Spatial Ecology of a Resident Avian Predator During the Non-Breeding Period in Managed Habitats of Southeastern Europe
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
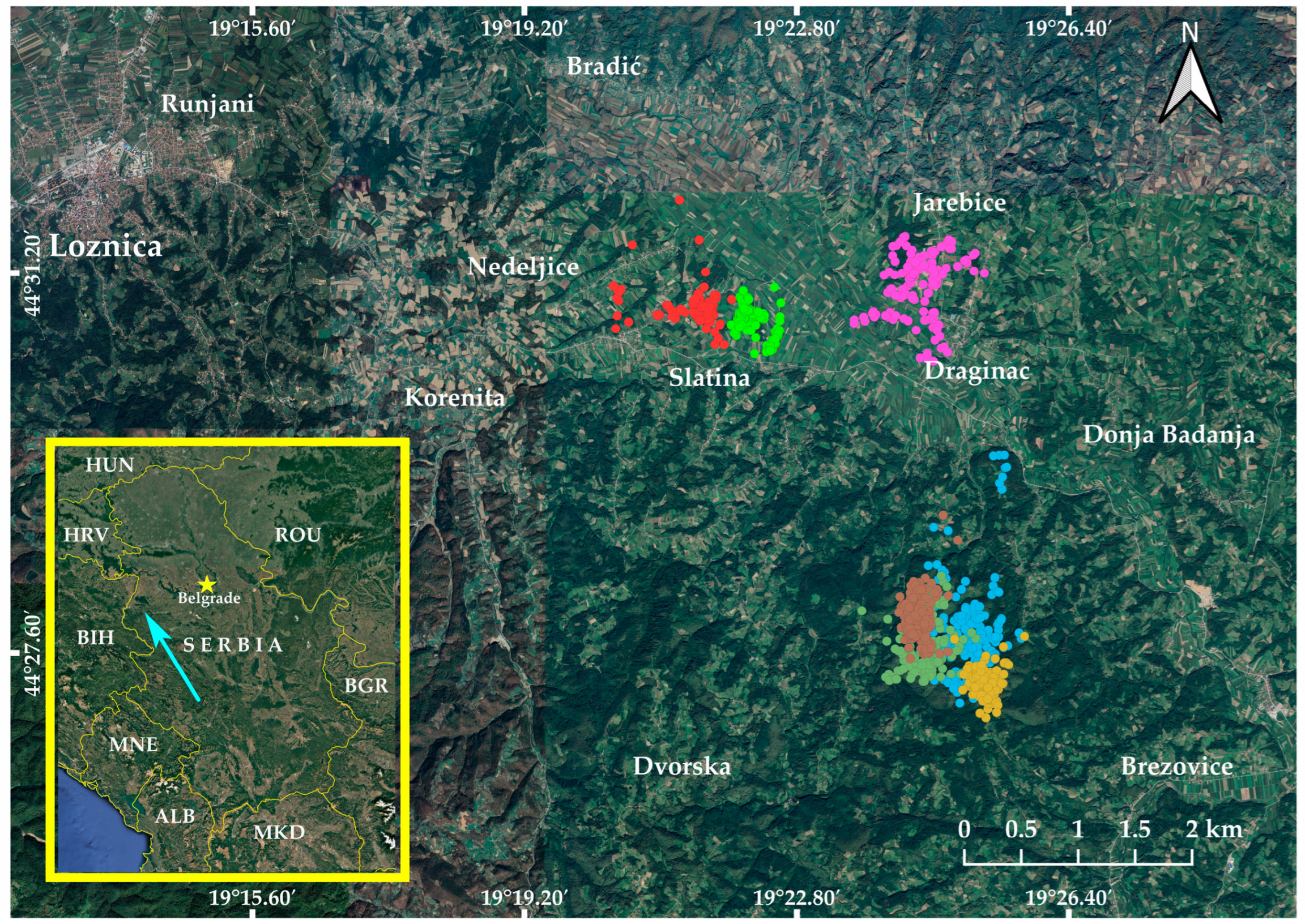
2.1. Study Area
2.2. Capturing, Tagging, and Data Collection
2.3. Estimation of Home Ranges and Spatial Overlap
2.4. Defining Habitat Selection and Roosting Sites
2.5. Additional Statistical Testing
3. Results
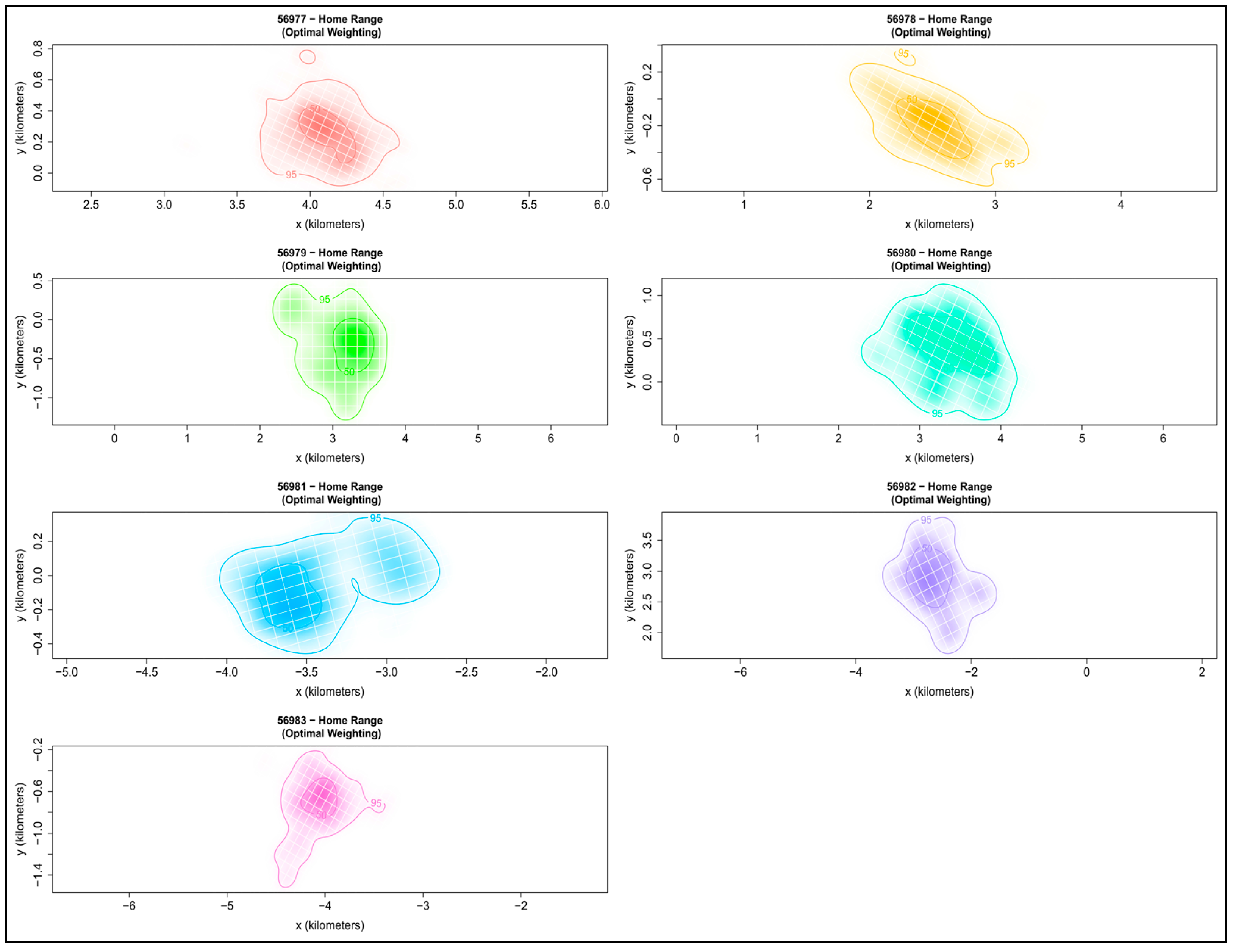
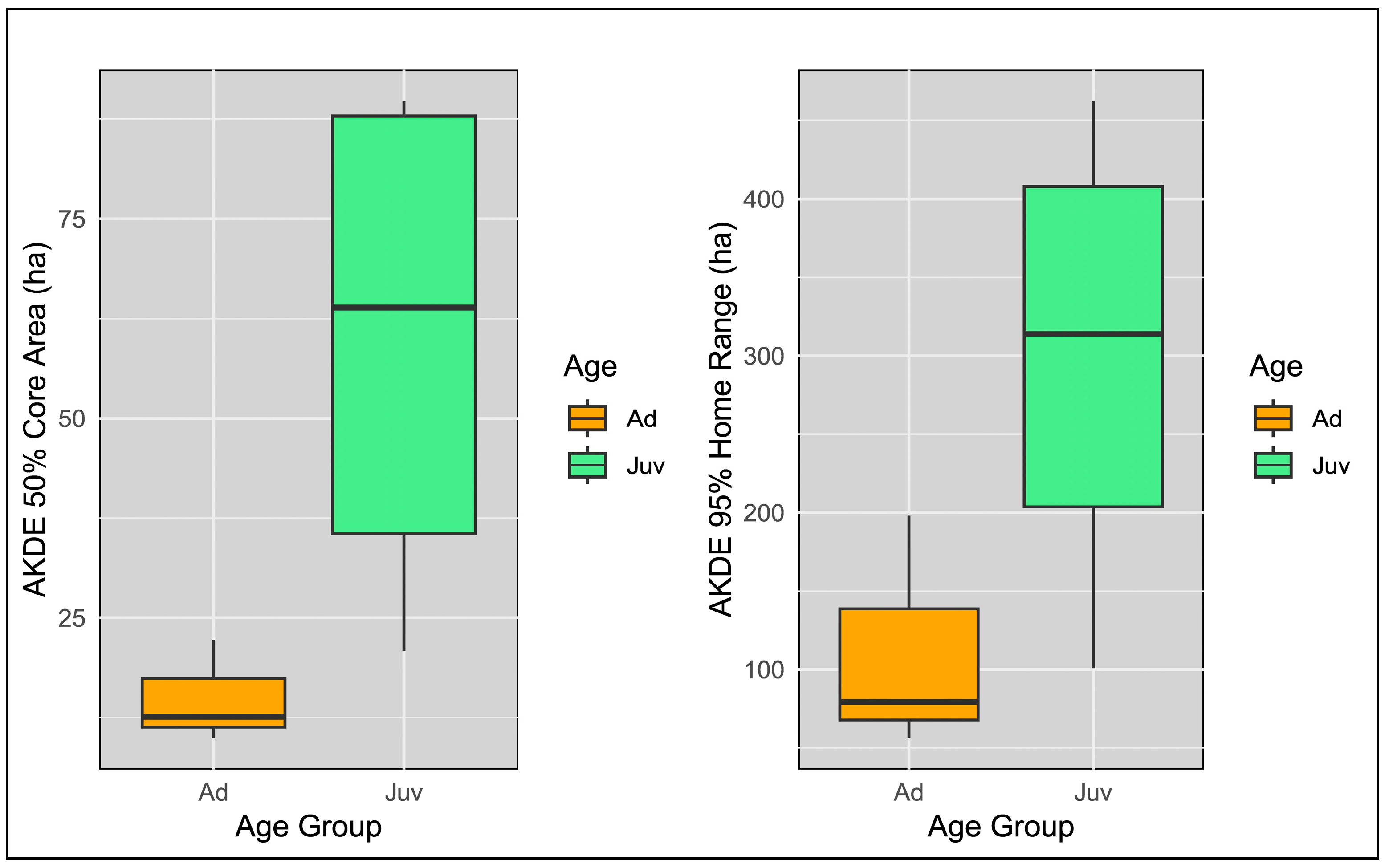
3.1. Home Range and Core Area Estimates
3.2. Habitat Selection
3.3. Roosts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, D.W. Toward an ecological synthesis: A case for habitat selection. Oecologia 2003, 136, 1–13. [Google Scholar] [CrossRef]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems, 4th ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 31–45. [Google Scholar]
- Cassini, M.H. Distribution Ecology: From Individual Habitat Use to Species Biogeographical Range, 1st ed.; Springer: New York, NY, USA, 2013; pp. 4–13. [Google Scholar]
- Tilman, D.; Lehman, C.L.; Kareiva, P. Population Dynamics in Spatial Habitats; Tilman, D., Kareiva, P., Eds.; Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions; Princeton University Press: Princeton, NJ, USA, 2018; pp. 3–20. [Google Scholar]
- Jarić, I.; Lennox, R.J.; Prchalová, M.; Monk, C.T.; Říha, M.; Nathan, R.; Arlinghaus, R. The power and promise of interdisciplinary international research networks to advance movement ecology. Mov. Ecol. 2023, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Crane, M.; Suwanwaree, P.; Strine, C.; Goode, M. Using dynamic Brownian Bridge Movement Models to identify home range size and movement patterns in king cobras. PLoS ONE 2018, 13, e0203449. [Google Scholar] [CrossRef] [PubMed]
- Slaght, J.C.; Surmach, S.G. Blakiston’s Fish-owl Bubo blakistoni and logging: Applying resource selection information to endangered species conservation in Russia. Bird Conserv. Int. 2016, 26, 214–224. [Google Scholar] [CrossRef]
- Horne, J.S.; Fieberg, J.; Börger, L.; Rachlow, J.L.; Calabrese, J.M.; Fleming, C.H. Animal home ranges: Concepts, uses, and estimation. In Population Ecology: In Practice, 1st ed.; Murray, D.L., Sandercock, B.K., Eds.; Wiley Blackwell: New York, NY, USA, 2020; pp. 315–332. [Google Scholar]
- Barnosky, A.D.; Hadly, E.A.; Bascompte, J.; Berlow, E.L.; Brown, J.H.; Fortelius, M.; Getz, W.M.; Harte, J.; Hastings, A.; Marquet, P.A.; et al. Approaching a state shift in Earth’s biosphere. Nature 2012, 486, 52–58. [Google Scholar] [CrossRef]
- Ellis, E.C.; Kaplan, J.O.; Fuller, D.Q.; Vavrus, S.; Goldewijk, K.K.; Verburg, P.H. Used planet: A global history. Proc. Natl. Acad. Sci. USA 2013, 110, 7978–7985. [Google Scholar] [CrossRef] [PubMed]
- Burt, W.H. Territoriality and home range concepts as applied to mammals. J. Mammal. 1943, 24, 346–352. [Google Scholar] [CrossRef]
- McNab, B.K. Bioenergetics and the Determination of Home Range Size. Am. Nat. 1963, 97, 133–140. [Google Scholar] [CrossRef]
- Börger, L.; Dalziel, B.D.; Fryxell, J.M. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol. Lett. 2008, 11, 637–650. [Google Scholar] [CrossRef]
- Maher, C.R.; Lott, D.F. A Review of Ecological Determinants of Territoriality within Vertebrate Species. Am. Midl. Nat. 2000, 143, 1–29. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds, 1st ed.; Academic Press: London, UK, 1998; pp. 45–94. [Google Scholar]
- Watson, J. The Golden Eagle, 2nd ed.; Bloomsbury Publishing: London, UK, 2010; pp. 126–133. [Google Scholar]
- Johansson, Ö.; Rauset, G.R.; Samelius, G.; McCarthy, T.; Andrén, H.; Tumursukh, L.; Mishra, C. Land sharing is essential for snow leopard conservation. Biol. Conserv. 2016, 203, 1–7. [Google Scholar] [CrossRef]
- Jacobson, O.T.; Crofoot, M.C.; Perry, S.; Kosmas, H.; Barrett, B.J.; Finerty, G. The Importance of Representative Sampling for Home Range Estimation in Field Primatology. Int. J. Primatol. 2024, 45, 213–245. [Google Scholar] [CrossRef]
- Bühler, R.; Schalcher, K.; Séchaud, R.; Michler, S.; Apolloni, N.; Roulin, A.; Almasi, B. Influence of prey availability on habitat selection during the non-breeding period in a resident bird of prey. Mov. Ecol. 2023, 11, 14. [Google Scholar] [CrossRef]
- Williams, P.J.; Gutiérrez, R.J.; Whitmore, S.A. Home range and habitat selection of spotted owls in the central Sierra Nevada. J. Wildl. Manag. 2011, 75, 333–343. [Google Scholar] [CrossRef]
- Forsman, E.D. Home Range and Habitat Selection by Northern Spotted Owls on the Eastern Slope of the Cascade Mountains, Washington. JRR 2015, 49, 109–128. [Google Scholar] [CrossRef]
- Monsarrat, S.; Benhamou, S.; Sarrazin, F.; Bessa-Gomes, C.; Bouten, W.; Duriez, O. How Predictability of Feeding Patches Affects Home Range and Foraging Habitat Selection in Avian Social Scavengers? PLoS ONE 2013, 8, e53077. [Google Scholar] [CrossRef]
- Krausman, P.R. Some Basic Principles of Habitat Use. In Proceedings—Grazing Behaviour of Livestock and Wildlife; Launchbaugh, K.L., Sanders, K.D., Mosley, J.L., Eds.; Idaho Forest, Wildlife and Range Experiment Station Collection; University of Idaho: Moscow, ID, USA, 1999; pp. 85–90. [Google Scholar]
- Rutz, C. Home range size, habitat use, activity patterns and hunting behaviour of urban-breeding Northern Goshawks Accipiter gentilis. Ardea 2006, 94, 185–202. [Google Scholar]
- Lok, T.; van der Geest, M.; de Goeij, P.; Rakhimberdiev, E.; Piersma, T. Sex-specific nest attendance rhythm and foraging habitat use in a colony-breeding waterbird. Behav. Ecol. 2024, 35, arae020. [Google Scholar] [CrossRef]
- Lowe, A.; Rogers, A.C.; Durrant, K.L. Effect of human disturbance on long-term habitat use and breeding success of the European Nightjar, Caprimulgus europaeus. ACE 2014, 9, 6. [Google Scholar] [CrossRef]
- Yuan, B.D.; Jiang, A.W.; Li, X.D.; Lu, C.H. Roost habitat selection by birds: A review. Chin. J Ecol. 2012, 31, 2145–2151. [Google Scholar]
- Morant, J.; Abad-Gómez, J.M.; Álvarez, T.; Sánchez, Á.; Zuberogoitia, I.; López-López, P. Winter movement patterns of a globally endangered avian scavenger in south-western Europe. Sci. Rep. 2020, 10, 17690. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, J. Field tests of theories concerning distributional control. Am. Nat. 1917, 51, 115–128. [Google Scholar] [CrossRef]
- Svärdson, G. Competition and habitat selection in birds. Oikos 1949, 1, 157–174. [Google Scholar] [CrossRef]
- Hildén, O. Habitat selection in birds: A review. Ann. Zool. Fenn. 1965, 2, 53–75. [Google Scholar]
- Kenward, R.E. Wildlife Radio Tagging: Equipment, Field Techniques and Data Analysis, 1st ed.; Academic Press: London, UK, 1987; pp. vii–ix. [Google Scholar]
- Kays, R.; Crofoot, M.C.; Jetz, W.; Wikelski, M. Terrestrial animal tracking as an eye on life and planet. Science 2015, 348, aaa2478. [Google Scholar] [CrossRef]
- Benson, E.S. Trackable life: Data, sequence, and organism in movement ecology. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2016, 57, 137–147. [Google Scholar] [CrossRef]
- Hebblewhite, M.; Haydon, D.T. Distinguishing technology from biology: A critical review of the use of GPS telemetry data in ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.A.; Thomas, L.; Wilcox, C.; Ovaskainen, O.; Matthiopoulos, L. State–space models of individual animal movement. Trends Ecol. Evol. 2008, 23, 87–94. [Google Scholar] [CrossRef]
- García-Ripollés, C.; López-López, P.; Urios, V. Ranging behaviour of non-breeding Eurasian Griffon Vultures Gyps fulvus: A GPS-telemetry study. Acta Ornithol. 2011, 46, 127–134. [Google Scholar] [CrossRef]
- Vignali, S.; Lörcher, F.; Hegglin, D.; Arlettaz, R.; Braunisch, V. Modelling the habitat selection of the bearded vulture to predict areas of potential conflict with wind energy development in the Swiss Alps. GECCO 2021, 25, e01405. [Google Scholar] [CrossRef]
- Morollón, S.; Urios, V.; López-López, P. Home-Range Size and Space Use of Territorial Bonelli’s Eagles (Aquila fasciata) Tracked by High-Resolution GPS/GSM Telemetry. Diversity 2022, 14, 1082. [Google Scholar] [CrossRef]
- Ronconi, R.A.; Lieske, D.J.; McFarlane Tranquilla, L.A.; Abbott, S.; Allard, K.A.; Allen, B.; Black, A.L.; Bolduc, F.; Davoren, G.K.; Diamond, A.W.; et al. Predicting Seabird Foraging Habitat for Conservation Planning in Atlantic Canada: Integrating Telemetry and Survey Data Across Thousands of Colonies. Front. Mar. Sci. 2022, 9, 816794. [Google Scholar] [CrossRef]
- König, C.; Weick, F.; Becking, J.-H. Owls—A Guide to the Owls of the World, 1st ed.; Pica Press: Sussex, UK, 1999; pp. 333–335. [Google Scholar]
- Butyev, V.T.; Zubkov, N.I.; Ivanchev, V.P.; Koblik, E.A.; Kovshar, A.F.; Kotyukov, Y.V.; Lyuleeva, D.S.; Nazarov, Y.N.; Nechaev, V.A.; Priklonsky, S.G.; et al. Strigiformes, Caprimulgiformes, Apodiformes, Coraciiformes, Upupiformes, Piciformes; KMK Scientific Publications: Moscow, Russia, 2005; p. 487. [Google Scholar]
- Mikkola, H. Owls of the Europe; T&AD Poyser: Calton UK, 1983; pp. 136–156. [Google Scholar]
- BirdLife International. Strix aluco (Europe assessment). The IUCN Red List of Threatened Species 2021: e.T22725469A166431700. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T22725469A166431700.en (accessed on 18 November 2024).
- Brito, P.H. The influence of Pleistocene glacial refugia on tawny owl genetic diversity and phylogeography in western Europe. Mol. Ecol. 2005, 14, 3077–3094. [Google Scholar] [CrossRef] [PubMed]
- Derlink, M.; Wernham, C.; Bertoncelj, I.; Kovács, A.; Saurola, P.; Duke, G.; Movalli, P.; Vrezec, A. A review of raptor and owl monitoring activity across Europe: Its implications for capacity building towards pan-European monitoring. Bird Study 2018, 65 (Suppl. S1), S4–S20. [Google Scholar] [CrossRef]
- Obuch, J. Spatial and temporal diversity of the diet of the tawny owl (Strix aluco). Slovak Raptor J. 2011, 5, 1–120. [Google Scholar] [CrossRef]
- Petty, S.J.; Shaw, G.; Anderson, D.I.K. Value of nest boxes for population studies and conservation of owls in coniferous forests in Britain. J. Raptor Res. 1994, 28, 134–142. [Google Scholar]
- Southern, H.N. The natural control of a population of tawny owls (Strix aluco). J. Zool. 1970, 162, 197–285. [Google Scholar] [CrossRef]
- Coles, C.F. Breeding, Survival, Movements and Foraging of Tawny Owls Strix aluco in a Managed Spruce Forest: A Spatial Approach. Ph.D. Thesis, Durham University, Durham, UK, October 2000. [Google Scholar]
- Solonen, T.; af Ursin, K. Breeding of Tawny Owls Strix aluco in rural and urban habitats in southern Finland. Bird Study 2008, 55, 216–221. [Google Scholar] [CrossRef]
- Redpath, S.M. Censusing Tawny Owls Strix aluco by the use of imitation calls. Bird Study 1994, 41, 192–198. [Google Scholar] [CrossRef]
- Zuberogoitia, I.A.; Martínez Clement, J.A. Methods for surveying Tawny Owl Strix aluco populations in large areas. Biota 2000, 1, 79–94. [Google Scholar]
- Vrezec, A.; Bertoncelj, I. Territory monitoring of Tawny Owls Strix aluco using playback calls is a reliable population monitoring method. Bird Study 2018, 65 (Suppl. S1), S52–S62. [Google Scholar] [CrossRef]
- Astaras, C.; Valeta, C.; Vasileiadis, I. Acoustic ecology of tawny owl (Strix aluco) in the Greek Rhodope Mountains using passive acoustic monitoring methods. Folia Oecologica 2022, 49, 110–116. [Google Scholar] [CrossRef]
- Pagaldai, N.; Arizaga, J.; Jiménez-Franco, M.V.; Zuberogoitia, I. Colonization of Urban Habitats: Tawny Owl Abundance Is Conditioned by Urbanization Structure. Animals 2021, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- López-Peinado, A.; Lis, Á.; Perona, A.M.; López-López, P. Habitat Preferences of the Tawny Owl (Strix aluco) in a Special Conservancy Area of Eastern Spain. J. Raptor Res. 2000, 54, 402–413. [Google Scholar] [CrossRef]
- Redpath, S.M. Habitat fragmentation and the individual: Tawny owls Strix aluco in woodland patches. J. Anim. Ecol. 1995, 64, 652–661. [Google Scholar] [CrossRef]
- Burgos, G.; Zuberogoitia, I. A telemetry study to discriminate between home range and territory size in Tawny Owls. Bioacoustics 2018, 29, 109–121. [Google Scholar] [CrossRef]
- Sunde, P.; Bølstad, M.S. A telemetry study of the social organization of a tawny owl (Strix aluco) population. J. Zool. 2004, 263, 65–76. [Google Scholar] [CrossRef]
- Sunde, P. What do we know about territorial behaviour and its consequences in Tawny Owls? In Ecology and Conservation of European Forest-Dwelling Raptors; Zuberogoitia, I., Je, M., Eds.; Diputación Foral de Bizkaia: Bilbao, Spain, 2011; pp. 253–260. [Google Scholar]
- Gajić, M.; Vujadinović, S.; Šabić, D. Contemporary demographic and functional transformations in the settlement net of Jadar. Glas. Srp. Geogr. Drus. 2011, 91, 43–68. [Google Scholar] [CrossRef]
- RHMZ. Meteorološki Godišnjak 1: Klimatološki Podaci; Republički Hidrometeorološki Zavod: Beograd, Serbia, 2022; p. 168. [Google Scholar]
- Lengagne, T.; Slater, P.J.B. The effects of rain on acoustic communication: Tawny owls have good reason for calling less in wet weather. Proc. R. Soc. B Biol. Sci. 2002, 269, 2121–2125. [Google Scholar] [CrossRef]
- Worthington-Hill, J.; Conway, G. Tawny Owl Strix aluco response to call-broadcasting and implications for survey design. Bird Study 2017, 64, 205–210. [Google Scholar] [CrossRef]
- Redpath, S.M.; Wyllie, I. Traps for Capturing Territorial Owls. J. Raptor Res. 1994, 28, 115–117. [Google Scholar]
- Demongin, L. Identification Guide to Birds in the Hand, 1st ed.; Laurent Demongin: Beauregard-Vendon, France, 2016; pp. 193–194. [Google Scholar]
- Kenward, R.E. Raptor Radio-Tracking and Telemetry; ICBP Technical Publication 5; ICBP: Cambridge, UK, 1985; pp. 409–420. [Google Scholar]
- Buehler, D.A.; Fraser, J.D.; Fuller, M.R.; McAllister, L.S.; Seegar, J.K.D. Captive and field-tested radio transmitter attachments for Bald Eagles. J. Field Ornithol. 1995, 66, 173–180. [Google Scholar]
- Jones, T.M.; Cooper, N.W.; Haradon, H.A.; Brunner, A.R.; Dossman, B.C.; Ward, M.P.; Sillett, T.S.; Kaiser, S.A. Considerations for radio-transmitter specifications on songbirds: Color and antenna length matter too. J. Field Ornithol. 2024, 95, 7. [Google Scholar] [CrossRef]
- Barron, D.G.; Brawn, J.D.; Weatherhead, P.J. Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 2010, 1, 180–187. [Google Scholar] [CrossRef]
- Gaunt, A.S.; Oring, L.W. Guidelines to the Use of Wild Birds in Research, 2nd ed.; Ornithological Council: Washington, DC, USA, 1999; p. 52. [Google Scholar]
- Geen, G.R.; Robinson, R.A.; Baillie, S.R. Effects of tracking devices on individual birds—A review of the evidence. J. Avian Biol. 2019, 50. [Google Scholar] [CrossRef]
- Sunde, P. Effects of Backpack Radio Tags on Tawny Owls. J. Wildl. Manag. 2006, 70, 594–599. [Google Scholar] [CrossRef]
- Walter, W.D.; Fischer, J.W.; Baruch-Mordo, S.; VerCauteren, K.C. What is the proper method to delineate home range of an animal using today’s advanced GPS telemetry systems: The initial step. Mod. Telem. 2011, 68, 249–268. [Google Scholar]
- Fleming, C.H.; Drescher-Lehman, J.; Noonan, M.J.; Akre, T.S.B.; Brown, D.J.; Cochrane, M.M.; Dejid, N.; DeNicola, V.; DePerno, C.S.; Dunlop, J.N.; et al. A comprehensive framework for handling location error in animal tracking data. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mohr, C.O. Table of Equivalent Populations of North American Small Mammals. Am. Nat. 1947, 37, 223–249. [Google Scholar] [CrossRef]
- Worton, B.J. A review of models of home range for animal movement. Ecol. Model. 1987, 38, 277–298. [Google Scholar] [CrossRef]
- Fleming, C.H.; Sheldon, D.; Fagan, W.F.; Leimgruber, P.; Mueller, T.; Nandintsetseg, D.; Noonan, M.J.; Olson, K.A.; Setyawan, E.; Sianipar, A.; et al. Correcting for missing and irregular data in home-range estimation. Ecol. App. 2018, 28, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Burgman, M.A.; Fox, J.C. Bias in species range estimates from minimum convex polygons: Implications for conservation and options for improved planning. Anim. Conserv. 2003, 6, 19–28. [Google Scholar] [CrossRef]
- Walter, W.D.; Onorato, D.P.; Fischer, J.W. Is there a single best estimator? Selection of home range estimators using area-under-the-curve. Mov. Ecol. 2015, 3, 10. [Google Scholar] [CrossRef]
- van Winkle, W. Comparison of several probabilistic home-range models. J. Wildl. Manag. 1975, 39, 118–123. [Google Scholar] [CrossRef]
- Worton, B.J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Fleming, C.H.; Fagan, W.F.; Mueller, T.; Olson, K.A.; Leimgruber, P.; Calabrese, J.M. Rigorous home range estimation with movement data: A new autocorrelated kernel density estimator. Ecology 2015, 96, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Noonan, M.J.; Tucker, M.A.; Fleming, C.H.; Akre, T.S.; Alberts, S.C.; Ali, A.H.; Altmann, J.; Antunes, P.C.; Belant, J.L.; Beyer, D.; et al. A comprehensive analysis of autocorrelation and bias in home range estimation. Ecol. Monogr. 2019, 89, e01344. [Google Scholar] [CrossRef]
- Silva, I.; Fleming, C.H.; Noonan, M.J.; Alston, J.; Folta, C.; Fagan, W.F.; Calabrese, J.M. Autocorrelation-informed home range estimation: A review and practical guide. Methods Ecol. Evol. 2022, 13, 534–544. [Google Scholar] [CrossRef]
- Calabrese, J.M.; Fleming, C.H.; Gurarie, E. ctmm: An R package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol. Evol. 2016, 7, 1124–1132. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 17 January 2024).
- ctmmweb: A Shiny Web App for the ctmm Movement Analysis Package. Available online: https://github.com/ctmm-initiative/ctmmweb (accessed on 25 August 2024).
- Calabrese, J.M.; Fleming, C.H.; Noonan, M.J.; Dong, X. ctmmweb: A Graphical User Interface for Autocorrelation-Informed Home Range Estimation. Wildl. Soc. Bull. 2021, 45, 162–169. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 72–132. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Hijmans, R.J. Geosphere: Spherical Trigonometry. 2022. Available online: https://CRAN.R-project.org/package=geosphere (accessed on 19 July 2024).
- Fieberg, J.; Kochanny, C.O. Quantifying Home-Range Overlap: The Importance of the Utilization Distribution. J. Wildl. Manag. 2005, 69, 1346–1359. [Google Scholar] [CrossRef]
- Winner, K.; Noonan, M.J.; Fleming, C.H.; Olson, K.; Mueller, T.; Sheldon, D.; Calabrese, J.M. Statistical inference for home range overlap. Methods Ecol. Evol. 2018, 9, 1679–1691. [Google Scholar] [CrossRef]
- Zvidzai, M.; Zengeya, F.M.; Masocha, M.; Ndaimani, H.; Murwira, A. Application of GPS occurrence data to understand African white-backed vultures Gyps africanus spatial home range overlaps. Ecol. Evol. 2022, 12, e8778. [Google Scholar] [CrossRef]
- Johnson, D.H. The comparison of usage and availability measurements for evaluating resource preference. Ecology 1980, 61, 65–71. [Google Scholar] [CrossRef]
- Lele, S.R.; Keim, J.L. Weighted Distributions and Estimation of Resource Selection Probability Functions. Ecology 2006, 87, 3021–3028. [Google Scholar] [CrossRef]
- Nemes, S.; Hartel, T. Summary measures for binary classification systems in animal ecology. North-West. J. Zool. 2010, 6, 323–330. [Google Scholar]
- Maindonald, J.H.; John Braun, W. DAAG: Data Analysis and Graphics Data and Functions Version 1.25.6. 2024. Available online: https://cran.r-project.org/web/packages/DAAG/index.html (accessed on 22 July 2024).
- Jennions, M.D.; Møller, A.P. A survey of the statistical power of research in behavioral ecology and animal behavior. Behav. Ecol. 2003, 14, 438–445. [Google Scholar] [CrossRef]
- Kouba, M.; Bartoš, L.; Štastný, K. Differential Movement Patterns of Juvenile Tengmalms Owls (Aegolius funereus) during the Post-Fledging Dependence Period in Two Years with Contrasting Prey Abundance. PLoS ONE 2013, 8, e67034. [Google Scholar] [CrossRef]
- Belthoff, J.R.; Sparks, E.J.; Ritchison, G. Home ranges of adult and juvenile Eastern Screech-owls: Size, seasonal variation and extent of overlap. J. Raptor Res. 1993, 27, 8–15. [Google Scholar]
- Marzluff, J.M.; Kimsey, B.A.; Schueck, L.S.; McFadzen, M.E.; Vekasy, M.S.; Bednarz, J.C. The Influence of Habitat, Prey Abundance, Sex, and Breeding Success on the Ranging Behavior of Prairie Falcons. Condor 1997, 99, 567–584. [Google Scholar] [CrossRef]
- Mirski, P.; Grosberg, J.; Kull, T.; Mellov, P.; Tõnisalu, G.; Väli, V.; Väli, Ü. Movement of avian predators points to biodiversity hotspots in agricultural landscape. R. Soc. Open Sci. 2024, 11, 231543. [Google Scholar] [CrossRef] [PubMed]
- La Haye, M.; Mertens, F.; Nieuwenweg, S.; Baeyens, G. Habitat use of Tawny Owls Strix aluco in the Amsterdam Waterwork Dunes, as shown by telemetry. Limosa 1995, 68, 73–76. [Google Scholar]
- Choi, W.S.; Sung, H.C.; Park, J.C.; Kim, W.Y. A first Home range and habitat characteristics of a breeding pair tawny owl: Case study using direct tracking in the Korean Peninsula. J. Asia-Pac. Biodivers. 2020, 13, 169–174. [Google Scholar] [CrossRef]
- Sunde, P.; Redpath, S.M. Combining information from range use and habitat selection: Sex-specific spatial responses to habitat fragmentation in tawny owls Strix aluco. Ecography 2006, 29, 152–158. [Google Scholar] [CrossRef]
- Vrezec, A.; Tome, D. Altitudinal segregation between Ural Owl Strix uralensis and Tawny Owl, S. aluco: Evidence for competitive exclusion in raptorial birds. Bird Study 2004, 51, 264–269. [Google Scholar] [CrossRef]
- Đorđević, N.; Popović, Z.; Beuković, D.; Beuković, M.; Đorđević, M. The Importance of Arable Land in Serbia to the Feed Pheasant and Brown Hare and Number of Population. In Proceedings of the XXVI Conference of Agronomists, Veterinarians, Technologists andAgricultural Economists, Belgrade, Serbia, 22–23 February 2012. [Google Scholar]
- Pruitt, M.L. Thermal benefits of roost site selection in a small forest owl. Wilson J. Ornithol. 2024, 135, 520–532. [Google Scholar] [CrossRef]
- Sunde, P.; Bølstad, M.S.; Desfor, K.B. Diurnal exposure as a risk sensitive behaviour in tawny owls Strix aluco? J. Avian Biol. 2003, 34, 409–418. [Google Scholar] [CrossRef]
- Yatsiuk, Y.; Wesołowski, T. Diversity and abundance of large tree holes used by Tawny Owls Strix aluco in lowland temperate forests. Bird Study 2021, 67, 331–343. [Google Scholar] [CrossRef]

| Individual ID | Capture Date | Age | Sex | Body Mass (g) | Habitat | Collected Fixes (n) | Tag Model | Transmission Period (Days) |
|---|---|---|---|---|---|---|---|---|
| Male_56977 | 28 October 2023 | Ad | Male | 488 | HFL | 269 | 240 | 55 |
| Male_56978 | 28 October 2023 | Juv | Male | 474 | HFL | 264 | 240 | 54 |
| Male_56979 | 29 October 2023 | Juv | Male | 446 | HFL | 180 | 240 | 46 |
| Male_56980 | 29 October 2023 | Juv | Male | 417 | HFL | 281 | 240 | 58 |
| Male_56981 | 30 October 2023 | Ad | Male | 486 | LAL | 213 | 120 | 45 |
| Male_56982 | 30 October 2023 | Juv | Male | 436 | LAL | 162 | 120 | 37 |
| Male_56983 | 2 November 2023 | Ad | Male | 467 | LAL | 157 | 120 | 35 |
| Individual ID | MCP (ha) | AKDE Core Area (ha) | AKDE Home Range (ha) |
|---|---|---|---|
| Male_56977 | 38.60 | 9.95 | 56.36 |
| Male_56978 | 64.33 | 20.80 | 100.84 |
| Male_56979 | 170.50 | 40.43 | 238.08 |
| Male_56980 | 285.65 | 87.33 | 462.23 |
| Male_56981 | 60.65 | 12.59 | 79.22 |
| Male_56982 | 254.52 | 89.74 | 389.85 |
| Male_56983 | 225.05 | 22.20 | 197.87 |
| Dyad | Individual I | Individual II | Home Range Overlap | 95% CI |
|---|---|---|---|---|
| 1 | Male_56977 | Male_56978 | 0 | 0 |
| 2 | Male_56977 | Male_56979 | 0.02 | 0.01–0.04 |
| 3 | Male_56977 | Male_56980 | 0.32 | 0.27–0.37 |
| 4 | Male_56977 | Male_56981 | 0 | - |
| 5 | Male_56977 | Male_56982 | 0 | - |
| 6 | Male_56977 | Male_56983 | 0 | - |
| 7 | Male_56978 | Male_56979 | 0.45 | 0.37–0.53 |
| 8 | Male_56978 | Male_56980 | 0.11 | 0.08–0.15 |
| 9 | Male_56978 | Male_56981 | 0 | - |
| 10 | Male_56978 | Male_56982 | 0 | - |
| 11 | Male_56978 | Male_56983 | 0 | - |
| 12 | Male_56979 | Male_56980 | 0.43 | 0.34–0.52 |
| 13 | Male_56979 | Male_56981 | 0 | - |
| 14 | Male_56979 | Male_56982 | 0 | - |
| 15 | Male_56979 | Male_56983 | 0 | - |
| 16 | Male_56980 | Male_56981 | 0 | - |
| 17 | Male_56980 | Male_56982 | 0 | - |
| 18 | Male_56980 | Male_56983 | 0 | - |
| 19 | Male_56981 | Male_56982 | 0 | - |
| 20 | Male_56981 | Male_56983 | 0.03 | 0.02–0.04 |
| 21 | Male_56982 | Male_56983 | 0 | - |
| Model No | Model Variables | AIC | ΔiAIC | Akaike w i | AUC | CV (%) |
|---|---|---|---|---|---|---|
| I | Cultivated land | 3926.127 | 2.336 | 0.119 | 0.595 | 0.602 |
| II | Suburban | 4083.266 | 159.475 | <0.001 | 0.515 | 0.602 |
| III | Altitude | 4079.353 | 155.562 | <0.001 | 0.545 | 0.602 |
| IV | Cultivated land + Suburban | 3923.791 | 0 | 3.841 | 0.601 | 0.602 |
| V | Cultivated land + Altitude | 3924.980 | 1.189 | 0.212 | 0.607 | 0.602 |
| VI | Suburban + Altitude | 4059.943 | 136.152 | <0.001 | 0.566 | 0.602 |
| VII | Cultivated land + Suburban + Altitude | 3924.391 | 0.600 | 0.284 | 0.607 | 0.602 |
| Covariate | Estimate | SE | z | p Value |
|---|---|---|---|---|
| (Intercept) | 0.263 | 0.043 | 6.117 | <0.001 |
| Suburban | 0.355 | 0.173 | 2.054 | 0.040 |
| Cultivated land | −1.231 | 0.101 | −12.118 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajković, D.Z.; Stanković, D.; Šeat, J.; Stevanović, D.S.; Andrejević Stošović, M.V.; Skorić, S. Spatial Ecology of a Resident Avian Predator During the Non-Breeding Period in Managed Habitats of Southeastern Europe. Animals 2024, 14, 3338. https://doi.org/10.3390/ani14223338
Rajković DZ, Stanković D, Šeat J, Stevanović DS, Andrejević Stošović MV, Skorić S. Spatial Ecology of a Resident Avian Predator During the Non-Breeding Period in Managed Habitats of Southeastern Europe. Animals. 2024; 14(22):3338. https://doi.org/10.3390/ani14223338
Chicago/Turabian StyleRajković, Draženko Z., Daliborka Stanković, Jelena Šeat, Dejan S. Stevanović, Miona V. Andrejević Stošović, and Stefan Skorić. 2024. "Spatial Ecology of a Resident Avian Predator During the Non-Breeding Period in Managed Habitats of Southeastern Europe" Animals 14, no. 22: 3338. https://doi.org/10.3390/ani14223338
APA StyleRajković, D. Z., Stanković, D., Šeat, J., Stevanović, D. S., Andrejević Stošović, M. V., & Skorić, S. (2024). Spatial Ecology of a Resident Avian Predator During the Non-Breeding Period in Managed Habitats of Southeastern Europe. Animals, 14(22), 3338. https://doi.org/10.3390/ani14223338






