Circulating Endocannabinoids in Canine Cutaneous Mast Cell Tumor
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Endocannabinoids Analysis
2.3. Statistical Analysis
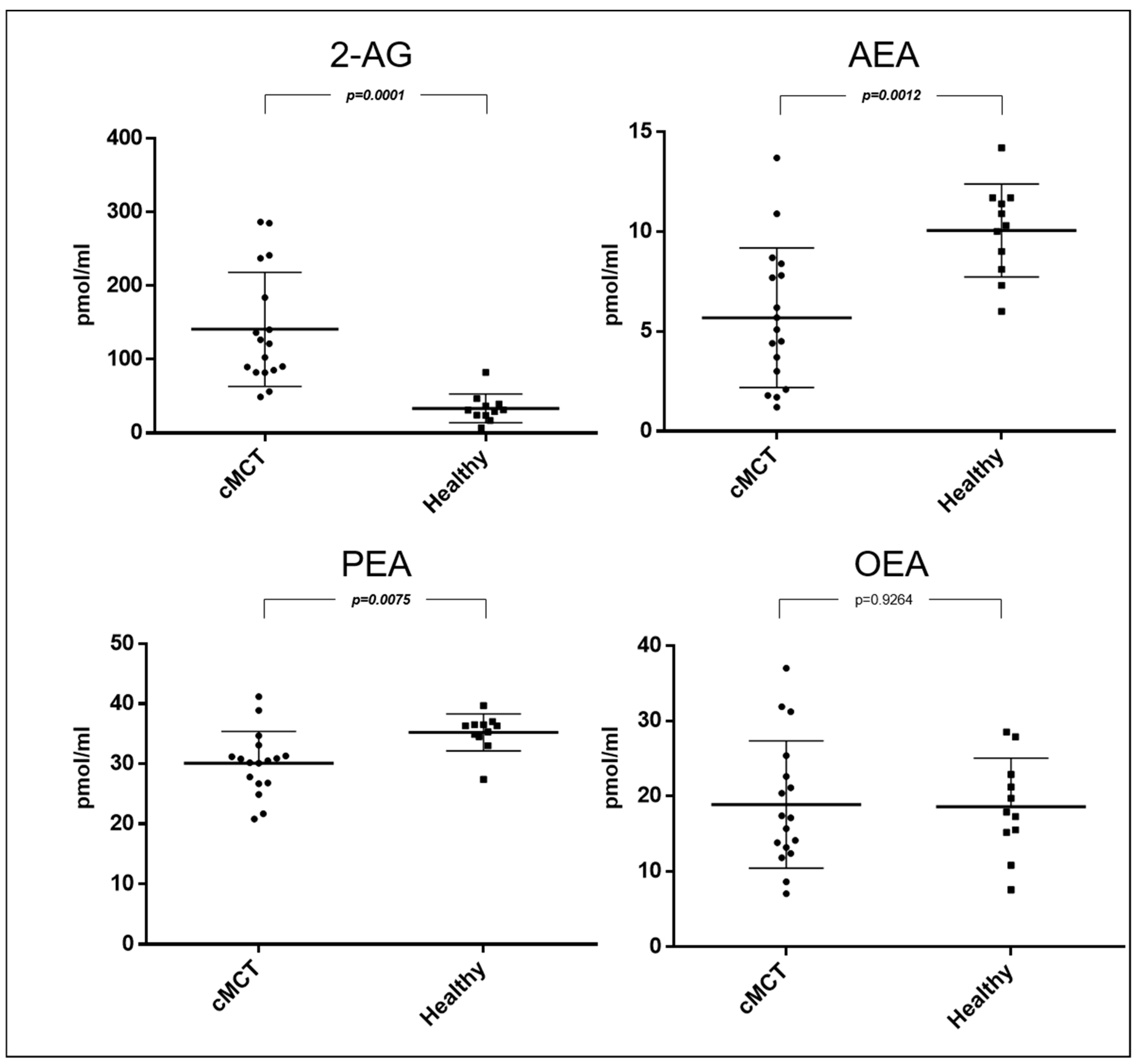
3. Results
3.1. Patient Population
3.2. Endocannabinoids and Related NAEs in Canine Plasma Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackwood, L.; Murphy, S.; Buracco, P.; De Vos, J.P.; De Fornel-Thibaud, P.; Hirschberger, J.; Kessler, M.; Pastor, J.; Ponce, F.; Savary-Bataille, K.; et al. European Consensus Document on Mast Cell Tumours in Dogs and Cats. Vet. Comp. Oncol. 2012, 10, e1–e29. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.M.; Scase, T.J. Advances in the Diagnosis and Management of Cutaneous Mast Cell Tumours in Dogs. J. Small Anim. Pract. 2007, 48, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.N. Veterinary Public Health Unit & WHO Collaborating Center for Comparative Oncology. In TNM Classification of Tumours in Domestic Animals; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- Kiupel, M.; Webster, J.D.; Bailey, K.L.; Best, S.; DeLay, J.; Detrisac, C.J.; Fitzgerald, S.D.; Gamble, D.; Ginn, P.E.; Goldschmidt, M.H.; et al. Proposal of a 2-Tier Histologic Grading System for Canine Cutaneous Mast Cell Tumors to More Accurately Predict Biological Behavior. Vet. Pathol. 2011, 48, 147–155. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Ehler, W.J.; MacEwen, E.G. Canine Cutaneous Mast Cell Tumor: Morphologic Grading and Survival Time in 83 Dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Sparkes, A.H.; Blunden, A.S.; Brearley, M.J.; Smith, K.C. Effects of Stage and Number of Tumours on Prognosis of Dogs with Cutaneous Mast Cell Tumours. Vet. Rec. 2006, 158, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Berlato, D.; Bulman-Fleming, J.; Clifford, C.A.; Garrett, L.; Intile, J.; Jones, P.; Kamstock, D.A.; Liptak, J.M.; Pavuk, A.; Powell, R.; et al. Value, Limitations, and Recommendations for Grading of Canine Cutaneous Mast Cell Tumors: A Consensus of the Oncology-Pathology Working Group. Vet. Pathol. 2021, 58, 858–863. [Google Scholar] [CrossRef]
- Kim, S.; Matsuyama, A. Canine Mast Cell Tumors: When to Worry about Aggressive Behavior Pre-Surgically. Can. Vet. J. 2022, 63, 1261–1263. [Google Scholar]
- Cheryl, A.L.; Douglas, H. Thamm Mast Cell Tumoors. In Small Animal Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 382–403. ISBN 978-0-323-59496-7. [Google Scholar]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid Metabolism in Cancer: New Perspectives and Emerging Mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef]
- Portavella, M.; Rodriguez-Espinosa, N.; Galeano, P.; Blanco, E.; Romero, J.I.; Holubiec, M.I.; Rodriguez de Fonseca, F.; Fernández-Espejo, E. Oleoylethanolamide and Palmitoylethanolamide Protect Cultured Cortical Neurons Against Hypoxia. Cannabis Cannabinoid Res. 2018, 3, 171–178. [Google Scholar] [CrossRef]
- Thabuis, C.; Tissot-Favre, D.; Bezelgues, J.-B.; Martin, J.-C.; Cruz-Hernandez, C.; Dionisi, F.; Destaillats, F. Biological Functions and Metabolism of Oleoylethanolamide. Lipids 2008, 43, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Campora, L.; Miragliotta, V.; Ricci, E.; Cristino, L.; Di Marzo, V.; Albanese, F.; Federica Della Valle, M.; Abramo, F. Cannabinoid Receptor Type 1 and 2 Expression in the Skin of Healthy Dogs and Dogs with Atopic Dermatitis. Am. J. Vet. Res. 2012, 73, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Río, C.D.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The Endocannabinoid System of the Skin. A Potential Approach for the Treatment of Skin Disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar] [CrossRef]
- Biener, I.; Mueller, T.T.; Lin, J.; Bao, H.; Steffen, J.; Hoerl, M.; Biere, K.; Matzel, S.; Woehrle, T.; König, S.; et al. Endocannabinoids, Endocannabinoid-like Compounds and Cortisone in Head Hair of Health Care Workers as Markers of Stress and Resilience during the Early COVID-19 Pandemic. Transl. Psychiatry 2024, 14, 71. [Google Scholar] [CrossRef]
- Febo, E.; Crisi, P.E.; Oddi, S.; Pietra, M.; Galiazzo, G.; Piscitelli, F.; Gramenzi, A.; Prinzio, R.D.; Di Tommaso, M.; Bernabò, N.; et al. Circulating Endocannabinoids as Diagnostic Markers of Canine Chronic Enteropathies: A Pilot Study. Front. Vet. Sci. 2021, 8, 655311. [Google Scholar] [CrossRef]
- Hay, J.K.; Hocker, S.E.; Monteith, G.; Woods, J.P. Circulating Endocannabinoids in Canine Multicentric Lymphoma Patients. Front. Vet. Sci. 2022, 9, 828095. [Google Scholar] [CrossRef]
- Sailler, S.; Schmitz, K.; Jäger, E.; Ferreiros, N.; Wicker, S.; Zschiebsch, K.; Pickert, G.; Geisslinger, G.; Walter, C.; Tegeder, I.; et al. Regulation of Circulating Endocannabinoids Associated with Cancer and Metastases in Mice and Humans. Oncoscience 2014, 1, 272–282. [Google Scholar] [CrossRef]
- Rinaldi, V.; Ressel, L.; Bongiovanni, L.; Crisi, P.E.; Boari, A.; Killick, D.; Chiocchetti, R.; Finotello, R. Cannabinoid Receptor-2 Expression in Canine Multicentric Diffuse Large B-Cell Lymphoma: An Immunohistochemical, Digital Pathology and Clinical Analysis. Res. Veter-Sci. 2024, 180, 105411. [Google Scholar] [CrossRef]
- Rinaldi, V.; Boari, A.; Ressel, L.; Bongiovanni, L.; Crisi, P.E.; Cabibbo, E.; Finotello, R. Expression of Cannabinoid Receptors CB1 and CB2 in Canine Cutaneous Mast Cell Tumours. Res. Vet. Sci. 2022, 152, 530–536. [Google Scholar] [CrossRef]
- Finotello, R.; Pasquini, A.; Meucci, V.; Lippi, I.; Rota, A.; Guidi, G.; Marchetti, V. Redox Status Evaluation in Dogs Affected by Mast Cell Tumour. Vet. Comp. Oncol. 2014, 12, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.J.; Wood, G.A.; Foster, R.A.; Coomber, B.L. Beclin-1 Is a Novel Predictive Biomarker for Canine Cutaneous and Subcutaneous Mast Cell Tumors. Vet. Pathol. 2022, 59, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Zamarian, V.; Ferrari, R.; Stefanello, D.; Ceciliani, F.; Grieco, V.; Minozzi, G.; Chiti, L.E.; Arigoni, M.; Calogero, R.; Lecchi, C. miRNA Profiles of Canine Cutaneous Mast Cell Tumours with Early Nodal Metastasis and Evaluation as Potential Biomarkers. Sci. Rep. 2020, 10, 18918. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Miller, R.A.; Kaneene, J.B.; Kiupel, M. Cellular Proliferation in Canine Cutaneous Mast Cell Tumors: Associations with c-KIT and Its Role in Prognostication. Vet. Pathol. 2007, 44, 298–308. [Google Scholar] [CrossRef]
- El-Agamy, D.S. Targeting C-Kit in the Therapy of Mast Cell Disorders: Current Update. Eur. J. Pharmacol. 2012, 690, 1–3. [Google Scholar] [CrossRef]
- London, C.A.; Seguin, B. Mast Cell Tumors in the Dog. Vet. Clin. North. Am. Small Anim. Pract. 2003, 33, 473–489. [Google Scholar] [CrossRef]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The Levels of the Endocannabinoid Receptor CB2 and Its Ligand 2-Arachidonoylglycerol Are Elevated in Endometrial Carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef]
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible Endocannabinoid Control of Colorectal Cancer Growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Fezza, F.; Theodoropoulou, M.; Grübler, Y.; Stalla, J.; Arzberger, T.; Milone, A.; Losa, M.; Di Marzo, V.; et al. Normal Human Pituitary Gland and Pituitary Adenomas Express Cannabinoid Receptor Type 1 and Synthesize Endogenous Cannabinoids: First Evidence for a Direct Role of Cannabinoids on Hormone Modulation at the Human Pituitary Level. J. Clin. Endocrinol. Metab. 2001, 86, 2687–2696. [Google Scholar] [CrossRef]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of Endocannabinoid System in Human Gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef]
- Yang, J.; Tian, Y.; Zheng, R.; Li, L.; Qiu, F. Endocannabinoid System and the Expression of Endogenous Ceramides in Human Hepatocellular Carcinoma. Oncol. Lett. 2019, 18, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Medina-Cleghorn, D.; Bernal-Mizrachi, L.; Bracci, P.M.; Hubbard, A.; Conde, L.; Riby, J.; Nomura, D.K.; Skibola, C.F. The Potential Relevance of the Endocannabinoid, 2-Arachidonoylglycerol, in Diffuse Large B-Cell Lymphoma. Oncoscience 2016, 3, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and Endocannabinoid-Related Mediators: Targets, Metabolism and Role in Neurological Disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide Counteracts Substance P-Induced Mast Cell Activation In Vitro by Stimulating Diacylglycerol Lipase Activity. J. Neuroinflamm. 2019, 16, 274. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The Nuclear Receptor Peroxisome Proliferator-Activated Receptor-Alpha Mediates the Anti-Inflammatory Actions of Palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Sarnelli, G.; Gigli, S.; Capoccia, E.; Iuvone, T.; Cirillo, C.; Seguella, L.; Nobile, N.; D’Alessandro, A.; Pesce, M.; Steardo, L.; et al. Palmitoylethanolamide Exerts Antiproliferative Effect and Downregulates VEGF Signaling in Caco-2 Human Colon Carcinoma Cell Line Through a Selective PPAR-α-Dependent Inhibition of Akt/mTOR Pathway. Phytother. Res. 2016, 30, 963–970. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Yang, K.; Chi, T.; Liao, Z.; Wei, P. PPAR-α Modulators as Current and Potential Cancer Treatments. Front. Oncol. 2021, 11, 599995. [Google Scholar] [CrossRef]
- Blaukat, A.; Barac, A.; Cross, M.J.; Offermanns, S.; Dikic, I. G Protein-Coupled Receptor-Mediated Mitogen-Activated Protein Kinase Activation through Cooperation of Galpha(q) and Galpha(i) Signals. Mol. Cell Biol. 2000, 20, 6837–6848. [Google Scholar] [CrossRef]
- Grommes, C.; Karlo, J.C.; Caprariello, A.; Blankenship, D.; Dechant, A.; Landreth, G.E. The PPARγ Agonist Pioglitazone Crosses the Blood-Brain Barrier and Reduces Tumor Growth in a Human Xenograft Model. Cancer Chemother. Pharmacol. 2013, 71, 929–936. [Google Scholar] [CrossRef]
- Pang, X.; Wei, Y.; Zhang, Y.; Zhang, M.; Lu, Y.; Shen, P. Peroxisome Proliferator-activated Receptor-γ Activation Inhibits Hepatocellular Carcinoma Cell Invasion by Upregulating Plasminogen Activator Inhibitor-1. Cancer Sci. 2013, 104, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Corsato Alvarenga, I.; Panickar, K.S.; Hess, H.; McGrath, S. Scientific Validation of Cannabidiol for Management of Dog and Cat Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Ukai, M.; McGrath, S.; Wakshlag, J. The Clinical Use of Cannabidiol and Cannabidiolic Acid-Rich Hemp in Veterinary Medicine and Lessons from Human Medicine. J. Am. Vet. Med. Assoc. 2023, 261, 623–631. [Google Scholar] [CrossRef] [PubMed]

| # | Breed | Sex | Age (Years) | Body Weight (Kg) | BCS | Histology | Antihistaminic Treatment | WHO Stage | Ulcer | Size |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cross breed | M | 13 | 25 | 6 | MCT high-grade | No | II | Yes | >3 cm |
| 2 | Labrador Retriever | F | 5 | 27 | 5 | MCT low-grade | No | I | No | <3 cm |
| 3 | Cross breed | NM | 10 | 25 | 7 | MCT low-grade | No | III | No | <3 cm |
| 4 | Boxer | M | 9 | 31 | 5 | MCT low-grade | No | I | No | <3 cm |
| 5 | Cross breed | M | 10 | 20 | 8 | MCT low-grade | No | I | No | <3 cm |
| 6 | Cross breed | F | 7 | 18 | 5 | MCT low-grade | No | III | Yes | >3 cm |
| 7 | Cross breed | SF | 10 | 23 | 5 | MCT low-grade | No | I | No | <3 cm |
| 8 | Cross breed | SF | 11 | 12 | 5 | MCT high-grade | Yes | II | No | <3 cm |
| 9 | Cross breed | SF | 7 | 5 | 6 | MCT low-grade | No | I | No | <3 cm |
| 10 | Poodle | SF | 6 | 3 | 5 | MCT low-grade | Yes | III | No | <3 cm |
| 11 | Shar-pei | NM | 5 | 30 | 6 | MCT high-grade | No | II | Yes | >3 cm |
| 12 | Pug | SF | 13 | 9 | 8 | MCT low-grade | No | III | Yes | <3 cm |
| 13 | Galgo | SF | 10 | 22 | 5 | MCT low-grade | Yes | I | No | <3 cm |
| 14 | Cross breed | SF | 6 | 7 | 5 | MCT low-grade | No | II | No | <3 cm |
| 15 | Cross breed | NM | 5 | 19 | 5 | MCT low-grade | Yes | III | No | <3 cm |
| 16 | English Setter | NM | 10 | 25 | 7 | MCT low-grade | No | III | No | <3 cm |
| 17 | Maltese | M | 9 | 6 | 7 | MCT low-grade | No | I | Yes | <3 cm |
| 2-AG | AEA | PEA | |
|---|---|---|---|
| Area | 0.9840 | 0.8529 | 0.8182 |
| Std. Error | 0.01921 | 0.07246 | 0.08843 |
| 95% Confidence Interval | 0.9463 to 1.022 | 0.7109 to 0.9950 | 0.6448 to 0.9915 |
| p Value | <0.0001 | 0.001916 | 0.005147 |
| Value | >52.75 | <6.750 | <32.15 |
| Sensitivity (%) | 94.12 | 64.71 | 76.47 |
| 95% CI | 71.31% to 99.85% | 38.33% to 85.79% | 50.10% to 93.19% |
| Specificity (%) | 90.91 | 90.91 | 90.91 |
| 95% CI | 58.72% to 99.77% | 58.72% to 99.77% | 58.72% to 99.77% |
| Likelihood Ratio | 10.35 | 7.118 | 8.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, V.; Piscitelli, F.; Boari, A.; Verde, R.; Crisi, P.E.; Bisogno, T. Circulating Endocannabinoids in Canine Cutaneous Mast Cell Tumor. Animals 2024, 14, 2986. https://doi.org/10.3390/ani14202986
Rinaldi V, Piscitelli F, Boari A, Verde R, Crisi PE, Bisogno T. Circulating Endocannabinoids in Canine Cutaneous Mast Cell Tumor. Animals. 2024; 14(20):2986. https://doi.org/10.3390/ani14202986
Chicago/Turabian StyleRinaldi, Valentina, Fabiana Piscitelli, Andrea Boari, Roberta Verde, Paolo Emidio Crisi, and Tiziana Bisogno. 2024. "Circulating Endocannabinoids in Canine Cutaneous Mast Cell Tumor" Animals 14, no. 20: 2986. https://doi.org/10.3390/ani14202986
APA StyleRinaldi, V., Piscitelli, F., Boari, A., Verde, R., Crisi, P. E., & Bisogno, T. (2024). Circulating Endocannabinoids in Canine Cutaneous Mast Cell Tumor. Animals, 14(20), 2986. https://doi.org/10.3390/ani14202986







