Simple Summary
Microsporidia are a group of fungus-related eukaryotes with worldwide distribution. The microsporidian species Encephalitozoon cuniculi and E. hellem occur in mammals, birds and even humans. Knowledge of their relevance in wild rabbits is very limited so far. Thus, the aim of the present study was to investigate the occurrence of E. cuniculi and E. hellem in wild rabbit populations in southern Germany (Bavaria). Therefore, blood and organ samples of 158 wild rabbits were investigated by serological and PCR-based assays. Antibodies to E. cuniculi were detected in 24 of the 158 (15.2%) wild rabbits, while DNA of E. cuniculi was found in 7 (4.4%) and DNA of E. hellem was found in 3 (1.9%). Sequencing identified E. cuniculi genotype 1. This study provides the first E. cuniculi genotype determination in free-living wild rabbits worldwide and the first evidence of E. hellem in rabbits worldwide. Wild rabbits should, therefore, be regarded as a reservoir for both pathogens and as a source of infection for domestic rabbits, other animals and humans.
Abstract
Encephalitozoon cuniculi and Encephalitozoon hellem are fungus-related, obligate intracellular pathogens belonging to the microsporidia. Both microorganisms occur in mammals, birds and even humans, thus revealing a zoonotic potential. Knowledge of their relevance in wild rabbits is very limited so far. Thus, the aim of the present study was to investigate the occurrence of E. cuniculi and E. hellem in wild rabbit populations in southern Germany (Bavaria). Therefore, blood and organ samples (brain/kidney) of 158 wild rabbits were investigated by immunofluorescence and PCR-based assays. Antibodies to E. cuniculi were detected in 24 of the 158 (15.2%) wild rabbits, while DNA of E. cuniculi was found in 7 (4.4%) and DNA of E. hellem was found in 3 (1.9%). Sequencing identified E. cuniculi genotype 1. This study provides the first E. cuniculi genotype determination in free-living wild rabbits worldwide and the first evidence of E. hellem in rabbits worldwide. Wild rabbits should, therefore, be regarded as a reservoir for both pathogens and, on the basis of molecular evidence from kidney tissue and presumed urine excretion, also as a source of infection for E. cuniculi for animals and humans.
1. Introduction
Encephalitozoon cuniculi and Encephalitozoon hellem are obligate intracellular, spore-forming pathogens belonging to the group of microsporidia. E. cuniculi was first described by Wright and Craighead in 1922. They discovered it by chance during experimental research as the causative agent of motor paralysis in young rabbits [1]. The pathogen is mainly associated with rabbits, but it has a very broad host range and may occur in many other mammals including humans, as well as in birds [2,3,4].
So far, four different genotypes of E. cuniculi have been identified, which may be differentiated based on molecular genetic investigations of the ITS region of the rRNA gene. Those genotypes are called “rabbit strain” (type I), “mouse strain” (type II), “dog strain” (type III) and “human strain” (type IV) [5,6], but are of low host-specificity. For example, in rabbits, natural infections with genotypes I, II and III have been detected [7,8]. In humans, in addition to further genotype IV [6], infections with all of the other three genotypes were also identified, revealing a zoonotic potential independent of the genotype involved [3,9].
Horizontal infections with E. cuniculi occur primarily through oral ingestion of spores, which are excreted intermittently by infected animals or humans, mainly via the urine but also with faeces [10,11]. The oral route with ingestion of contaminated food and water is the most probable route of transmission of microsporidian spores to humans [3,12].
Infections with E. cuniculi in rabbits are usually clinically asymptomatic, but immunosuppression may lead to clinical signs and even death [13]. After ingestion, the spores enter the intestine, where they infect the epithelium and then reach the bloodstream via the gut-associated lymphoid tissue. Via the blood, the pathogen reaches various organs—either as a free spore or within infected monocytes [14]. The final predilection sites are the brain and the kidneys [10]. In addition, ocular transmission of the pathogen during the intrauterine period is possible [15]. Cerebral infection typically results in non-suppurative, focal to multifocal granulomatous (meningo-) encephalitis [16,17,18,19]. In the kidneys, E. cuniculi multiplies primarily in the tubular epithelial cells. Histologically, the kidneys typically show focal, multifocal or segmental non-suppurative granulomatous interstitial nephritis with macroscopic scarring due to fibrosis [17,18,19,20,21]. Clinically, infections manifest in neurological symptoms (vestibular dysfunction, e.g., head tilt, ataxia, nystagmus, rotations around the longitudinal axis), signs of renal insufficiency (non-specific symptoms such as apathy, anorexia, weight loss, polydypsia, polyuria) or ocular signs (phacoclastic uveitis, consecutive cataracts) [22,23,24]. Clinical manifestation in humans usually occurs in immunocompromised individuals only. Diarrhea and abdominal pain, hepatitis, peritonitis, liver failure, renal failure, disseminated disease with fever, persistent cough and endocarditis have been described [12].
E. hellem was first described in humans in 1990 as a cause of keratoconjunctivitis in AIDS patients [25,26]. Morphologically and ultrastructurally, E. hellem and E. cuniculi do not differ. However, immunological and molecular testing methods make it possible to distinguish between the two species [25]. In animals, E. hellem is most widespread among birds, both wild and companion birds, but the pathogen has also been occasionally detected in mammals, including rodents, carnivores and monkeys [4]. In Lagomorpha, natural infection with E. hellem has only been detected in the kidney tissue of a single European brown hare so far, which was simultaneously infected with E. intestinalis [27].
Three different genotypes (genotypes 1, 2 and 3) of E. hellem have been described by Mathis et al. based on molecular genetic analyses of the ITS region of the rDNA gene [28]. Subsequently, Xiao et al. were able to subdivide genotype 1 into 1A, 1B and 1C and genotype 2 into 2A and 2B by additionally analysing the gene locus of the polar tube protein and the gene of the small subunit of the rRNA; it has been recommended to rename genotype 3 as 2C [29].
E. hellem infections in birds may cause a variety of clinical symptoms ranging from mild to fatal [30,31]. However, most infections are probably asymptomatic [32,33]. Increased stunting and high mortality have been observed in budgerigar chicks [30]. Necropsy of infected birds revealed abnormalities such as significant muscle wasting, loss of body fat and lesions, especially in the kidneys, liver, intestines and eyes [34]. Clinical manifestations in humans, as with E. cuniculi, occur predominantly in immunocompromised individuals. Keratoconjunctivitis in particular, but also nephritis, pneumonia, bronchitis and disseminated disease with renal failure have been reported [12].
Since hunted European wild rabbits (Oryctolagus cuniculus) in Germany and elsewhere are partially intended for consumption and usually enter the human food chain without prior hygiene control in accordance with country-specific legislation, there is a need to identify possible zoonotic agents such as E. cuniculi and E. hellem. In the past, the serological prevalence of E. cuniculi in domestic rabbits was found to vary between 7.7 [35] and 81.7% [36] worldwide. However, significantly fewer studies have been conducted in wild rabbits, and there is no current data on E. cuniculi and a complete lack of data on E. hellem for Central Europe. Previous serologic studies on E. cuniculi in Germany on wild rabbits have shown contradictory results. The first study in southern Germany in 1988 showed a seroprevalence of 18.1% in 155 wild rabbits investigated [37], but in a subsequent study in northern Germany in 1996, none of 100 wild rabbits tested positive [38]. Molecular studies to detect the nucleic acids of the pathogens had not yet been carried out in wild rabbits in Germany so far.
The aim of this study was, therefore, to gain updated insight into the prevalence of E. cuniculi using serological and molecular assays and to investigate the occurrence of E. hellem in wild rabbits in Germany for the first time in order to assess whether wild rabbits represent a reservoir for both pathogens and thus a potential source of infection.
2. Materials and Methods
2.1. Ethics
The Ethics Committee of the Faculty of Veterinary Medicine at LMU Munich approved this study, and no ethical concerns were raised (reference number 317-30-06-2022). Most of the samples were collected from legally hunted wild rabbits during the hunting seasons. Three samples originated from wild rabbits from a shelter that died or had to be euthanised due to severe disease independently of the current investigation. One of the samples came from a wild rabbit that had been found freshly dead, presumably after an accident with a car.
2.2. Study Area and Sample Collection
This study was conducted between 2021 and 2023. A total of 158 European wild rabbits (Oryctolagus cuniculus) originating from all seven administrative districts of Bavaria, a federal state located in the south of Germany, were sampled (Figure 1). Data on age (juvenile (≤200 days [39], not fully grown)/adult), gender, location of origin and sampling year were recorded for each wild rabbit included in the investigation (Table 1, Supplementary Table S1).
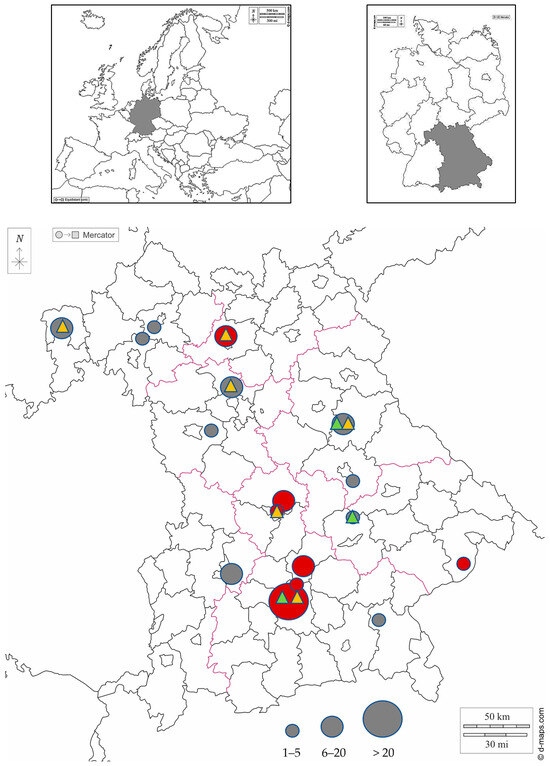
Figure 1.
Rural administrative district map of Bavaria showing the collection areas of wild rabbits. Numbers of sampled rabbits per administrative district are semiquantitatively indicated by differing circle diameters. Areas in which wild rabbits were tested seropositive for E. cuniculi are marked in red, while areas in which all tested wild rabbits were seronegative are marked in dark gray. Areas in which E. cuniculi DNA has been detected in wild rabbits using molecular testing methods are highlighted with a yellow triangle. Areas in which E. hellem DNA has been detected using molecular testing methods are highlighted with a light green triangle. Maps were taken from d-maps.com (https://d-maps.com/carte.php?num_car=6121&lang=de, https://d-maps.com/carte.php?num_car=17879&lang=de, https://d-maps.com/carte.php?num_car=2233&lang=de; accessed on 1 August 2024).

Table 1.
Age and gender of the sampled European wild rabbits and sampling year.
Brain, kidney and blood samples were taken from each individual. Only rabbits with both blood and tissue samples available were included in this study. Sampling was either performed by the hunters themselves immediately after the hunt according to previous instructions, or the carcasses were provided whole and tissue and blood samples were taken in the laboratory. The fresh clinical samples or the carcasses were generally submitted to the laboratory within 1–3 days. For 46 of the 158 carcasses/samples, however, no immediate transfer could take place due to logistical circumstances, thus they were frozen at −18 °C by the hunters in the meantime.
Tissue samples were placed in sterile plastic containers (125 mL) and blood samples were collected in 4 mL serum tubes. The tissue samples were stored at −20 °C until analysis. The serum tubes were centrifuged after clotting, and the supernatant was pipetted off and transferred to sterile 1.5 mL Eppendorf tubes. The Eppendorf tubes containing the serum samples were stored at −20 °C until used for E. cuniculi antibody testing.
2.3. Indirect Immunofluorescence Antibody Test
The indirect immunofluorescence antibody test (IFAT) for the detection of E. cuniculi antibodies was carried out according to Chalupsky et al. [40] by a commercial company (SYNLAB.vet GmbH, Augsburg, Germany). Both IgG and IgM antibodies were tested. The highest serum dilution level evaluated as positive was given as a titer. Titers of ≥1:80 were considered positive for both antibody isotypes. Possible associations between seropositivity and the age or gender of the rabbits were verified using the Chi-Square test.
2.4. DNA Extraction
A total of 500 mg of brain or kidney tissue was added to 150 µL of sterile phosphate-buffered saline (pH 7.2) and then homogenised with the aid of SiLibeads Typ ZS 1.4–1.6 mm (Sigmund Lindner GmbH, Warmensteinach, Germany) using a vibrating mill (Retsch, Haan, Germany) for 5 min with a frequency of 30 Hz. A total of 200 mg of the homogenised tissue material was weighed into a microcentrifuge tube, mixed with 600 µL of sterile phosphate-buffered saline (pH 7.2) by pulse-vortexing and then clarified by centrifugation at 16,200× g for 2 min. Nucleic acid was extracted from 200 µL of the supernatant using the IndiSpin® Pathogen Kit (Indical Bioscience GmbH, Leipzig, Germany) according to the manufacturer’s instructions, except that proteinase K digestion was performed at 56 °C for one hour instead of 20–25 °C for 15 min.
2.5. Real-Time PCR
The extracted DNA was tested for E. cuniculi and E. hellem in a duplex real-time PCR analysis, as described before by Leipig et al. [41], using the E. cuniculi forward primer MSP-3 (5′-TTGCGATGAAGGACGAAGG-3′), the E. hellem forward primer MSP-4 (5′-TGATGAAGGACGAAGG-3′), a reverse primer (MSP-5: 5′-TCTTGCGAGCGTACTATCC-3′) and the molecular beacon fluorescent probes for E. cuniculi (MSP-S3: 5′-FAM-CGCGATCGACTGGACGGGACNGTGTGTGTTGTCCATGAGAAAGATCGCG-BHQ-1-3′) and E. hellem (MSP-S4: 5′-HEX-CGCGATCGACTGGACGGGACTGTTTTAGTGTTGTCCGAGAGAAAGATCGCG-BHQ-1-3′). Primers and probes were synthesised by Metabion (Planegg, Germany).
Real-time PCR was performed using 2.5 u AllTaq DNA polymerase (Qiagen, Hilden, Germany), 0.5 μM MSP-3, 0.5 µM MSP-4, 1 μM MSP-5, 0.5 μM MSP-S3, 1 µM MSP-S4, 0.25 mM, each, dNTPs, 2.5 μg bovine serum albumin, 5.5 mM MgCl2, 1× key buffer (Qiagen, Hilden, Germany) and 2.5 μL of DNA in a total volume of 25 μL. The real-time PCR assay was performed using a G8830A AriaMx Real-time PCR System (Agilent Technologies, Santa Clara, CA, USA) using an initial denaturation for 15 min at 95 °C, followed by fifty cycles of denaturation for 30 s at 95 °C, annealing for 60 s at 53 °C and elongation for 30 s at 72 °C. Samples that yielded Ct values calculated by the Aria data analysis software and revealed a sigmoid shape of the fluorescence curve were then further analysed in conventional nested PCR assays for confirmation and genotyping.
2.6. Nested PCR I
In order to confirm the results of the real-time PCR assay obtained for E. cuniculi and E. hellem and thus to identify the species, a nested PCR I was performed according to Katzwinkel-Wladarsch et al. [42] with minor modifications. The primers used were generic microsporidia primers. For the first round, forward primer MSP-1 (5′-TGAATGKGTCCCTGT-3′) and reverse primer MSP-2a (5′-TCACTCGCCGCTACT-3′) were applied, and in the second round, forward primer MSP-3 (5′GGAATTCACACACCGCCCGTCRYTAT-3′) and reverse primer MSP-4a (5′-CCAAGCTTATGCTTAAGTYMAARGGGT-3′). The primers were synthesised by Metabion (Planegg, Germany).
The first PCR round contained 1.25 u AllTaq DNA polymerase (Qiagen, Hilden, Germany), 0.5 μM of each respective primer, 0.25 mM, each, dNTPs, 1.0 mM MgCl2, 1× key buffer (Qiagen, Hilden, Germany) and 2.5 μL DNA in a total volume of 25 μL.
The second PCR round contained 1.25 u AllTaq DNA polymerase (Qiagen, Hilden, Germany), 0.5 μM of each respective primer, 0.25 mM, each, dNTPs, 0.5 mM MgCl2, 1× key buffer (Qiagen, Hilden, Germany) and 1 μL of the first PCR round in a total volume of 25 μL. The nested PCR assay was performed using a SensoQuest LabCycler (SensoQuest GmbH, Göttingen, Germany). For both rounds, identical temperature profiles were used. The initial denaturation was carried out for 2 min at 96 °C, followed by fifty cycles of denaturation for 60 s at 92 °C, annealing for 60 s at 58 °C, elongation for 90 s at 72 °C and a final extension step for 7 min at 72 °C.
PCR products were visualised under UV light after electrophoresis in 2% agarose gels with ethidium bromide.
2.7. Nested PCR II
The nested PCR II aimed at determining the genotypes of E. cuniculi involved. All samples for which real-time PCR had previously yielded Ct values for E. cuniculi were included. The first round of the nested PCR assay was performed according to Asakura et al. [43] with minor changes, using the E. cuniculi forward primer F2 (5′-TCCTAGTAATAGCGGCTGAC-3′) and the E. cuniculi reversed primer int580r (5′-TTTCACTCGCCGCTACTCAG-3′), which was originally designed by Didier et al. [5]. Primers were synthesised by Metabion (Planegg, Germany). The PCR reaction contained 0.625 u AllTaq DNA polymerase (Qiagen, Hilden, Germany), 0.2 μM of each primer, 0.2 mM, each, dNTPs, 1× key buffer (Qiagen, Hilden, Germany) and 2.5 μL of DNA in a total volume of 25 μL. The PCR assay was performed with an initial denaturation for 2 min at 95 °C, followed by thirty-five cycles of denaturation for 30 s at 94 °C, annealing for 30 s at 56 °C, elongation for 30 s at 72 °C and a final extension step for 5 min at 72 °C.
For the second round of nested PCR II E. cuniculi, forward primer EcF3 (5′-AAGATGACGCACTGGACGAA-3′) and reverse primer EcR3 (5′-GTGCACACCGCACACAATTC-3′) were designed for the present study based on the identification of rDNA genomic regions conserved for the varying E. cuniculi genotypes. Primers were synthesised by Metabion (Planegg, Germany). The PCR reactions contained 0.625 u AllTaq DNA polymerase (Qiagen, Hilden, Germany), 0.25 μM of each primer, 0.2 mM, each, dNTPs, 1× key buffer (Qiagen, Hilden, Germany), 1 μL of the first PCR round in a total volume of 25 μL. The initial denaturation was carried out for 2 min at 95 °C, followed by forty cycles of denaturation for 5 s at 95 °C, annealing for 15 s at 53 °C and elongation for 10 s at 72 °C.
PCR products were visualised under UV light after electrophoresis in 2% agarose gels with ethidium bromide.
2.8. Sequencing
PCR products of expected sizes were extracted from agarose gel using the QiaQuick gel extraction kit (Qiagen, Hilden, Germany) and sequenced by Sanger’s method at Eurofins Genomics (Ebersberg, Germany) from both sides, using the respective PCR primers of the second PCR rounds. The BLAST search tool provided by the National Centre for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi; access date: 3 May 2024) was used to determine the identity of the PCR products. To determine the genotype of E. cuniculi sequence alignments were carried out using CLUSTAL W, which is included in the DNAMAN software package (Lynnon Corporation, Quebec, QC, Canada), including sequences for the different E. cuniculi genotypes deposited in the NCBI GenBank.
3. Results
3.1. Indirect Immunofluorescence Antibody Test for E. cuniculi
Serum samples obtained from 24 of the 158 wild rabbits (15.2%) were positive for antibodies against E. cuniculi. No association was found between seropositivity and age (X2 (1, N = 158) = 1.116, p = 0.29)) or gender (X2 (1, N = 158) = 0.06, p = 0.80)) using the Chi-Square test.
In 12 of the samples, both IgM and IgG antibodies were found. As shown in Table 2, only IgG antibodies were detected in 10 samples, while only IgM antibodies were found in 2 samples. In 19 serum samples, low titres of 1:80 were detected. Higher titres ranging from 1:160 to >1:1280 were found in five samples (Table 2). Wild rabbits originating from 7 of the 17 locations in Bavaria included in this study tested positive (Table 2, Figure 1).

Table 2.
Results of indirect immunofluorescence antibody test for E. cuniculi with titres for IgG and IgM.
3.2. Genome detection by Real-Time PCR
Real-time PCR revealed Ct values for E. cuniculi in 10 of the 158 (6.3%) and for E. hellem in 4 of the 158 (2.5%) wild rabbits (Supplementary Tables S2 and S3). The Ct values for E. cuniculi ranged between 27.67 and 45.98, and those for E. hellem were between 42.93 and 48.23. Only the Ct values of those samples for which the curves showed a sigmoid shape were taken into account.
Positive reactivities for E. cuniculi were found in both the brain and the kidneys of 2 rabbits (Nos. 9 and 118) as well as in the brains only of 3 rabbits (Nos. 75, 79 and 155) and in the kidneys only of 5 rabbits (Nos. 66, 68, 97, 134 and 157) (Supplementary Table S2).
Regarding the 4 rabbits with Ct values for E. hellem-positive reactions, 1 rabbit (No. 118) originated from both the brain and kidney samples, while in 2 rabbits (Nos. 97 and 150), the kidney samples only, and in 1 rabbit (No. 109) the brain sample revealed fluorescence signals (Supplementary Table S3).
A total of 2 of the rabbits showed Ct values for both E. cuniculi and E. hellem, 1 rabbit (No. 118) in the brain and kidney samples and the other (No. 97) in the kidney sample.
3.3. Nested PCRs and Sequencing
Samples from rabbits that gave Ct values in the real-time PCR were subsequently analysed with nested PCRs for confirmation and genotyping.
The nested PCRs I yielded PCR products of the expected size for E. cuniculi in 7 of the 10 rabbits that had previously yielded Ct values in the real-time PCR (Supplementary Table S2, Supplementary Figure S1). For E. hellem, products of the expected size were obtained in three of the four rabbits that previously gave Ct values in the real-time PCR (Supplementary Table S3, Supplementary Figure S2).
Using nested PCR II, in 1 rabbit (No. 9), E. cuniculi genotype 1 characterised by three 5′-GTTT-3′ repeats in the ITS DNA sequence, [5] was found. Identical sequences were obtained from both brain and kidney samples of this rabbit. The sequences are available in GenBank under the accession numbers PQ214196 and PQ214197. For the remaining PCR products of nested PCR I and II, including those of E. hellem, direct Sanger sequencing did not result in evaluable nucleotide sequences, probably because of the low DNA amount or bad DNA quality of the PCR product. The genotype of E. hellem involved could thus not be determined.
4. Discussion
In the current study, antibodies against E. cuniculi were detected in 15.2% of the wild rabbits tested, which is comparable to the results of the first investigation in southern Germany [37]. In that previous study, published in 1988, 18.1% of the wild rabbits tested positive. However, the wild rabbits analysed at that time came from only two properties in Munich, which limited the significance of the results [37]. The current results show that the pathogen is circulating in various areas of Bavaria and, considering the former study, indicate the endemic occurrence of E. cuniculi in the wild rabbit population, at least in southern Germany. Whether this endemic occurrence also extends to other regions in Germany is still unclear, as in another study including wild rabbits from the northern part of Germany published in 1996, antibodies against the pathogen were not detected, and it was therefore assumed that wild rabbits probably do not play a role in the spread of the pathogen [38]. Further new investigations are thus necessary to answer this question.
In the present study, E. cuniculi genomic DNA was detected in the examined animals in addition to the presence of antibodies. The current investigation thus represents the first successful molecular detection of E. cuniculi genomes from organ material of free-living wild rabbits worldwide and demonstrates that the wild rabbits examined were not only exposed to the pathogen but were actually infected. In 10 of the 158 (6.3%) wild rabbits, Ct values for E. cuniculi were obtained in the real-time PCR assay used. These results were subsequently confirmed by nested PCR in 7 out of the 10 rabbits. In addition, the detection in the kidney material, in particular, indicates that wild rabbits are not only a reservoir of the pathogen but, as has been proven in domestic rabbits already [44] are most likely also a source of infection due to the potential excretion of spores via the urine. Some rabbits within the present investigation showed Ct values by real-time PCR and did not reveal detectable antibody titres in the IFAT at the same time. Similar results of lacking antibody detections have been reported already in the past [45,46]. The potential reasons may only be speculated. Besides false-negative IFAT results caused by individual serum-inherent inhibition and false-positive PCR results, which were excluded as far as possible in the current study by a further confirmatory PCR test, causes may be an as-yet undetectable antibody level, an insufficient amount of spores ingested for seroconversion [45] or immunosuppressive effects of other diseases that prevent antibody production [45,46]. Excessive binding of antibodies by the pathogen could also play a role [46].
Up to the investigation presented here, there have been three prevalence studies on E. cuniculi in European wild rabbits based on DNA testing, all of which were conducted in Spain (including Tenerife). In addition, molecular methods have been used in a prevalence study of Eastern cottontail rabbits in Italy [47]. In one of the three studies from Spain, E. cuniculi DNA was detected by PCR in 2 of the 50 faecal samples examined [48]. However, the positive faecal samples originated from wild rabbits temporarily housed on farms in close contact with each other, which means that there might have been an increased risk of transmission. In the same study, E. cuniculi could not be detected in faecal samples originating from free-living wild rabbits, but other unknown microsporidian species were detected in five of these rabbits [48]. In the two other studies from Spain (0%, 0/383 [49], 0/438 [50]), DNA from E. cuniculi could not be detected [49,50]. However, only kidney tissue [49] or faecal samples were analysed [50]. In a former investigation conducted in Italy [47] on Eastern cottontail rabbits, a lagomorph species introduced for hunting purposes from North America to Italy in the 1960s, E. cuniculi was detected from organ tissues by conventional PCR in almost 10% of individuals that were tested. In this study, in addition to the brain and kidneys, the pathogen was also detected in skeletal muscle [47], which provides an interesting finding with regard to the potential risk of infection for humans and animals through the consumption of muscle meat. In histopathological studies on 34 European wild rabbits from Spain [51] and 62 European wild rabbits from England [52], no spores of E. cuniculi were detected in any of the tissue samples examined. In the study carried out in Spain, a broad range of organs of the wild rabbits were screened [51]. In the study carried out in England, only the kidneys were screened [52]. In both studies, the tissue samples were stained with hematoxylin and eosin (HE) and examined using light microscopy [51,52].
E. cuniculi genotype 1 (“rabbit strain”) was detected in both the brain and kidney tissue of one rabbit in the present study. This represents the first description of E. cuniculi genotypes in free-living wild rabbits. In previous studies on wild rabbits, genotyping of E. cuniculi was only carried out in one study conducted in Spain from faecal samples. Genotype 1 was also detected in this study, but only in wild rabbits temporarily housed on farms, which may have an increased risk of infection and other possible sources of infection compared to those in the wild [48].
Studies in wild rabbits in which both antibody determination and molecular methods were used for comparison were not available so far. Our study allowed for the first time a comparison of both antibody determination and PCR testing to assess whether wild rabbits are affected by the pathogen and thus pose a potential risk of infection to other animals and humans.
Antibodies against E. cuniculi were detected in 24 of the 158 wild rabbits in the current study, in 12 rabbits with both IgG and IgM, in 10 rabbits with exclusively IgG and in 2 rabbits with exclusively IgM (Table 2). Most prevalence studies, both in domesticated and wild rabbits, are based on the detection of antibodies, partly because this investigation can easily be performed antemortem. The interpretation of antibody detection is difficult, as it basically only proves exposure to the pathogen in the first place [53]. Simultaneous testing of IgG and IgM antibodies can provide an indication of infection status. The exclusive detection of antibodies of the IgM isotype serves as an indication of an early, acute infection. If both IgM and IgG isotype antibodies are detected, this indicates an acute infection. If only antibodies of the IgG isotype are present, this is an indication of a latent/chronic infection [10,54,55]. In the current study, solely IgM antibodies were detected in two of the wild rabbits examined, suggesting an early infection. Previous antibody determinations in wild rabbits were most often tested only for IgG antibodies, possibly leaving early-stage infections undetected.
Previous global serological studies on E. cuniculi in wild rabbits reported seroprevalences ranging from 3.9 to 100% in the UK (100%; 3/3) [56], Germany (18.1%, 28/155) [37], France (3.9%; 8/204) [57], Australia (24.7%; 20/81) [58] and Slovakia (44.7%; 21/47) [59], but no seropositive rabbits at all were found in further studies in Australia (0%, 0/823) [60], New Zealand (0%, 0/57) [60], the UK (0%, 0/175 [61]; 0/27 [62]; 0/60 [63]) and Germany (0%, 0/100) [38], suggesting that wild rabbits do not generally serve as a reservoir for the pathogen. In comparison to these varying results in wild rabbits, antibodies are regularly observed in domestic rabbits, especially pet rabbits, and often with high prevalence [64]. Due to the primarily high seroprevalences among domestic rabbits, some authors have suggested that the pathogen may be transmitted from domestic rabbits to wild rabbits [38,65]. In contrast, other researchers even suspect that wild rabbits are the natural reservoir of the pathogen, as they tested wild rabbits as seropositive, in which contact with domestic rabbits was virtually ruled out [57]. The partially lower seroprevalence in wild rabbits could be due to a lower population density and a consequently lower infection risk compared to domestic rabbits, as high prevalence has been found in domestic rabbits, particularly in connection with overstocking and presumably associated urine contamination [66]. An additional factor to consider that could limit the prevalence in the wild population is that wild rabbits with manifest encephalitozoonosis are most likely more susceptible to predation and, therefore, may not be among the rabbits studied [38]. This would also partially limit the possibility of diseased rabbits continuing to excrete spores, which in turn could infect other rabbits.
In the current study, E. cuniculi DNA was detected in the brain and kidneys of only 2 of the 24 rabbits that tested positive for antibodies using IFAT. This could occur due to very low spore concentration and/or uneven distribution in the tissue under study [45]. It is possible that the animals were still in the early phase of infection or were exposed to the pathogen but were able to fight off the infection, which may have prevented high spore numbers and their detection in the organs. Some authors report that spores are less abundant in the tissues concerned when infection progresses [19,20], which could also lead to a lower positive rate.
E. hellem is a potential pathogen that occurs mainly in birds and humans, but occasionally also in mammals. In Lagomorpha, both domesticated and wild, natural infection with E. hellem has so far only been detected in the kidney tissue of a single European brown hare, which was simultaneously infected with E. intestinalis. The researchers had not expected to find these pathogens, as the kidney lesions initially indicated an infection with E. cuniculi [27].
To the best of our knowledge, the present study presents the first successful detection of E. hellem in rabbits. A total of 4 of the 158 wild rabbits showed Ct values using real-time PCR. Although the Ct values are relatively high (Supplementary Table S3), the real-time PCR used is very sensitive, and no non-specific reactions between E. hellem and E. cuniculi have been documented [41]. The nested PCR used in this investigation also confirmed these results with corresponding bands in three of the four rabbits visible after agarose gel electrophoresis. For the identification of E. hellem, only molecular methods were used here, as there is no established antibody test for this pathogen in rabbits available. In general, knowledge of E. hellem in rabbits is very limited so far. The few studies published originate from Spain (including Tenerife). They were also based on molecular testing methods, and E. hellem could not be detected (0%, 0/383 [49], 0/50 [48], 0/438 [50]).
Further large-scale studies on this pathogen—for both domesticated and wild rabbits—could provide an important insight into the actual spread of E. hellem and also supply information on a possible relevance as a pathogen for rabbits.
There were some limitations of the present study that should be mentioned. The sample areas of this study were distributed as far as possible across different regions of Bavaria. However, an even distribution of samples was not achieved. Wild rabbits in Bavaria, as well as in many other states in Germany, have been eradicated or at least very severely decimated in some areas due to epidemics such as rabbit haemorrhagic disease or myxomatosis [67]. The largest proportion of the samples, therefore, originated from the Munich area, as there are still quite large wild rabbit populations in this region. Nevertheless, many counties could be included in the investigation (Figure 1).
The sample material used from the brain and kidneys is very suitable for testing for E. cuniculi, but E. hellem has so far been isolated primarily from faecal samples, even if there is also evidence from organ material [4]. It is, therefore, possible that faeces or intestinal tissue are more appropriate for the detection of this pathogen and should, therefore, be additionally included in future investigations. The inclusion of the eyes of the animals in the sample material specifically for the detection of E. cuniculi could possibly further increase the detection rate.
5. Conclusions
The results of this study show that both E. cuniculi and E. hellem are present in wild rabbits in Bavaria. This study provides the first E. cuniculi genotype determination in free-living wild rabbits worldwide and, in addition, the first evidence of E. hellem in rabbits worldwide. Wild rabbits should, therefore, be regarded as a reservoir for both pathogens and, on the basis of molecular evidence from kidney tissue and presumed urine excretion, also as a source of infection for E. cuniculi for animals and humans. For domestic rabbits, the possibility of infection via wild rabbits by outdoor housing or contaminated fresh feed from meadows arises. For immunocompromised persons (YOPI group) in particular, hygienic safety precautions should be considered when in contact with wild rabbits, their food products and excretions. Further studies covering larger parts of Germany would be desirable to gain deeper insights into the circulation of the pathogens in the wild rabbit population.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani14192880/s1, Table S1. Location and number of rabbits sampled from these locations; Table S2. Real-time PCR, Ct values of samples tested positive for E. cuniculi DNA; Table S3. Real-time PCR, Ct values of samples tested positive for E. hellem DNA; Figure S1. Agarose gel electrophoresis of the second round of nested PCR II showing specific PCR products of E. cuniculi strain (expected size about 0.3 kb); Figure S2. Agarose gel electrophoresis of the second round of nested PCR I showing specific PCR products of E. hellem strain (expected size about 0.3 kb).
Author Contributions
Conceptualization, K.B., M.R. and R.K.; methodology, K.B. and M.R.; investigation, K.B. and M.R.; data curation, K.B. and M.R.; writing—original draft preparation, K.B., M.R. and R.K.; writing—review and editing, K.B., M.R. and R.K.; supervision, R.K.; funding acquisition, K.B. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bavarian Academy for Hunting and Nature, grant number 8200483.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Ludwig-Maximilians-Universität in Munich (protocol code 317-30-06-2022, 26.08.2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials. Sequence information is found in GenBank (accession numbers PQ214196 and PQ214197).
Acknowledgments
First of all, we would like to thank the Bavarian Academy for Hunting and Nature (BAJN) for partial funding of the study and the Bavarian Hunting Society for their outstanding support in providing the wild rabbit samples for testing. We also gratefully acknowledge the most fruitful support by J. Reddemann and the BAJN board. Also, we would like to thank SYNLAB.vet GmbH (Augsburg), especially I. Schwarze, for the continuous and most helpful cooperation and professional exchange. Last but not least, we would like to thank our colleagues at the laboratory and pathology section of the Clinic for Birds, Small Mammals, Reptiles and Ornamental Fish of LMU Munich for their great support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wright, J.H.; Craighead, E.M. Infectious motor paralysis in young rabbits. J. Exp. Med. 1922, 36, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Mathis, A.; Weber, R. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 2000, 6, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Weber, R.; Deplazes, P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005, 18, 423–445. [Google Scholar] [CrossRef]
- Hinney, B.; Sak, B.; Joachim, A.; Kváč, M. More than a rabbit’s tale—Encephalitozoon spp. in wild mammals and birds. Int. J. Parasitol. Parasites Wildl. 2016, 5, 76–87. [Google Scholar] [CrossRef]
- Didier, E.S.; Vossbrinck, C.R.; Baker, M.D.; Rogers, L.B.; Bertucci, C.D.; Shadduck, J.A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology 1995, 111, 411–421. [Google Scholar] [CrossRef]
- Talabani, H.; Sarfati, C.; Pillebout, E.; van Gool, T.; Derouin, F.; Menotti, J. Disseminated infection with a new genovar of Encephalitozoon cuniculi in a renal transplant recipient. J. Clin. Microbiol. 2010, 48, 2651–2653. [Google Scholar] [CrossRef] [PubMed]
- Valencakova, A.; Balent, P.; Ravaszova, P.; Horak, A.; Obornik, M.; Halanova, M.; Malcekova, B.; Novotny, F.; Goldova, M. Molecular identification and genotyping of Microsporidia in selected hosts. Parasitol. Res. 2012, 110, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chai, Y.; Xiang, L.; Wang, W.; Zhou, Z.; Liu, H.; Zhong, Z.; Fu, H.; Peng, G. First identification and genotyping of Enterocytozoon bieneusi and Encephalitozoon spp. in pet rabbits in China. BMC Vet. Res. 2020, 16, 212. [Google Scholar] [CrossRef]
- Sokolova, O.I.; Demyanov, A.V.; Bowers, L.C.; Didier, E.S.; Yakovlev, A.V.; Skarlato, S.O.; Sokolova, Y.Y. Emerging microsporidian infections in Russian HIV-infected patients. J. Clin. Microbiol. 2011, 49, 2102–2108. [Google Scholar] [CrossRef]
- Cox, J.C.; Hamilton, R.C.; Attwood, H.D. An investigation of the route and progression of Encephalitozoon cuniculi infection in adult rabbits. J. Protozool. 1979, 26, 260–265. [Google Scholar] [CrossRef]
- Kimura, M.; Aoki, M.; Ichikawa-Seki, M.; Matsuo, K.; Yagita, K.; Itagaki, T. Detection and genotype of Encephalitozoon cuniculi DNA from urine and feces of pet rabbits in Japan. J. Vet. Med. Sci. 2013, 75, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in Humans. Clin. Microbiol. Rev. 2021, 34, e0001020. [Google Scholar] [CrossRef] [PubMed]
- Harcourt-Brown, F. Encephalitozoon cuniculi infection in rabbits. Semin. Avian. Exot. Pet. Med. 2004, 13, 86–93. [Google Scholar] [CrossRef]
- Marcato, P.S.; Rosmini, R. Patologia del Coniglio e della Lepre: Atlante a Colori e Compendio; Società Editrice Esculapio: Bologna, Italy, 1986. [Google Scholar]
- Ozkan, O.; Karagoz, A.; Kocak, N. First molecular evidence of ocular transmission of Encephalitozoonosis during the intrauterine period in rabbits. Parasitol. Int. 2019, 71, 1–4. [Google Scholar] [CrossRef]
- Scharmann, W.; Reblin, L.; Griem, W. Untersuchungen über die Infektion von Kaninchen durch Enzephalitozoon cuniculi. Berl. Münch. Tierärztl. Wschr. 1986, 99, 20–24. [Google Scholar]
- Cox, J.C.; Gallichio, H.A. Serological and histological studies on adult rabbits with recent, naturally acquired encephalitozoonosis. Res. Vet. Sci. 1978, 24, 260–261. [Google Scholar] [CrossRef]
- Eröksüz, H.; Eröksüz, Y.; Metin, N.; Özer, H. Morphologic examinations of cases of naturally acquired encephalitozoonosis in a rabbit colony. Turk. J. Vet. Anim. Sci. 1999, 23, 191–196. [Google Scholar]
- Csokai, J.; Gruber, A.; Künzel, F.; Tichy, A.; Joachim, A. Encephalitozoonosis in pet rabbits (Oryctolagus cuniculus): Pathohistological findings in animals with latent infection versus clinical manifestation. Parasitol. Res. 2009, 104, 629–635. [Google Scholar] [CrossRef]
- Flatt, R.E.; Jackson, S.J. Renal nosematosis in young rabbits. Path. Vet. 1970, 7, 492–497. [Google Scholar]
- Rodríguez-Tovar, L.E.; Nevárez-Garza, A.M.; Trejo-Chávez, A.; Hernández-Martínez, C.A.; Hernández-Vidal, G.; Zarate-Ramos, J.J.; Castillo-Velázquez, U. Encephalitozoon cuniculi: Grading the histological lesions in brain, kidney, and liver during primoinfection outbreak in rabbits. J. Pathog. 2016, 2016, 5768428. [Google Scholar] [CrossRef]
- Harcourt-Brown, F.M.; Holloway, H.K.R. Encephalitozoon cuniculi in pet rabbits. Vet. Rec. 2003, 152, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Weigt, A.; Vercelli, A.; Rondena, M.; Grilli, G.; Giudice, C. Immunohistochemical identification of Encephalitozoon cuniculi in phacoclastic uveitis in four rabbits. Vet. Ophthalmol. 2005, 8, 271–275. [Google Scholar] [CrossRef]
- Künzel, F.; Gruber, A.; Tichy, A.; Edelhofer, R.; Nell, B.; Hassan, J.; Leschnik, M.; Thalhammer, J.G.; Joachim, A. Clinical symptoms and diagnosis of encephalitozoonosis in pet rabbits. Vet. Parasitol. 2008, 151, 115–124. [Google Scholar] [CrossRef]
- Didier, E.S.; Didier, P.J.; Friedberg, D.N.; Stenson, S.M.; Orenstein, J.M.; Yee, R.W.; Tio, F.O.; Davis, R.M.; Vossbrinck, C.; Millichamp, N. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J. Infect. Dis. 1991, 163, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.W.; Tio, F.O.; Martinez, J.A.; Held, K.S.; Shadduck, J.A.; Didier, E.S. Resolution of microsporidial epithelial keratopathy in a patient with AIDS. Ophthalmology 1991, 98, 196–201. [Google Scholar] [CrossRef]
- de Bosschere, H.; Wang, Z.; Orlandi, P.A. First diagnosis of Encephalitozoon intestinalis and E. Hellem in a European brown hare (Lepus europaeus) with kidney lesions. Zoonoses Public Health 2007, 54, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Tanner, I.; Weber, R.; Deplazes, P. Genetic and phenotypic intraspecific variation in the microsporidian Encephalitozoon hellem. Int. J. Parasitol. 1999, 29, 767–770. [Google Scholar] [CrossRef]
- Xiao, L.; Li, L.; Moura, H.; Sulaiman, I.; Lal, A.A.; Gatti, S.; Scaglia, M.; Didier, E.S.; Visvesvara, G.S. Genotyping Encephalitozoon hellem isolates by analysis of the polar tube protein gene. J. Clin. Microbiol. 2001, 39, 2191–2196. [Google Scholar] [CrossRef]
- Black, S.S.; Steinohrt, L.A.; Bertucci, D.C.; Rogers, L.B.; Didier, E.S. Encephalitozoon hellem in budgerigars (Melopsittacus undulatus). Vet. Pathol. 1997, 34, 189–198. [Google Scholar] [CrossRef]
- Phalen, D.N.; Logan, K.S.; Snowden, K.F. Encephalitozoon hellem infection as the cause of a unilateral chronic keratoconjunctivitis in an umbrella cockatoo (Cacatua alba). Vet. Ophthalmol. 2006, 9, 59–63. [Google Scholar] [CrossRef]
- Barton, C.E.; Phalen, D.N.; Snowden, K.F. Prevalence of Microsporidian Spores Shed by Asymptomatic Lovebirds: Evidence for a Potential Emerging Zoonosis. J. Avian Med. Surg. 2003, 17, 197–202. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, S.-S.; Lyoo, Y.S.; Park, H.-M. DNA detection and genotypic identification of potentially human-pathogenic microsporidia from asymptomatic pet parrots in South Korea as a risk factor for zoonotic emergence. Appl. Environ. Microbiol. 2011, 77, 8442–8444. [Google Scholar] [CrossRef] [PubMed]
- Snowden, K.; Phalen, D.N. Encephalitozoon infection in birds. Semin. Avian. Exot. Pet. Med. 2004, 13, 94–99. [Google Scholar] [CrossRef]
- Ozkan, O.; Ozkan, A.T.; Zafer, K. Encephalitozoonosis in New Zealand rabbits and potential transmission risk. Vet. Parasitol. 2011, 179, 234–237. [Google Scholar] [CrossRef]
- Berger Baldotto, S.; Cray, C.; Giannico, A.T.; Reifur, L.; Montiani-Ferreira, F. Seroprevalence of Encephalitozoon cuniculi Infection in Pet Rabbits in Brazil. J. Exot. Pet. Med. 2015, 24, 435–440. [Google Scholar] [CrossRef]
- Neuwirt, E. Ein Beitrag zur Diagnose der Encephalitozoonose (Nosematose) beim Kaninchen; Vergleich zwischen direkten und indirekten Nachweismethoden. Doctoral Thesis, Ludwig-Maximilians-Universität, München, Germany, 1988. [Google Scholar]
- Meyer-Breckwoldt, A. Epidemiologische und klinische Untersuchungen zur Enzephalitozoonose bei Zwergkaninchen. Doctoral Thesis, Tierärztliche Hochschule Hannover, Hannover, Germany, 1996. [Google Scholar]
- Fa, J.E.; Sharples, C.M.; Bell, D.J.; DeAngelis, D. An individual-based model of rabbit viral haemorrhagic disease in European wild rabbits (Oryctolagus cuniculus). Ecol. Model. 2001, 144, 121–138. [Google Scholar] [CrossRef]
- Chalupský, J.; Vávra, J.; Bedrník, P. Detection of antibodies to Encephalitozoon cuniculi in rabbits by the indirect immunofluorescent antibody test. Folia Parasitol. 1973, 20, 281–284. [Google Scholar]
- Leipig, M.; Matiasek, K.; Rinder, H.; Janik, D.; Emrich, D.; Baiker, K.; Hermanns, W. Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J. Vet. Diagn. Investig. 2013, 25, 16–26. [Google Scholar] [CrossRef]
- Katzwinkel-Wladarsch, S.; Lieb, M.; Helse, W.; Löscher, T.; Rinder, H. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1996, 1, 373–378. [Google Scholar] [CrossRef]
- Asakura, T.; Nakamura, S.; Ohta, M.; Une, Y.; Furuya, K. Genetically unique microsporidian Encephalitozoon cuniculi strain type III isolated from squirrel monkeys. Parasitol. Int. 2006, 55, 159–162. [Google Scholar] [CrossRef]
- Jeklova, E.; Leva, L.; Kovarcik, K.; Matiasovic, J.; Kummer, V.; Maskova, J.; Skoric, M.; Faldyna, M. Experimental oral and ocular Encephalitozoon cuniculi infection in rabbits. Parasitology 2010, 137, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Csokai, J.; Joachim, A.; Gruber, A.; Tichy, A.; Pakozdy, A.; Künzel, F. Diagnostic markers for encephalitozoonosis in pet rabbits. Vet. Parasitol. 2009, 163, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.; Flock, U.; Sauter-Louis, C.; Hartmann, K. Encephalitozoon cuniculi in rabbits in Germany: Prevalence and sensitivity of antibody testing. Vet. Rec. 2014, 174, 350. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Palese, V.; Trisciuoglio, A.; Cantón Alonso, C.; Ferroglio, E. Encephalitozoon cuniculi, Toxoplasma gondii and Neospora caninum infection in invasive Eastern Cottontail Rabbits Sylvilagus floridanus in Northwestern Italy. Vet. Parasitol. 2013, 197, 682–684. [Google Scholar] [CrossRef]
- Baz-González, E.; Martin-Carrillo, N.; García-Livia, K.; Abreu-Acosta, N.; Foronda, P. Molecular Detection of Microsporidia in Rabbits (Oryctolagus cuniculus) in Tenerife, Canary Islands, Spain. Biology 2022, 11, 1796. [Google Scholar] [CrossRef]
- Martínez-Padilla, A.; Caballero-Gómez, J.; Magnet, Á.; Gómez-Guillamón, F.; Izquierdo, F.; Camacho-Sillero, L.; Jiménez-Ruiz, S.; Del Águila, C.; García-Bocanegra, I. Zoonotic Microsporidia in Wild Lagomorphs in Southern Spain. Animals 2020, 10, 2218. [Google Scholar] [CrossRef]
- Rego, L.; Castro-Scholten, S.; Cano, C.; Jiménez-Martín, D.; Köster, P.C.; Caballero-Gómez, J.; Bailo, B.; Dashti, A.; Hernández-Castro, C.; Cano-Terriza, D.; et al. Iberian wild leporidae as hosts of zoonotic enteroparasites in Mediterranean ecosystems of Southern Spain. Zoonoses Public Health 2023, 70, 223–237. [Google Scholar] [CrossRef]
- Espinosa, J.; Ferreras, M.C.; Benavides, J.; Cuesta, N.; Pérez, C.; García Iglesias, M.J.; García Marín, J.F.; Pérez, V. Causes of Mortality and Disease in Rabbits and Hares: A Retrospective Study. Animals 2020, 10, 158. [Google Scholar] [CrossRef]
- Lamalle, A.; Haverson, V.A.; Hughes, K. Renal pathology in wild European rabbits. Vet. Rec. 2023, 193, e2948. [Google Scholar] [CrossRef]
- Latney, L.V.; Bradley, C.W.; Wyre, N.R. Encephalitozoon cuniculi in pet rabbits: Diagnosis and optimal management. Vet. Med. Res. Rep. 2014, 5, 169–180. [Google Scholar] [CrossRef]
- Kunstýř, I.; Lev, L.; Naumann, S. Humoral antibody response of rabbits to experimental infection with Encephalitozoon cuniculi. Vet. Parasitol. 1986, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Jeklova, E.; Jekl, V.; Kovarcik, K.; Hauptman, K.; Koudela, B.; Neumayerova, H.; Knotek, Z.; Faldyna, M. Usefulness of detection of specific IgM and IgG antibodies for diagnosis of clinical encephalitozoonosis in pet rabbits. Vet. Parasitol. 2010, 170, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M. Encephalitozoon cuniculi in wild European rabbits and a fox. Res. Vet. Sci. 1979, 26, 114. [Google Scholar] [CrossRef]
- Chalupský, J.; Vávrá, J.; Gaudin, J.C.; Vandewalle, P.; Arthur, C.P.; Guenezan, M.; Launay, H. Serological evidence of the occurrence of encephalitozoonosis and toxoplasmosis in the European wild rabbit (Oryctolagus cuniculus) in France. Bull. Soc. fr. parasitol. 1990, 8, 91–95. [Google Scholar]
- Thomas, C.; Finn, M.; Twigg, L.; Deplazes, P.; Thompson, R.C. Microsporidia (Encephalitozoon cuniculi) in wild rabbits in Australia. Aust. Vet. J. 1997, 75, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Balent, P.; Halanova, M.; Sedlakova, T.; Valencakova, A.; Cislakova, L. Encephalitozoon cuniculi infection in rabbits and laboratory mice in Eastern Slovakia. Bull. Vet. Inst. Pulawy 2004, 48, 113–116. [Google Scholar]
- Cox, J.C.; Pye, D.; Edmonds, J.W.; Shepherd, R. An investigation of Encephalitozoon cuniculi in the wild rabbit Oryctolagus cuniculus in Victoria, Australia. Epidemiol. Infect. 1980, 84, 295–300. [Google Scholar] [CrossRef]
- Cox, J.C.; Ross, J. A serological survey of Encephalitozoon cuniculi infection in the wild rabbit in England and Scotland. Res. Vet. Sci. 1980, 28, 396. [Google Scholar] [CrossRef]
- Blevins, M. Prevalence of Encephalitozoon cuniculi in a population of wild rabbits in Norfolk, England. Zoomed 2007, 7, 28–36. [Google Scholar]
- Bose, H.M.; Woodhouse, M.A.; Powell, R. Absence of Encephalitozoon cuniculi antibodies in wild rabbits in England. Vet. Rec. 2015, 177, 48. [Google Scholar] [CrossRef]
- Magalhães, T.R.; Pinto, F.F.; Queiroga, F.L. A multidisciplinary review about Encephalitozoon cuniculi in a One Health perspective. Parasitol. Res. 2022, 121, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Keeble, E.J.; Shaw, D.J. Seroprevalence of antibodies to Encephalitozoon cuniculi in domestic rabbits in the United Kingdom. Vet. Rec. 2006, 158, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Gannon, J. A survey of Encephalitozoon cuniculi in laboratory animal colonies in the United Kingdom. Lab. Anim. 1980, 14, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Frölich, K.; Thiede, S.; Kozikowski, T.; Jakob, W. A review of mutual transmission of important infectious diseases between livestock and wildlife in Europe. Ann. N. Y. Acad. Sci. 2002, 969, 4–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).