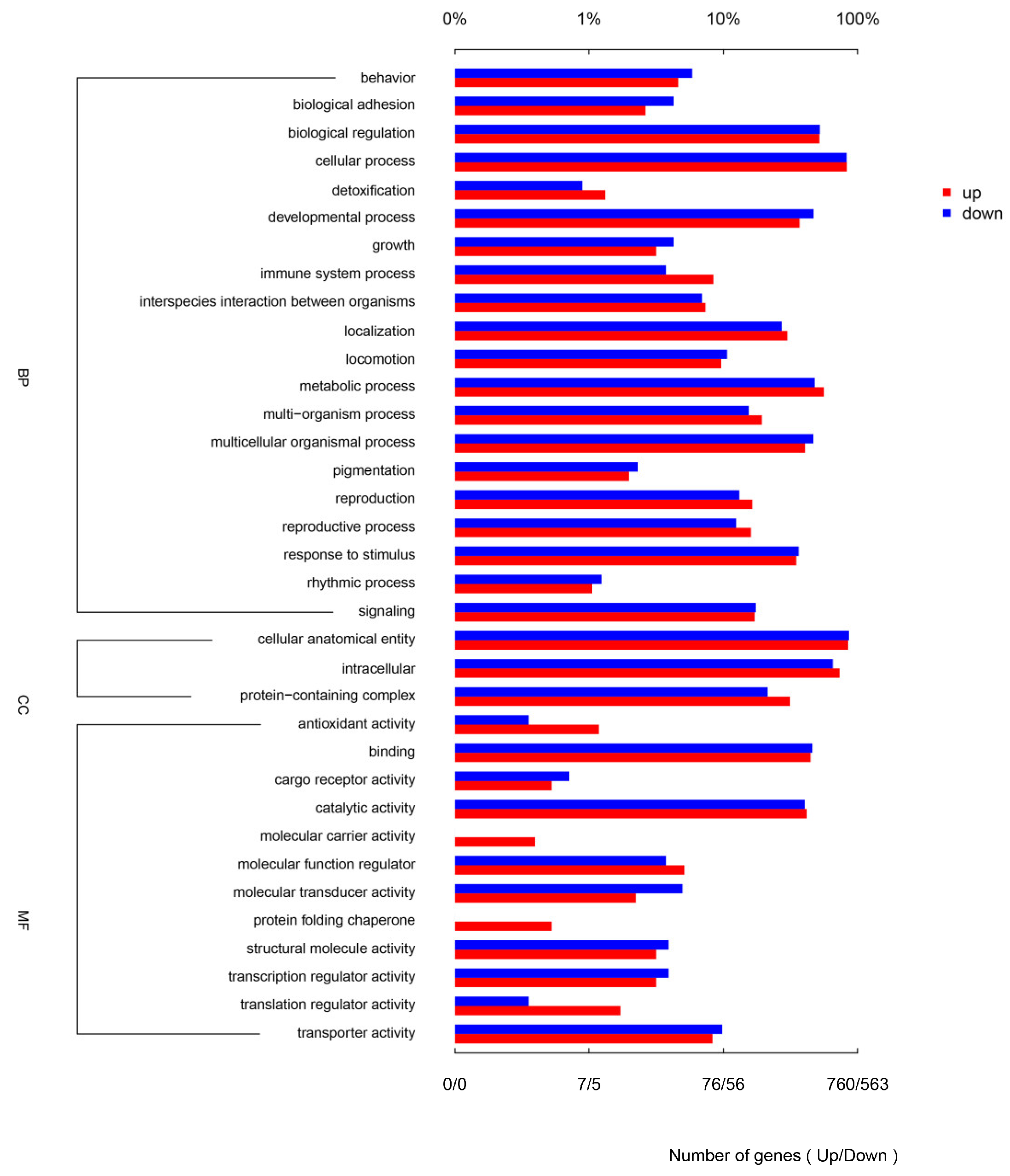
3.4. GO and KEGG Enrichment Analysis of DEGs in Response to DIV1 Infection
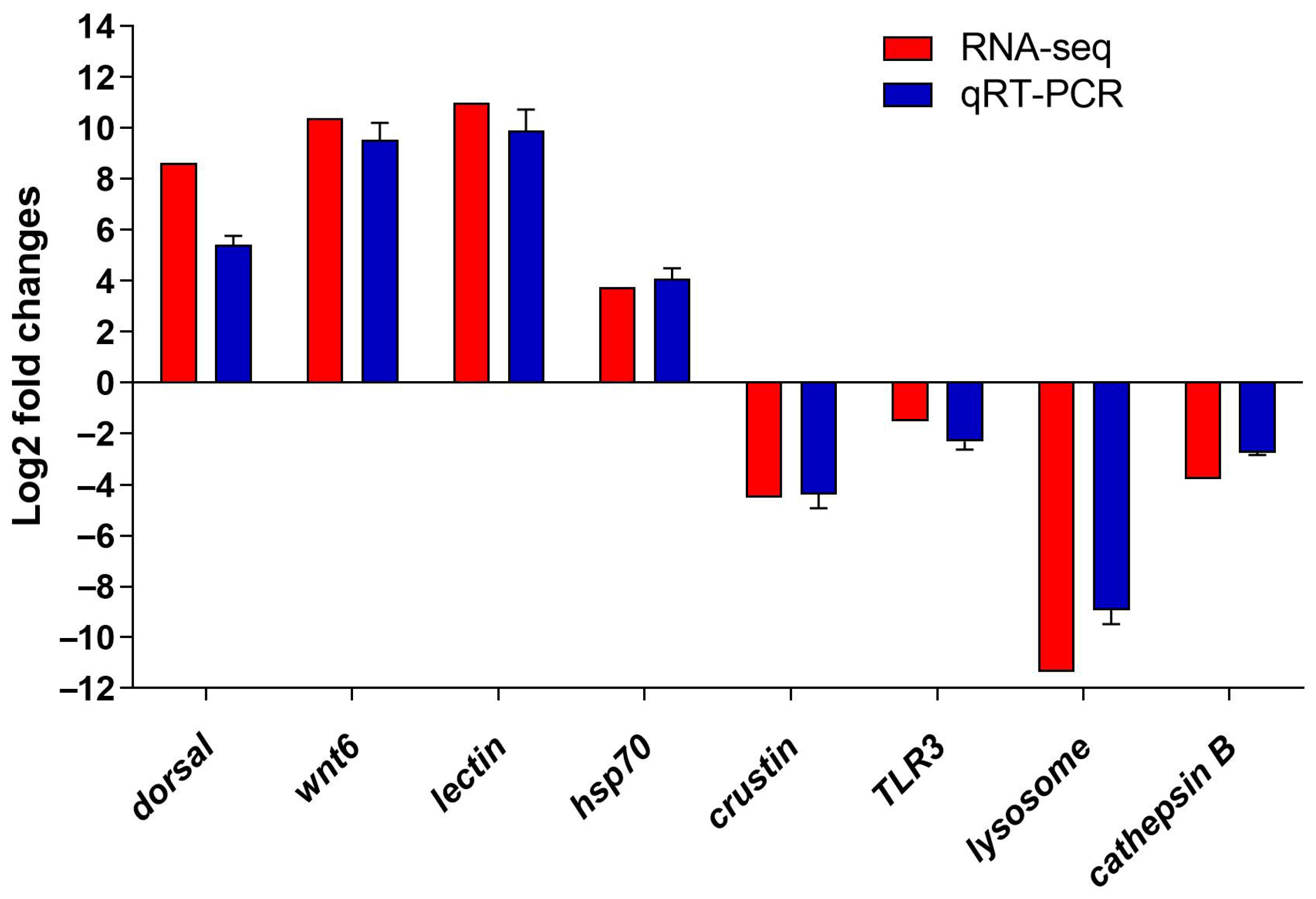
3.5. Verification of the DEGs by qRT-PCR
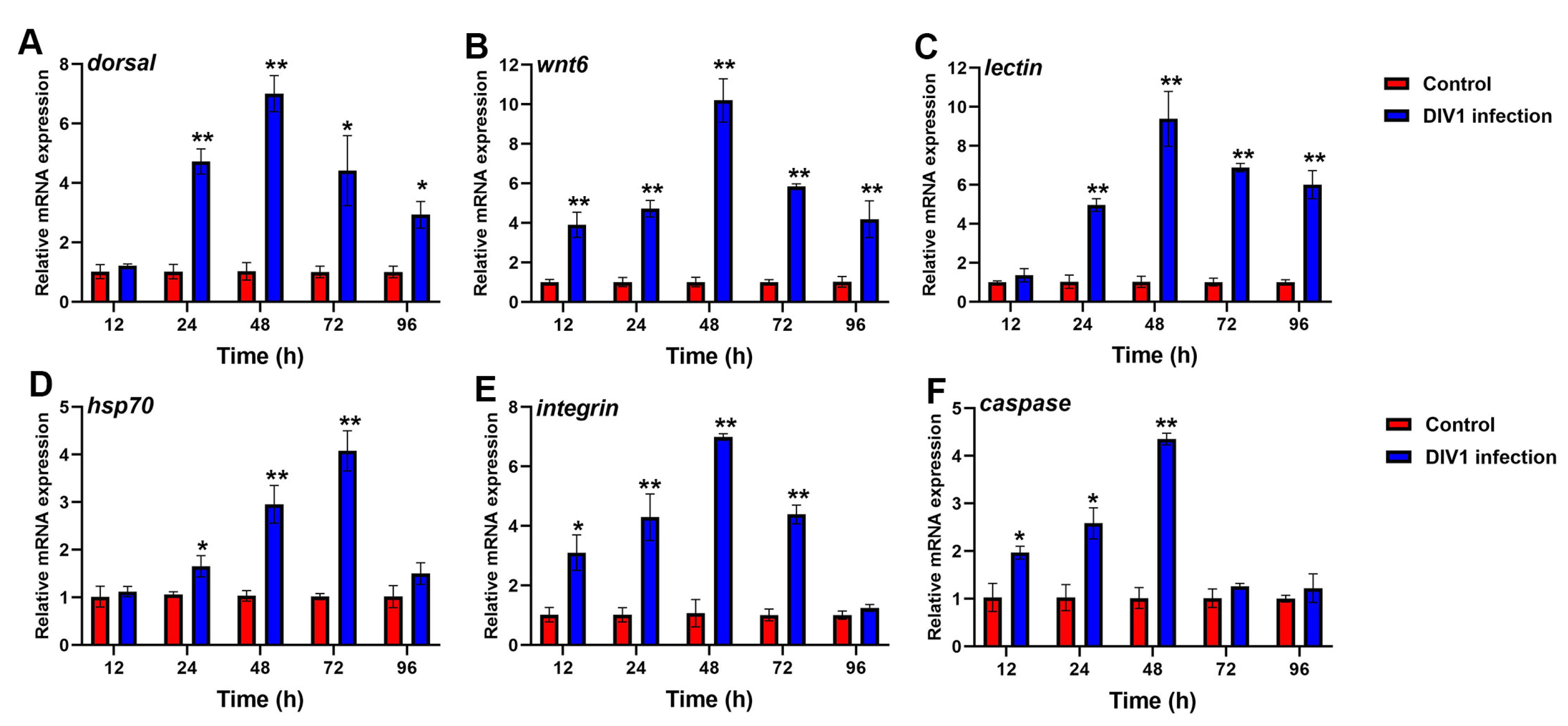
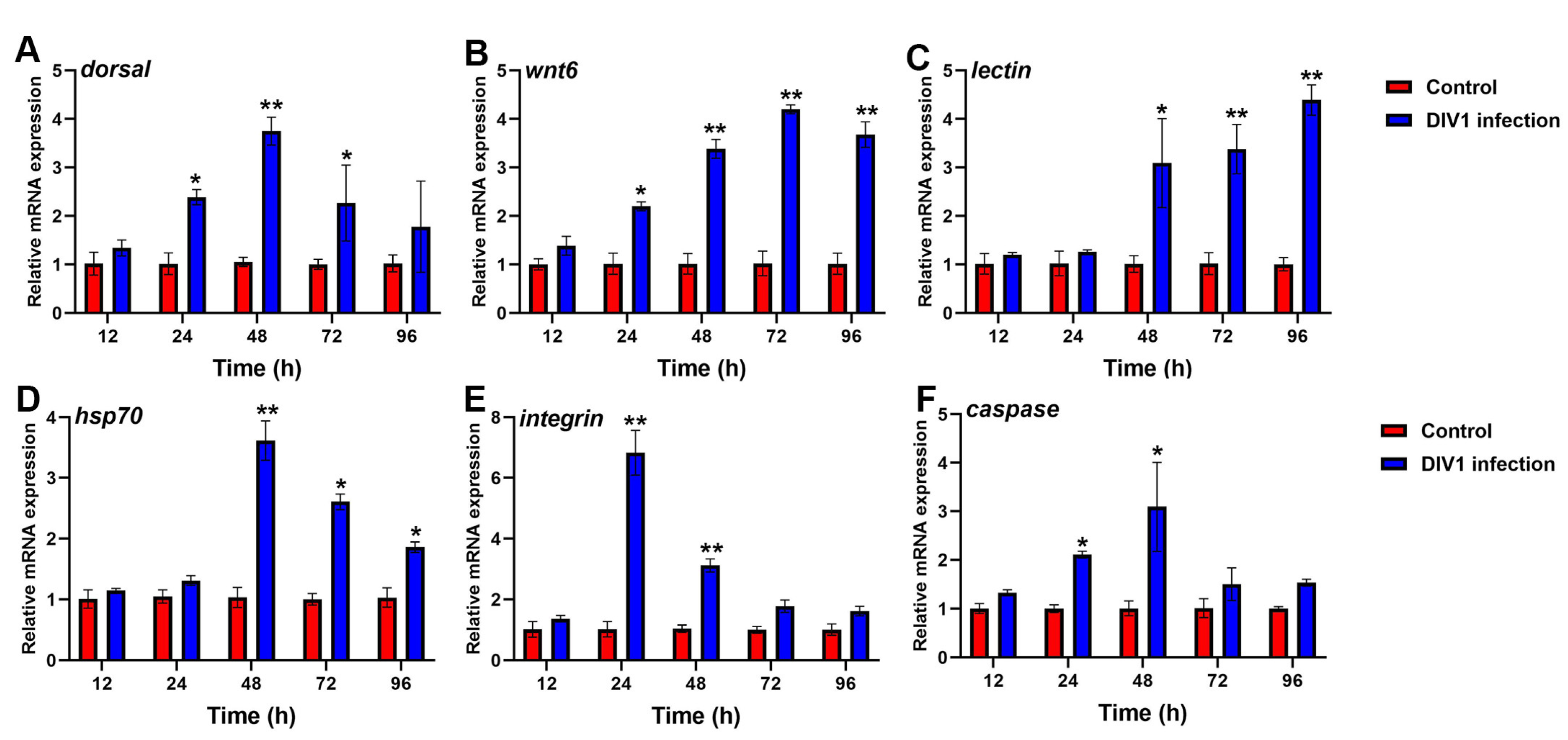
3.6. Expression Profiles of Immune-Related Genes Expression after Different Hours Post-Infection
3.6.1. Immune-Related Gene Expression in Hepatopancreas after Different Hours Post-Infection
3.6.2. Immune-Related Gene Expression in Hemocytes after Different Hours Post-Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, S.B.; Zhang, W.Y.; Xiong, Y.W.; Fu, H.T. Recent progress of male sexual differentiation and development in the oriental river prawn (Macrobrachium nipponense): A review. Rev. Aquac. 2023, 15, 305–317. [Google Scholar] [CrossRef]
- Fisheries Bureau, Department of Agriculture of China. 2023 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024; p. 24. [Google Scholar]
- Wang, W.F.; Yang, H.; Liu, F.; Chen, X.L.; Lv, Y.J.; Ning, Q.J. A novel effect of imidazole derivative KK-42 on increasing survival of Aeromonas hydrophila challenged prawn Macrobrachium nipponense. Fish Shellfish Immunol. 2013, 34, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Lv, X.T.; Chen, D.D.; Sun, B.; Guo, L.F.; Wang, S.Q.; Ru, Y.Y.; Wang, H.; Zeng, Q.F. Transcriptome analysis of the Macrobrachium nipponense hepatopancreas provides insights into immunoregulation under Aeromonas veronii infection. Ecotoxicol. Environ. Saf. 2021, 208, 111503. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Mei, J.H.; Chen, A.T.; Chen, Z.; Wang, J.; Gao, X.J.; Jiang, Q.; Zhang, X.J. Pathogenicity of Aeromonas salmonicida and protection effect of Bacillus velezensis on Macrobrachium nipponense against A. salmonicida. Aquac. Rep. 2023, 31, 101677. [Google Scholar] [CrossRef]
- Li, X.X.; Yang, H.; Gao, X.J.; Zhang, H.H.; Chen, N.; Miao, Z.; Liu, X.D.; Zhang, X.J.F. The pathogenicity characterization of non-O1 Vibrio cholerae and its activation on immune system in freshwater shrimp Macrobrachium nipponense. Fish Shellfish Immunol. 2019, 87, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Gao, X.J.; Jiang, Q.; Zhu, X.H.; Zhou, Y.F.; Zhang, Z.R.; Zhou, L.Y.; Tang, H.Y.; Zhang, X.J. Genomic characterization and pathogenicity analysis of the Vibrio mimicus Y4 causing red body disease in Macrobrachium nipponense. Aquaculture 2022, 548, 737701. [Google Scholar] [CrossRef]
- Guma, S.; Jiang, Z.Y.; Zhang, Y.J.; Wu, C.C.; Chen, Z.; Xu, J.W.; Jiang, Q.; Zhang, X.; Wang, C.B.; Gao, X.J. The pathogenic characterization of Citrobacter freundii and its activation on immune related genes in Macrobrachium nipponense. Microb. Pathog. 2022, 169, 105682. [Google Scholar] [CrossRef]
- Qin, L.J.; Qian, Q.Q.; Chen, A.T.; Zhang, Y.J.; Tang, X.Z.; Yin, T.C.; Jiang, Q.; Zhang, X.; Gao, X.J. Isolation and the pathogenicity characterization of Decapod iridescent virus 1 (DIV1) from diseased Macrobrachium nipponense and its activation on host immune response. Fish Shellfish Immunol. 2024, 146, 109403. [Google Scholar] [CrossRef]
- Liao, X.Z.; He, J.G.; Li, C.Z. Decapod iridescent virus 1: An emerging viral pathogen in aquaculture. Rev. Aquac. 2022, 14, 1779–1789. [Google Scholar] [CrossRef]
- Xu, L.M.; Wang, T.T.; Li, F.; Yang, F. Isolation and preliminary characterization of a new pathogenic iridovirus from redclaw crayfish Cherax quadricarinatus. Dis. Aquat. Organ. 2016, 120, 17–26. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Wang, R.Y.; Cheng, D.Y.; Dong, X.; Yang, B.; Wang, X.H.; et al. Characterization of a new member of Iridoviridae, Shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei). Sci. Rep. 2017, 7, 11834. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.Q.; Zhou, Y.F.; Chen, Z.; Zhu, Y.J.; Xu, J.W.; Gao, X.J.; Jiang, Q.; Wang, J.; Zhang, X.J. Pathogenesis and complete genome sequence of Decapod iridescent virus 1 (DIV1) associated with mass mortality in Macrobrachium rosenbergii. Aquaculture 2023, 566, 739220. [Google Scholar] [CrossRef]
- He, Z.H.; Zhao, J.C.; Chen, X.Y.; Liao, M.Z.; Xue, Y.; Zhou, J.N.; Chen, H.Z.; Chen, G.L.; Zhang, S.; Sun, C.B. The molecular mechanism of hemocyte immune response in Marsupenaeus japonicus infected with Decapod iridescent virus 1. Front. Microbiol. 2021, 12, 710845. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.L.; Zhu, L.; Wang, X.R.; Li, H.; Hou, L.B.; Kong, X.H. Research progress of pattern recognition receptors in red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2023, 141, 109028. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.Z.; Liu, S.H.; Chen, S.H.; Shan, X.X.; He, J.G.; Li, C.Z. Transcriptomic analysis reveals the role of Glycolysis pathway in Litopenaeus vannamei during DIV1 infection. Fish Shellfish Immunol. 2023, 141, 109036. [Google Scholar] [CrossRef]
- He, Z.H.; Chen, X.Y.; Zhao, J.C.; Hou, D.Q.; Fu, Z.B.; Zhong, Y.Q.; Hu, X.Y.; Zhang, S.; Sun, C.B. Establishment of infection mode and Penaeus monodon hemocytes transcriptomics analysis under decapod iridescent virus 1 (DIV1) challenge. Aquaculture 2021, 542, 736816. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Wang, H.L.; Zou, P.Z.; Dong, X.; Li, F.H.; Huang, J. Susceptibility of Exopalaemon carinicauda to the infection with shrimp hemocyte iridescent virus (SHIV 20141215), a strain of decapod iridescent virus 1 (DIV1). Viruses 2019, 11, 387. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, X.; Gao, W.; Guo, X.M.; Xie, G.S.; Gong, M.; Yang, B.; Li, C.; Zhang, Q.L.; Huang, J. Confirmation of susceptibility of swimming crab to infection with decapod iridescent virus 1. Aquaculture 2022, 548, 737607. [Google Scholar] [CrossRef]
- Cho, H.; Park, K.H.; Jang, Y.; Cho, Y.; Heo, Y.K.; Kim, M.; Kim, Y.B. Identification and characterization of a Toll-like receptor gene from Macrobrachium nipponense. Fish Shellfish Immunol. 2021, 108, 109–115. [Google Scholar] [CrossRef]
- Cao, X.T.; Pan, X.Y.; Sun, M.; Liu, Y.; Lan, J.F. Hepatopancreas-specific lectin participates in the antibacterial immune response by regulating the expression of antibacterial proteins. Front. Immunol. 2021, 12, 679767. [Google Scholar] [CrossRef]
- Thaimuangphol, W.; Sanoamuang, L.; Wangkahart, E. The immune response of fairy shrimp Streptocephalus sirindhornae against bacterial black disease by de novo transcriptome analysis. Fish Shellfish Immunol. 2022, 121, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Mao, Y.; Wang, J.; Liu, M.; Zhang, M.; Su, Y. Transcriptome analysis of Kuruma shrimp (Marsupenaeus japonicus) hepatopancreas in response to white spot syndrome virus (WSSV) under experimental infection. Fish Shellfish Immunol. 2017, 70, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Zhang, Q.L.; Li, C.; Dong, X.; Yang, B.; Huang, J. Detection and quantification of shrimp hemocyte iridescent virus by TaqMan probe based real-time PCR. J. Invertebr. Pathol. 2018, 154, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Soudy, M.; Anwar, A.M.; Ahmed, E.A.; Osama, A.; Ezzeldin, S.; Mahgoub, S.; Magdeldin, S. UniprotR: Retrieving and visualizing protein sequence and functional information from Universal Protein Resource (UniProt knowledgebase). J. Proteom. 2020, 213, 103613. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiang, S.F.; Xiong, Y.W.; Zhang, L.J.; Zhang, W.Y.; Zheng, Y.L.; Wang, J.S.; Jin, S.B.; Gong, Y.S.; Wu, Y.; Qiao, H.; et al. Genetic diversity and population structure of wild Macrobrachium nipponense populations across China: Implication for population management. Mol. Biol. Rep. 2023, 50, 5069–5080. [Google Scholar] [CrossRef]
- Wang, F.X.; Li, S.H.; Li, F.H. Different immune responses of the lymphoid organ in shrimp at early challenge stage of Vibrio parahaemolyticus and WSSV. Animals 2021, 11, 2160. [Google Scholar] [CrossRef]
- Liao, X.Z.; Wang, C.G.; Wang, B.; Qin, H.P.; Hu, S.K.; Zhao, J.C.; He, Z.H.; Zhong, Y.Q.; Sun, C.B.; Zhang, S. Research into the hemocyte immune response of Fenneropenaeus merguiensis under decapod iridescent virus 1 (DIV1) challenge using transcriptome analysis. Fish Shellfish Immunol. 2020, 104, 8–17. [Google Scholar] [CrossRef]
- Licona-Jain, A.; Racotta, I.; Angulo, C.; Luna-González, A.; Escamilla-Montes, R.; Cortés-Jacinto, E.; Morelos-Castro, R.M.; Campa-Córdova, Á.I. Combined administration routes of marine yeasts enhanced immune-related genes and protection of white shrimp (Penaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2022, 124, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salgado, J.L.; Pereyra, M.A.; Alpuche-Osorno, J.J.; Zenteno, E. Pattern recognition receptors in the crustacean immune response against bacterial infections. Aquaculture 2021, 532, 735998. [Google Scholar] [CrossRef]
- Du, J.; Yue, K.D.; Peng, Y.X.; Ning, Q.J. Crucial roles of a novel exoskeletal-derived lectin in innate immunity of the oriental river prawn, Macrobrachium nipponense. J. Fish Dis. 2022, 45, 717–728. [Google Scholar] [CrossRef]
- Zheng, J.B.; Mao, Y.; Su, Y.Q.; Wang, J. Identification of two novel C-type lectins involved in immune defense against white spot syndrome virus and Vibrio parahaemolyticus from Marsupenaeus japonicus. Aquaculture 2020, 519, 734797. [Google Scholar] [CrossRef]
- Weng, K.J.; Zuo, H.L.; Zhu, Z.M.; Guo, Z.X.; Weng, S.P.; He, J.G.; Xu, X.P. Ftz-F1H promotes white spot syndrome virus infection in shrimp by suppressing the Dorsal pathway. Aquaculture 2022, 548, 737708. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Ren, Q. Dorsal transcription factor is involved in regulating expression of crustin genes during white spot syndrome virus infection. Dev. Comp. Immunol. 2016, 63, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fang, S.B.; Song, S.G.; Zheng, Y.D.; Tan, B.P.; Shi, L.L. Effect of changes in the activity of Wnt/β-catenin signalling pathway on the growth performance, immunity and transcriptome response in Litopenaeus vannamei. Aquac. Rep. 2021, 20, 100774. [Google Scholar] [CrossRef]
- Wang, K.Q.; Dai, X.L.; Zhang, C.; Cao, X.Y.; Zhang, R.D.; Zhang, Z.X.; Huang, X.; Ren, Q. Two Wnt genes regulate the expression levels of antimicrobial peptides during Vibrio infection in Macrobrachium nipponense. Fish Shellfish Immunol. 2020, 101, 225–233. [Google Scholar] [CrossRef]
- Janewanthanakul, S.; Supungul, P.; Tang, S.; Tassanakajon, A. Heat shock protein 70 from Litopenaeus vannamei (LvHSP70) is involved in the innate immune response against white spot syndrome virus (WSSV) infection. Dev. Comp. Immunol. 2020, 102, 103476. [Google Scholar] [CrossRef]
- Valentim-Neto, P.A.; Moser, J.R.; Fraga, A.P.M.; Marques, M.R.F. Hsp70 expression in shrimp Litopenaeus vannamei in response to IHHNV and WSSV infection. Virus Disease 2014, 25, 437–440. [Google Scholar] [CrossRef]
- Tran, N.T.; Liang, H.; Zhang, M.; Bakky, M.A.; Zhang, Y.; Li, S. Role of cellular receptors in the innate immune system of crustaceans in response to white spot syndrome virus. Viruses 2022, 14, 743. [Google Scholar] [CrossRef]
- Tang, X.; Zhai, F.; Sheng, X.; Xing, J.; Zhan, W. The Roles of β-Integrin of Chinese Shrimp (Fenneropenaeus chinensis) in WSSV Infection. Int. J. Mol. Sci. 2017, 14, 1465. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yang, N.; He, C.; Gao, Y.; Chang, L.R.; Li, T.; Si, L.J.; Yan, D.C. The immune response of Caspase-3 gene in infectious hypodermal and hematopoietic necrosis virus (IHHNV) infection of Litopenaeus vannamei. Aquaculture 2024, 581, 740472. [Google Scholar] [CrossRef]

| Target Gene | PCR Primers Sequence (5′-3′) | Product Size (bp) | Transcript ID |
|---|---|---|---|
| 18S rRNA | CAGGCTTATGTTGTCTTGAA | 159 | TRINITY_DN6546_c0_g1 |
| CTATGTTGGATGTTGCTGTT | |||
| dorsal | TATCTTCTTCTTCGGCAGTT | 240 | TRINITY_DN6071_c0_g1 |
| TACGGCTCCTCCATATTCT | |||
| wnt6 | TCCTAGAAGAGAAGTTCCTTAG | 197 | TRINITY_DN40081_c0_g1 |
| ACGCAGTCCTATGTTCCT | |||
| lectin | AACGGTTGCTTCAGAGAA | 203 | TRINITY_DN7682_c0_g2 |
| CTTCGCCAGGAGTTATCTT | |||
| caspase | AGACTCTGCGAACAACTC | 199 | TRINITY_DN1075_c0_g2 |
| TCTCCTCTTGTGCTCCAT | |||
| integrin | TGGAGGCAATATCATCTGTT | 245 | TRINITY_DN7473_c0_g1 |
| CGTTACTCTTGGTGTTATAGG | |||
| hsp70 | TGCTTCACACGACTCTTC | 213 | TRINITY_DN3715_c0_g1 |
| CACCATCACCAACGACAA | |||
| crustin | AGATGCGATGTTGCGTAT | 107 | TRINITY_DN12166_c0_g1 |
| AACCTCTGTGCTATGCTATA | |||
| TLR3 | TTGGTTATCGGACACATTCA | 145 | TRINITY_DN39664_c0_g1 |
| TGGCATCACTGGCATAGA | |||
| lysosome | AATGCGATAGTTGCGATTG | 194 | TRINITY_DN5716_c0_g2 |
| GATGCTTCCAGTTCTTGTAG | |||
| cathepsin B | ATACTGGACCGTGGCTATA | 102 | TRINITY_DN608_c0_g1 |
| CCTGGAGAATACTGGACATT |
| Groups | Raw Reads | Clean Reads | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|
| DIV1-1 | 41,164,510 | 40,917,484 | 98.93 | 96.05 | 44.15% |
| DIV1-2 | 39,738,020 | 38,774,382 | 98.11 | 95.96 | 45.32% |
| DIV1-3 | 40,491,956 | 40,883,392 | 98.96 | 96.16 | 45.23% |
| Control-1 | 41,630,056 | 41,367,100 | 98.44 | 96.56 | 44.71% |
| Control-2 | 39,738,020 | 39,593,902 | 98.73 | 95.21 | 44.68% |
| Control-3 | 40,491,956 | 40,285,660 | 98.80 | 96.61 | 45.55% |
| Category or Gene ID | Gene Description | Log2 FC |
|---|---|---|
| C-type lectin receptor signaling pathway | ||
| TRINITY_DN2095_c0_g1 | Ras | 2.14 |
| TRINITY_DN7682_c0_g2 | lectin | 10.98 |
| TRINITY_DN758_c0_g1 | C-type lectin-like | 1.01 |
| TRINITY_DN763_c0_g2 | C-type lectin | 2.72 |
| TRINITY_DN39944_c0_g1 | rho-related GTP-binding protein RhoA-C-like | 10.72 |
| TRINITY_DN3722_c0_g2 | Protein kinase domain | 1.60 |
| TRINITY_DN8278_c0_g1 | calmodulin-beta-like | −1.16 |
| TRINITY_DN350_c0_g1 | calmodulin | −12.15 |
| Antigen processing and presentation | ||
| TRINITY_DN3715_c0_g1 | heat shock protein 70 | 2.28 |
| TRINITY_DN3715_c2_g1 | 70 kD heat shock protein form 1 | 2.62 |
| TRINITY_DN13116_c0_g1 | heat shock protein | 2.29 |
| TRINITY_DN83_c0_g1 | calnexin | 5.76 |
| TRINITY_DN17419_c0_g1 | gamma-interferon-inducible lysosomal thiol reductase | 1.78 |
| TRINITY_DN37377_c0_g1 | gamma-interferon-inducible lysosomal thiol reductase-like | 3.18 |
| TRINITY_DN19149_c0_g1 | putative gamma-interferon-inducible lysosomal thiol reductase-like isoform X1 | 11.45 |
| TRINITY_DN2412_c0_g2 | gamma-interferon-inducible lysosomal thiol reductase-like | 4.92 |
| TRINITY_DN13933_c0_g1 | cathepsin L | 1.08 |
| TRINITY_DN608_c0_g1 | cathepsin B | −3.80 |
| TRINITY_DN1606_c0_g1 | cathepsin B | −3.37 |
| Complement and coagulation cascades | ||
| TRINITY_DN14365_c0_g1 | trypsin | 4.28 |
| TRINITY_DN8140_c0_g1 | trypsin-1-like isoform X1 | 3.26 |
| TRINITY_DN3116_c0_g1 | EGF-like domain | −1.83 |
| TRINITY_DN39290_c0_g1 | coagulation factor X | −11.19 |
| Phagosome | ||
| TRINITY_DN10442_c0_g1 | V-type proton ATPase e subuni | 1.08 |
| TRINITY_DN163_c0_g1 | V-type proton ATPase 21 kDa proteolipid subunit | 1.03 |
| TRINITY_DN83_c0_g1 | calnexin | 5.76 |
| TRINITY_DN472_c0_g3 | cytoplasmic-like | 1.044 |
| TRINITY_DN2887_c0_g1 | integrin beta-PS-like | 4.82 |
| TRINITY_DN7473_c0_g1 | integrin | 11.76 |
| Lysosome | ||
| TRINITY_DN536_c0_g1 | natural resistance-associated macrophage protein 2-like | 10.28 |
| TRINITY_DN563_c0_g1 | formylglycine-generating enzyme-like | 2.28 |
| TRINITY_DN163_c0_g1 | V-type proton ATPase 21 kDa proteolipid subunit | 1.03 |
| TRINITY_DN3997_c1_g1 | glucosylceramidase-like | −1.70 |
| TRINITY_DN547_c0_g1 | alpha-N-acetylgalactosaminidase | −2.28 |
| Apoptosis | ||
| TRINITY_DN1075_c0_g2 | caspase | 4.72 |
| TRINITY_DN9284_c0_g1 | caspase | 2.83 |
| TRINITY_DN10429_c0_g1 | caspase-3-like | 3.10 |
| TRINITY_DN2307_c0_g1 | caspase 4 | 2.18 |
| AMPK signaling pathway | ||
| TRINITY_DN3645_c0_g1 | calcium/calmodulin-dependent protein kinase type 1B-like | 2.35 |
| TRINITY_DN4459_c0_g1 | serine/threonine-protein kinase mTOR-like | 1.35 |
| TRINITY_DN6500_c0_g1 | serine/threonine-protein phosphatase 2A regulatory subunit B″ subunit delta-like isoform X2 | 1.19 |
| TRINITY_DN8478_c0_g1 | ras-related protein Rab-8B-like isoform X1 | 1.74 |
| TRINITY_DN3645_c0_g1 | calcium/calmodulin-dependent protein kinase type 1B-like | 2.35 |
| TRINITY_DN2871_c0_g1 | elongation factor 2 | 1.24 |
| TRINITY_DN14309_c0_g1 | elongation factor 2 | 2.90 |
| TRINITY_DN14309_c0_g2 | elongation factor 2 | 2.93 |
| TRINITY_DN1265_c0_g1 | Rab10 | 1.33 |
| Toll-like receptor signaling pathway | ||
| TRINITY_DN168_c0_g1 | Toll interacting protein | 12.99 |
| TRINITY_DN1659_c0_g1 | ras-related C3 botulinum toxin substrate 1-like isoform X5 | 2.47 |
| Wnt signaling pathway | ||
| TRINITY_DN40081_c0_g1 | Wnt6 | 10.39 |
| TRINITY_DN31_c0_g3 | casein kinase II subunit alpha | 2.04 |
| TRINITY_DN3956_c0_g1 | serine/threonine-protein kinase NLK2-like | 1.35 |
| TRINITY_DN618_c0_g1 | calcium/calmodulin-dependent protein kinase type II alpha chain-like | 11.94 |
| Toll and Imd signaling pathway | ||
| TRINITY_DN6071_c0_g1 | dorsal | 8.64 |
| TRINITY_DN1816_c0_g2 | relish | 2.51 |
| TRINITY_DN4154_c0_g1 | ubiquitin-conjugating enzyme E2-24 kDa | 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Zhu, Y.; Qian, Q.; Chen, A.; Qin, L.; Tang, X.; Jiang, Q.; Zhang, X. The Immune Defense Response and Immune-Related Genes Expression in Macrobrachium nipponense Infected with Decapod Iridescent Virus 1 (DIV1). Animals 2024, 14, 2864. https://doi.org/10.3390/ani14192864
Gao X, Zhu Y, Qian Q, Chen A, Qin L, Tang X, Jiang Q, Zhang X. The Immune Defense Response and Immune-Related Genes Expression in Macrobrachium nipponense Infected with Decapod Iridescent Virus 1 (DIV1). Animals. 2024; 14(19):2864. https://doi.org/10.3390/ani14192864
Chicago/Turabian StyleGao, Xiaojian, Yujie Zhu, Qieqi Qian, Anting Chen, Lijie Qin, Xinzhe Tang, Qun Jiang, and Xiaojun Zhang. 2024. "The Immune Defense Response and Immune-Related Genes Expression in Macrobrachium nipponense Infected with Decapod Iridescent Virus 1 (DIV1)" Animals 14, no. 19: 2864. https://doi.org/10.3390/ani14192864
APA StyleGao, X., Zhu, Y., Qian, Q., Chen, A., Qin, L., Tang, X., Jiang, Q., & Zhang, X. (2024). The Immune Defense Response and Immune-Related Genes Expression in Macrobrachium nipponense Infected with Decapod Iridescent Virus 1 (DIV1). Animals, 14(19), 2864. https://doi.org/10.3390/ani14192864





