A20 Alleviates the Inflammatory Response in Bovine Endometrial Epithelial Cells by Promoting Autophagy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Animal Treatments
2.3. BEECs Isolation and Treatments
2.4. Histological Assay
2.5. Immunohistochemistry (IHC) Assay
2.6. Quantitative Reverse Transcriptase-PCR (qRT-PCR)
2.7. Western Blotting
2.8. Immunofluorescence Staining
2.9. Statistical Analyses
3. Results
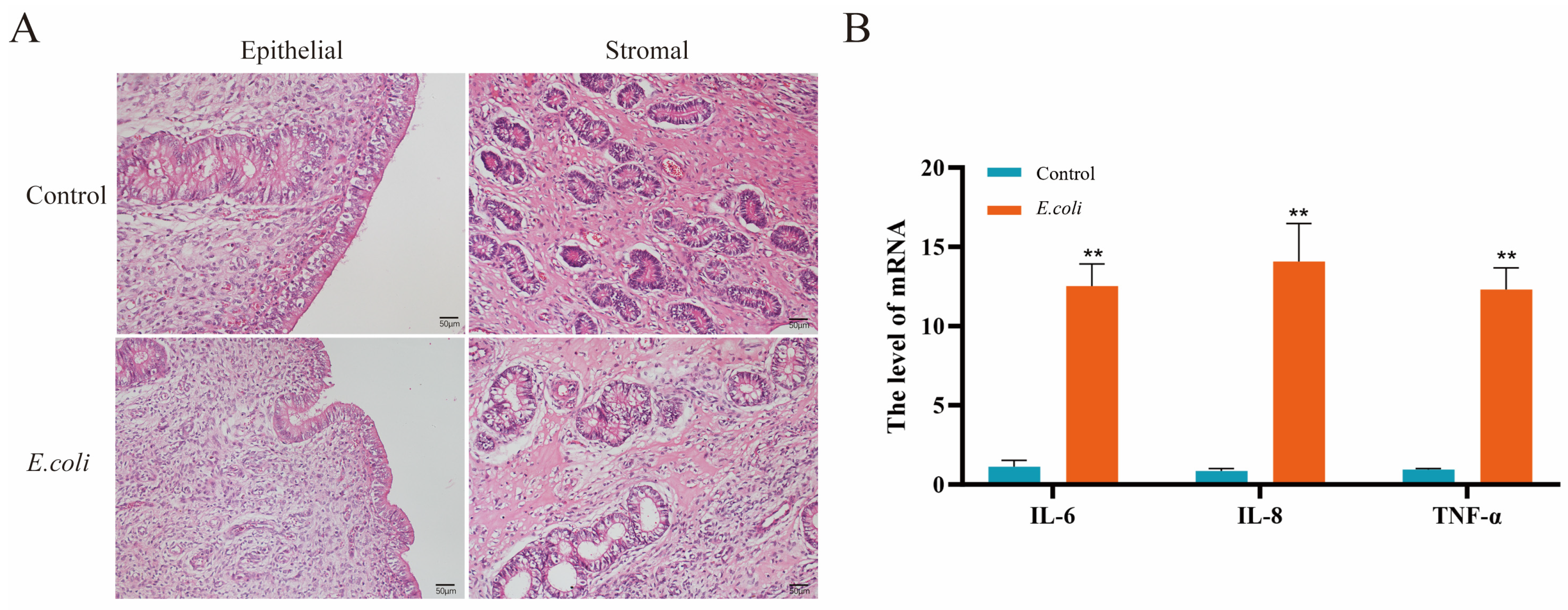
3.1. Inflammatory Damage in the Endometrium of Dairy Cows Was Caused by E. coli
3.2. Upregulated Expression of A20 in E. coli-Infected Endometrium and LPS-Stimulated BEECs
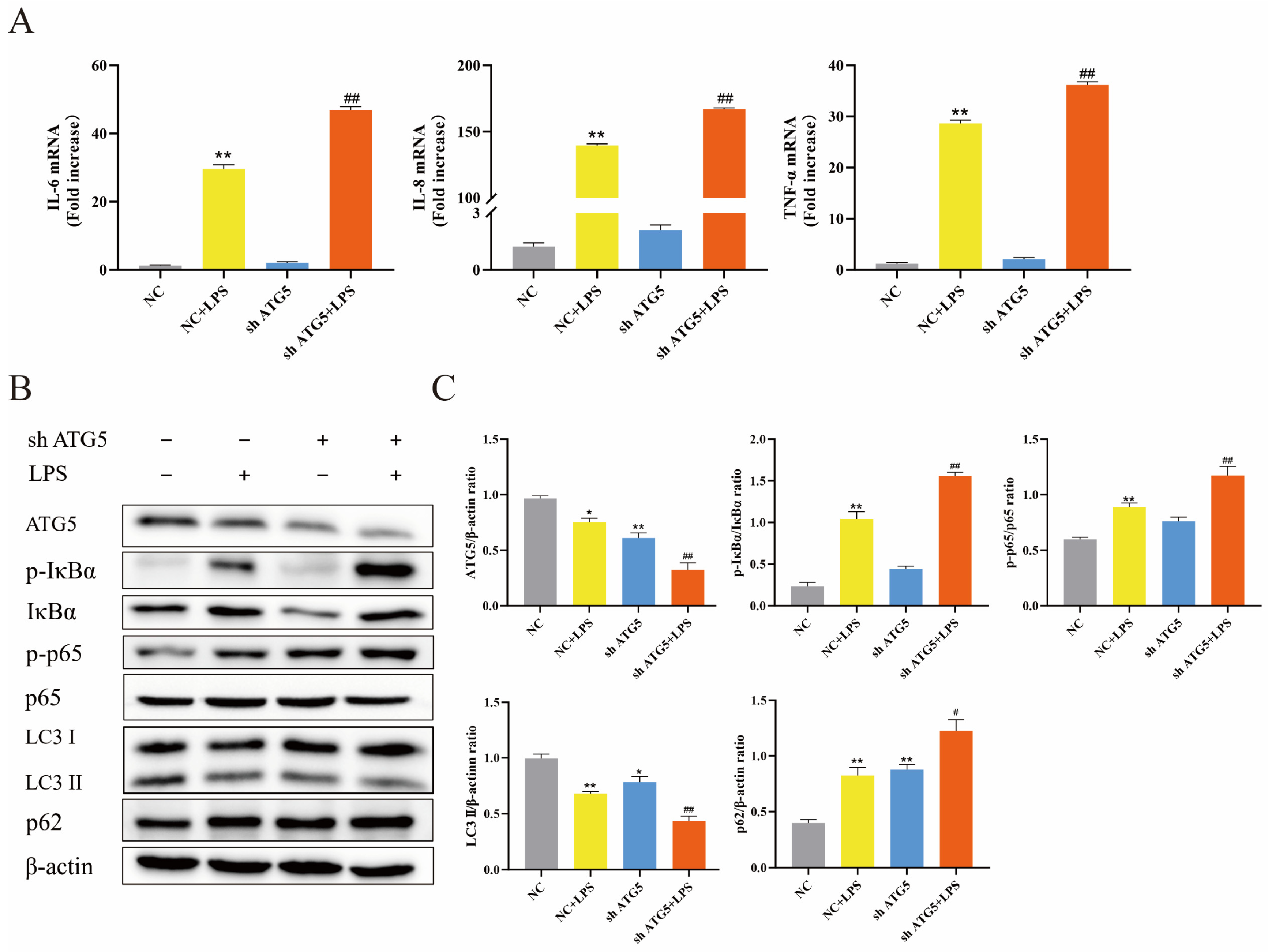
3.3. Inhibition of Autophagy Exacerbated Inflammatory Response of BEECs Induced by LPS
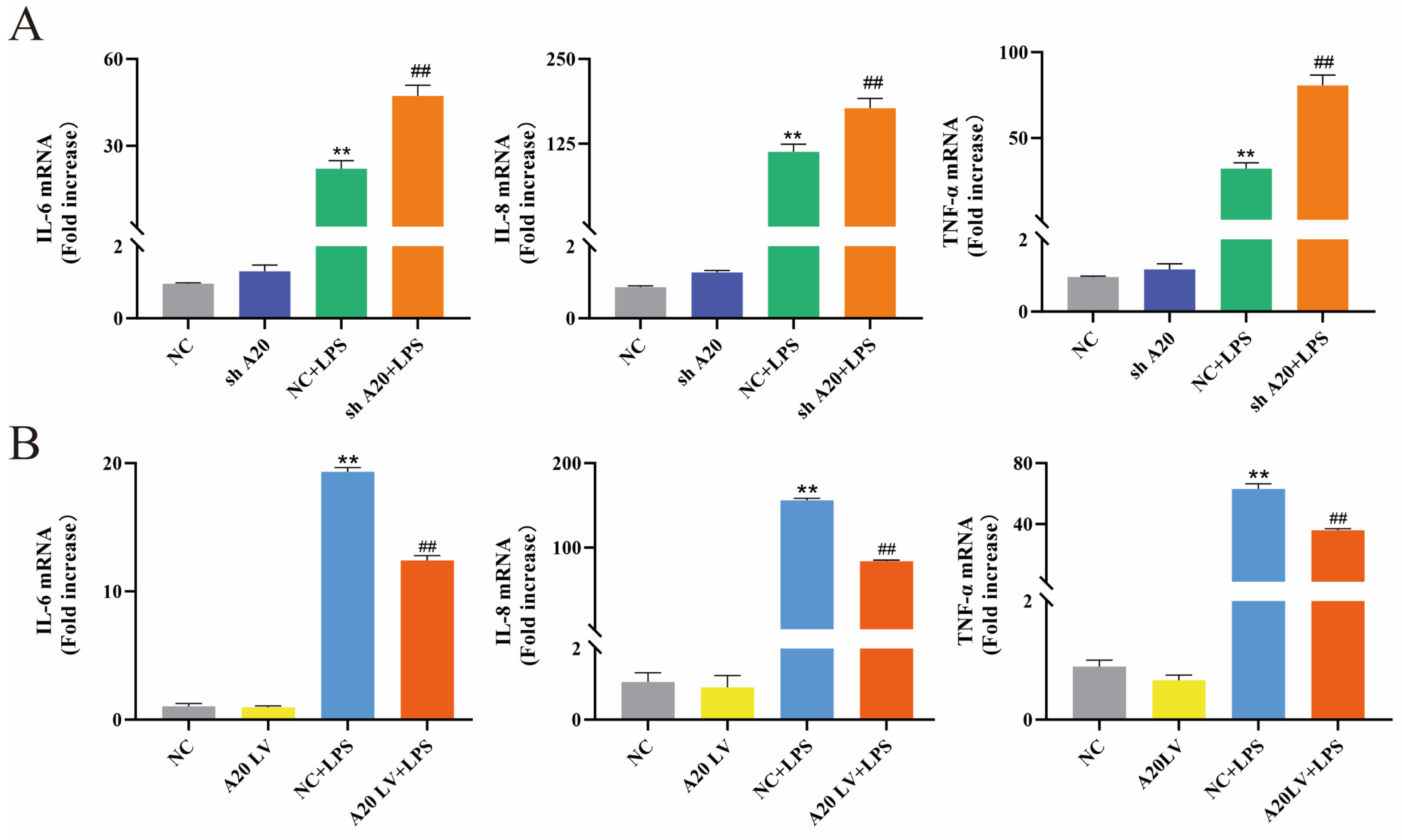
3.4. A20 Inhibited LPS-Induced Inflammatory Responses in BEECs
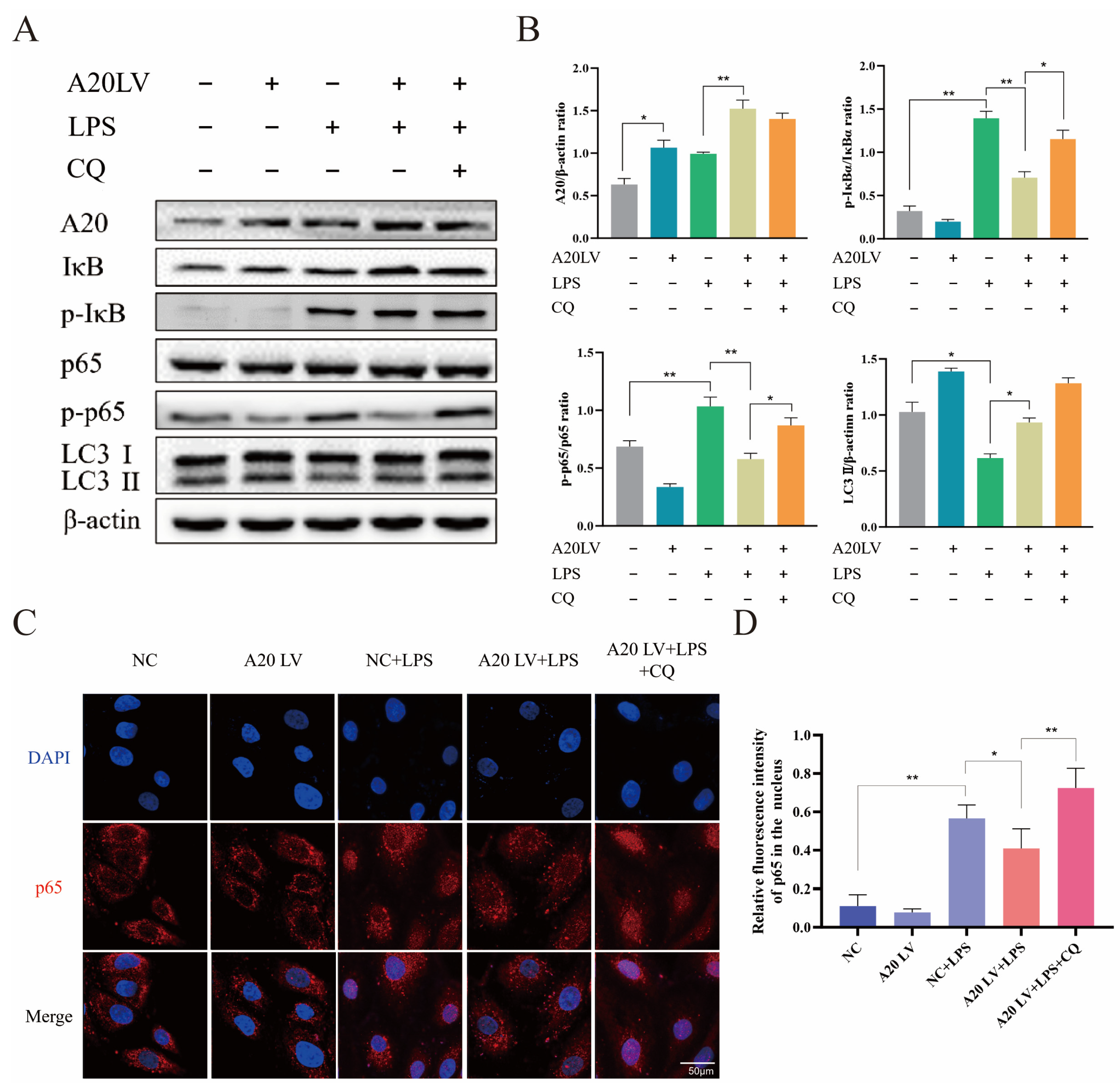
3.5. A20 Inhibited the NF-κB Pathway Activation by Promoting Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wu, Z.; Guo, S.; Zhang, T.; Ma, X.; Jiang, K.; Guo, X.; Deng, G. IFN-τ Attenuates LPS-Induced Endometritis by Restraining HMGB1/NF-κB Activation in bEECs. Inflammation 2021, 44, 1478–1489. [Google Scholar] [CrossRef]
- Piersanti, R.L.; Zimpel, R.; Molinari, P.C.C.; Dickson, M.J.; Ma, Z.; Jeong, K.C.; Santos, J.E.P.; Sheldon, I.M.; Bromfield, J.J. A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes. J. Dairy Sci. 2019, 102, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.; Sun, Y.; Dong, X.; Wang, Z.; Chen, J.; Dong, G. PGN and LTA from Staphylococcus aureus Induced Inflammation and Decreased Lactation through Regulating DNA Methylation and Histone H3 Acetylation in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Mandhwani, R.; Bhardwaz, A.; Kumar, S.; Shivhare, M.; Aich, R. Insights into bovine endometritis with special reference to phytotherapy. Vet. World 2017, 10, 1529–1532. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Guo, S.; Zhang, T.; Ma, X.; Wu, Z.; Jiang, K.; Zhang, X.; Guo, X.; Deng, G. MiR-505 as an anti-inflammatory regulator suppresses HMGB1/NF-κB pathway in lipopolysaccharide-mediated endometritis by targeting HMGB1. Int. Immunopharmacol. 2020, 88, 106912. [Google Scholar] [CrossRef]
- Koh, Y.Q.; Mitchell, M.D.; Almughlliq, F.B.; Vaswani, K.; Peiris, H.N. Regulation of inflammatory mediator expression in bovine endometrial cells: Effects of lipopolysaccharide, interleukin 1 beta, and tumor necrosis factor alpha. Physiol. Rep. 2018, 6, e13676. [Google Scholar] [CrossRef]
- Yan, C.; Lv, H.; Peng, Z.; Yang, D.; Shen, P.; Yu, J.; Tong, C.; Wang, X. Analysis of miRNA expression changes in bovine endometrial stromal cells treated with lipopolysaccharide. Theriogenology 2021, 167, 85–93. [Google Scholar] [CrossRef]
- Devos, M.; Mogilenko, D.A.; Fleury, S.; Gilbert, B.; Becquart, C.; Quemener, S.; Dehondt, H.; Tougaard, P.; Staels, B.; Bachert, C.; et al. Keratinocyte Expression of A20/TNFAIP3 Controls Skin Inflammation Associated with Atopic Dermatitis and Psoriasis. J. Investig. Dermatol. 2019, 139, 135–145. [Google Scholar] [CrossRef]
- Shembade, N.; Ma, A.; Harhaj, E.W. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 2010, 327, 1135–1139. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Yao, Y.; Liu, Z. Current research into A20 mediation of allergic respiratory diseases and its potential usefulness as a therapeutic target. Front. Immunol. 2023, 14, 1166928. [Google Scholar] [CrossRef] [PubMed]
- Malynn, B.A.; Ma, A. A20: A multifunctional tool for regulating immunity and preventing disease. Cell. Immunol. 2019, 340, 103914. [Google Scholar] [CrossRef] [PubMed]
- Duong, B.H.; Onizawa, M.; Oses-Prieto, J.A.; Advincula, R.; Burlingame, A.; Malynn, B.A.; Ma, A. A20 restricts ubiquitination of pro-interleukin-1β protein complexes and suppresses NLRP3 inflammasome activity. Immunity 2015, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox. Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Lim, M.C.C.; Maubach, G.; Birkl-Toeglhofer, A.M.; Haybaeck, J.; Vieth, M.; Naumann, M. A20 undermines alternative NF-κB activity and expression of anti-apoptotic genes in Helicobacter pylori infection. Cell. Mol. Life. Sci. 2022, 79, 102. [Google Scholar] [CrossRef]
- Wu, Y.F.; Li, Z.Y.; Dong, L.L.; Li, W.J.; Wu, Y.P.; Wang, J.; Chen, H.P.; Liu, H.W.; Li, M.; Jin, C.L.; et al. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy 2020, 16, 435–450. [Google Scholar] [CrossRef]
- Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016, 16, 661–675. [Google Scholar] [CrossRef]
- Chadha, S.; Behl, T.; Bungau, S.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Gupta, A.; Kandhwal, M.; Chandel, D. Focus on the Multimodal Role of Autophagy in Rheumatoid Arthritis. Inflammation 2021, 44, 1–12. [Google Scholar] [CrossRef]
- Kuenzig, M.E.; Yim, J.; Coward, S.; Eksteen, B.; Seow, C.H.; Barnabe, C.; Barkema, H.W.; Silverberg, M.S.; Lakatos, P.L.; Beck, P.L. The NOD2-Smoking Interaction in Crohn’s Disease is likely Specific to the 1007fs Mutation and may be Explained by Age at Diagnosis: A Meta-Analysis and Case-Only Study. eBioMedicine 2017, 21, 188–196. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Duijvestein, M.; Verhaar, A.P.; Vos, A.C.; van den Brink, G.R.; Hommes, D.W.; Wildenberg, M.E. Impaired autophagy leads to abnormal dendritic cell-epithelial cell interactions. J. Crohns. Colitis. 2013, 7, 534–541. [Google Scholar] [CrossRef]
- Zhai, Y.; Lin, P.; Feng, Z.; Lu, H.; Han, Q.; Chen, J.; Zhang, Y.; He, Q.; Nan, G.; Luo, X.; et al. TNFAIP3-DEPTOR complex regulates inflammasome secretion through autophagy in ankylosing spondylitis monocytes. Autophagy 2018, 14, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
- Socha, B.M.; Łada, P.; Szczepańska, A.A.; Łupicka, M.; Korzekwa, A.J. The influence of experimentally induced endometritis on the PPAR expression profile in the bovine endometrium. Theriogenology 2018, 122, 74–83. [Google Scholar] [CrossRef]
- Dong, J.; Ji, B.; Jiang, Y.; Liu, K.; Guo, L.; Cui, L.; Wang, H.; Li, B.; Li, J. Autophagy activation alleviates the LPS-induced inflammatory response in endometrial epithelial cells in dairy cows. Am. J. Reprod. Immunol. 2024, 91, e13820. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Lilly, S.T.; Fischer, D.P.; Williams, E.J.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2alpha to prostaglandin E2 in bovine endometrium. Endocrinology 2009, 150, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Fredericksen, F.; Villalba, M.; Olavarría, V.H. Characterization of bovine A20 gene: Expression mediated by NF-κB pathway in MDBK cells infected with bovine viral diarrhea virus-1. Gene 2016, 581, 117–129. [Google Scholar] [CrossRef]
- Husnain, A.; Arshad, U.; Poindexter, M.B.; Zimpel, R.; Marinho, M.N.; Perdomo, M.C.; Fan, P.; Jeong, K.C.; Nelson, C.D.; Sheldon, I.M.; et al. Induced endometritis in early lactation compromises production and reproduction in dairy cows. J. Dairy. Sci. 2023, 106, 4198–4213. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Molinari, P.C.C.; Ormsby, T.J.R.; Bromfield, J.J. Preventing postpartum uterine disease in dairy cattle depends on avoiding, tolerating and resisting pathogenic bacteria. Theriogenology 2020, 150, 158–165. [Google Scholar] [CrossRef]
- Yang, C.; Yang, C.; Zhang, J.; Guo, Y.; Chen, N.; Yin, B.; Zhou, Q.; Zhang, T.; Guo, S.; Deng, G. MicroRNA-211 regulates the expression of TAB1 and inhibits the NF-κB signaling pathway in lipopolysaccharide-induced endometritis. Int. Immunopharmacol. 2021, 96, 107668. [Google Scholar] [CrossRef]
- Li, Y.; Mooney, E.C.; Xia, X.J.; Gupta, N.; Sahingur, S.E. A20 Restricts Inflammatory Response and Desensitizes Gingival Keratinocytes to Apoptosis. Front. Immunol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Wang, W.; Bale, S.; Wei, J.; Yalavarthi, B.; Bhattacharyya, D.; Yan, J.J.; Abdala-Valencia, H.; Xu, D.; Sun, H.; Marangoni, R.G.; et al. Fibroblast A20 governs fibrosis susceptibility and its repression by DREAM promotes fibrosis in multiple organs. Nat. Commun. 2022, 13, 6358. [Google Scholar] [CrossRef]
- Kelly, C.; Shields, M.D.; Elborn, J.S.; Schock, B.C. A20 regulation of nuclear factor-κB: Perspectives for inflammatory lung disease. Am. J. Respir. Cell Mol. Biol. 2011, 44, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Boone, D.L.; Chai, S.; Libby, S.L.; Chien, M.; Lodolce, J.P.; Ma, A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 2000, 289, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Netea-Maier, R.T.; Plantinga, T.S.; van de Veerdonk, F.L.; Smit, J.W.; Netea, M.G. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy 2016, 12, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death. Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, W.; Wang, J.; Yan, J.; Shi, Y.; Zhang, C.; Ge, W.; Wu, J.; Du, P.; Chen, Y. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. eBioMedicine 2018, 35, 345–360. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, R.; Liu, R.; Song, P.; Lin, P.; Chen, H.; Zhou, D.; Wang, A.; Jin, Y. Autophagy Mediates Escherichia coli-Induced Cellular Inflammatory Injury by Regulating Calcium Mobilization, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2022, 23, 14174. [Google Scholar] [CrossRef]
- Silwal, P.; Paik, S.; Kim, J.K.; Yoshimori, T.; Jo, E.K. Regulatory Mechanisms of Autophagy-Targeted Antimicrobial Therapeutics Against Mycobacterial Infection. Front. Cell. Infect. Microbiol. 2021, 11, 633360. [Google Scholar] [CrossRef]
- Shu, Z.; Yuan, J.; Wang, H.; Zhang, J.; Li, S.; Zhang, H.; Liu, Y.; Yin, Y.; Zhang, X. Streptococcus pneumoniae PepO promotes host anti-infection defense via autophagy in a Toll-like receptor 2/4 dependent manner. Virulence 2020, 11, 270–282. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Kumar, A. Butyrate Ameliorates Intraocular Bacterial Infection by Promoting Autophagy and Attenuating the Inflammatory Response. Infect. Immun. 2023, 91, e0025222. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, L.; Shen, L.; Liu, W.; Li, Y.; Ma, H.; Wu, X. A20 ameliorates Aspergillus fumigatus keratitis by promoting autophagy and inhibiting NF-κB signaling. Int. J. Biol. Macromol. 2023, 253, 127640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yi, W.; Xia, H.; Lan, H.; Chen, J.; Yang, Z.; Han, F.; Tang, P.; Liu, B. A20 regulates inflammation through autophagy mediated by NF-κB pathway in human nucleus pulposus cells and ameliorates disc degeneration in vivo. Biochem. Biophys. Res. Commun. 2021, 549, 179–186. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers (5′→3′) | Product Size (bp) | Accession Number |
|---|---|---|---|
| β-actin | F: CATCACCATCGGCAATGAGC R: AGCACCGTGTTGGCGTAGAG | 156 | NM_173979.3 |
| A20 | F: TGCTGCAAAGTTGGATGAAG R: TTGGGACTTTCGTTTGGTTC | 281 | XM_005210987.2 |
| TNF-α | F: GGGCTTTACCTCATCTACTCACAG R: GATGGCAGACAGGATGTTGACC | 132 | NM_173966.3 |
| IL-6 | F: TGAAAGCAGCAAGGAGACACT R: TGATTGAACCCAGATTGGAAGC | 90 | NM_173923.2 |
| IL-8 | F: TTCCTCAGTAAAGATGCCAATG R: TGACAACCCTACACCAGACCCA | 86 | NM_173925.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Ji, B.; Jiang, Y.; Fei, F.; Guo, L.; Liu, K.; Cui, L.; Meng, X.; Li, J.; Wang, H. A20 Alleviates the Inflammatory Response in Bovine Endometrial Epithelial Cells by Promoting Autophagy. Animals 2024, 14, 2876. https://doi.org/10.3390/ani14192876
Dong J, Ji B, Jiang Y, Fei F, Guo L, Liu K, Cui L, Meng X, Li J, Wang H. A20 Alleviates the Inflammatory Response in Bovine Endometrial Epithelial Cells by Promoting Autophagy. Animals. 2024; 14(19):2876. https://doi.org/10.3390/ani14192876
Chicago/Turabian StyleDong, Junsheng, Bowen Ji, Yeqi Jiang, Fan Fei, Long Guo, Kangjun Liu, Luying Cui, Xia Meng, Jianji Li, and Heng Wang. 2024. "A20 Alleviates the Inflammatory Response in Bovine Endometrial Epithelial Cells by Promoting Autophagy" Animals 14, no. 19: 2876. https://doi.org/10.3390/ani14192876
APA StyleDong, J., Ji, B., Jiang, Y., Fei, F., Guo, L., Liu, K., Cui, L., Meng, X., Li, J., & Wang, H. (2024). A20 Alleviates the Inflammatory Response in Bovine Endometrial Epithelial Cells by Promoting Autophagy. Animals, 14(19), 2876. https://doi.org/10.3390/ani14192876







