Ecotoxicological Effects of Potassium Dichromate on the Tadpole Shrimp Triops longicaudatus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Test Solutions
2.2. Test Organisms
2.3. Exposure Assays
body weight (ABW) = (initial body weight + final body weight)/2
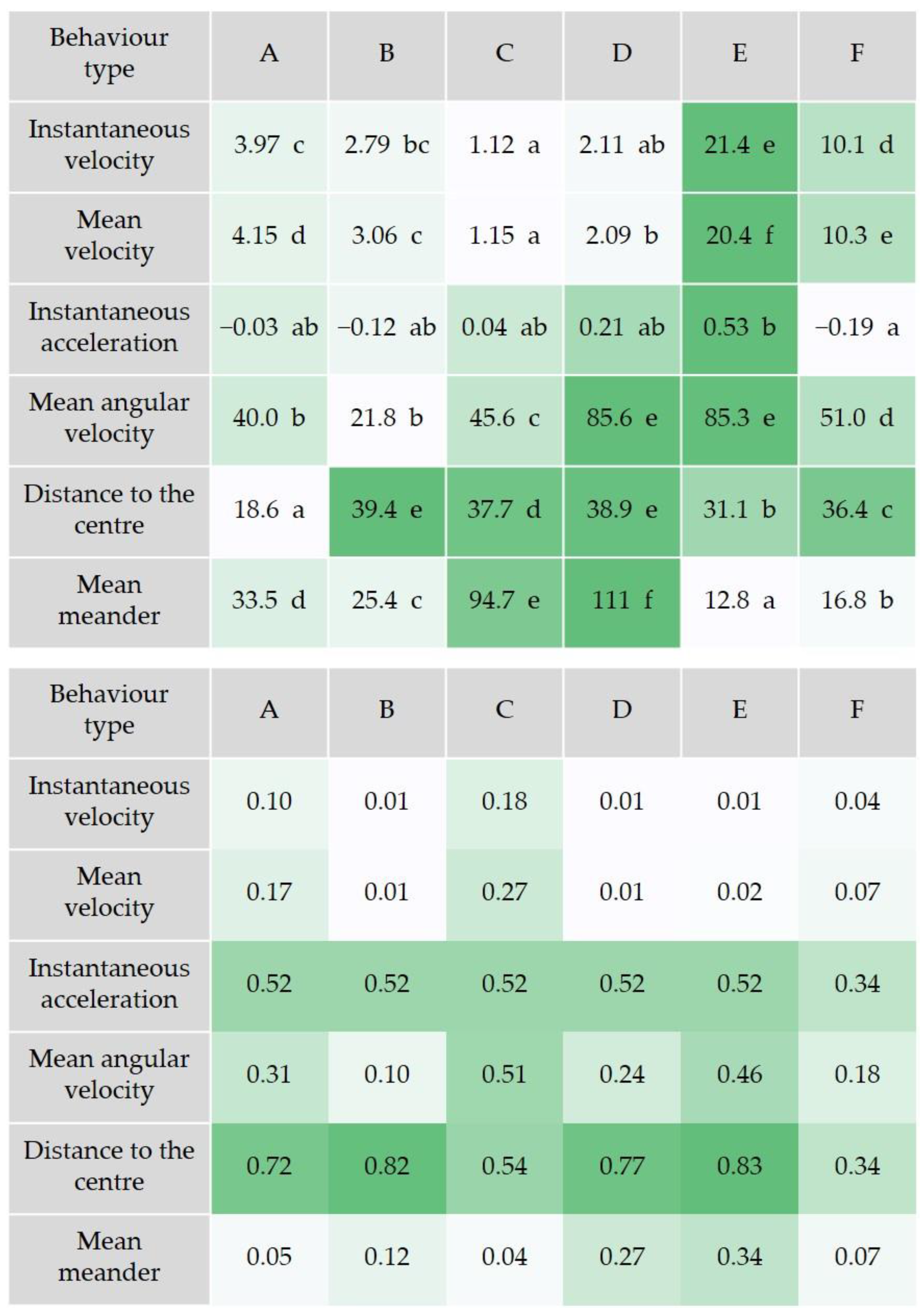
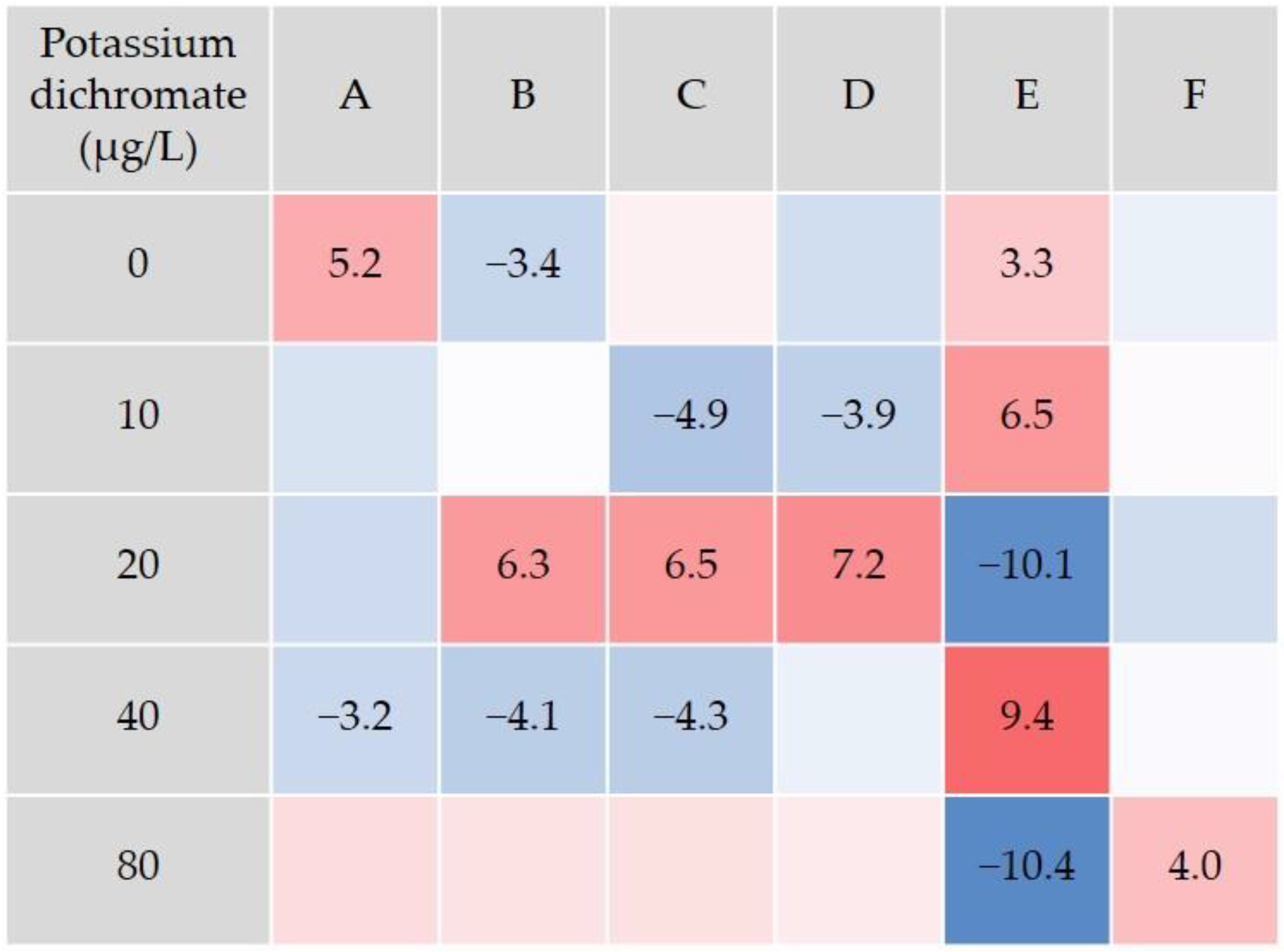
2.4. Locomotor Behavior Analysis
2.5. Post-Exposure Reproductive Assessment
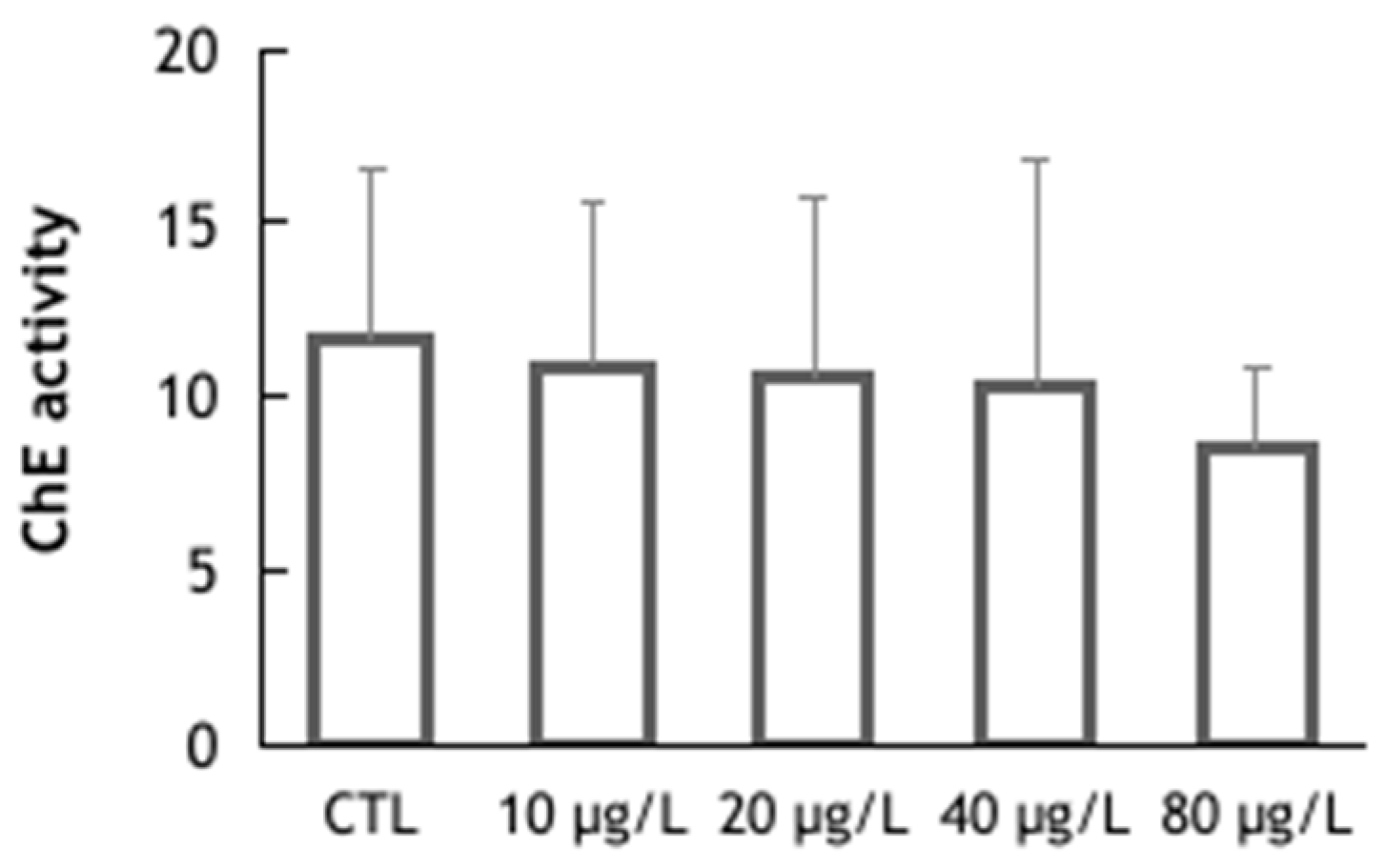
2.6. Cholinesterase (ChE) Activity Assessment
2.7. Statistical Analysis
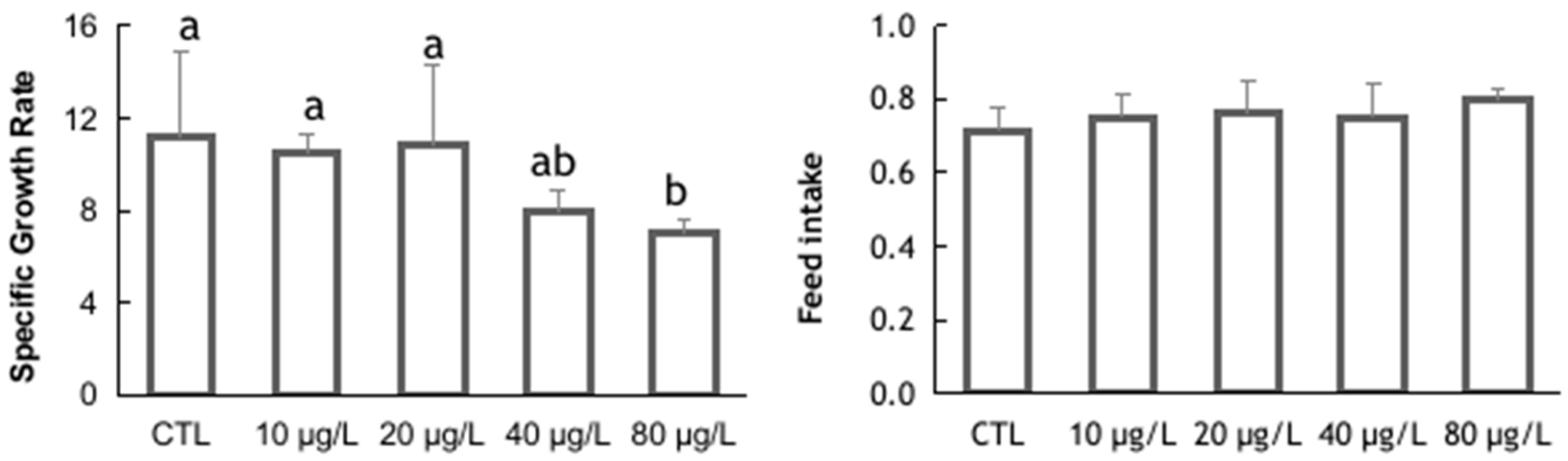
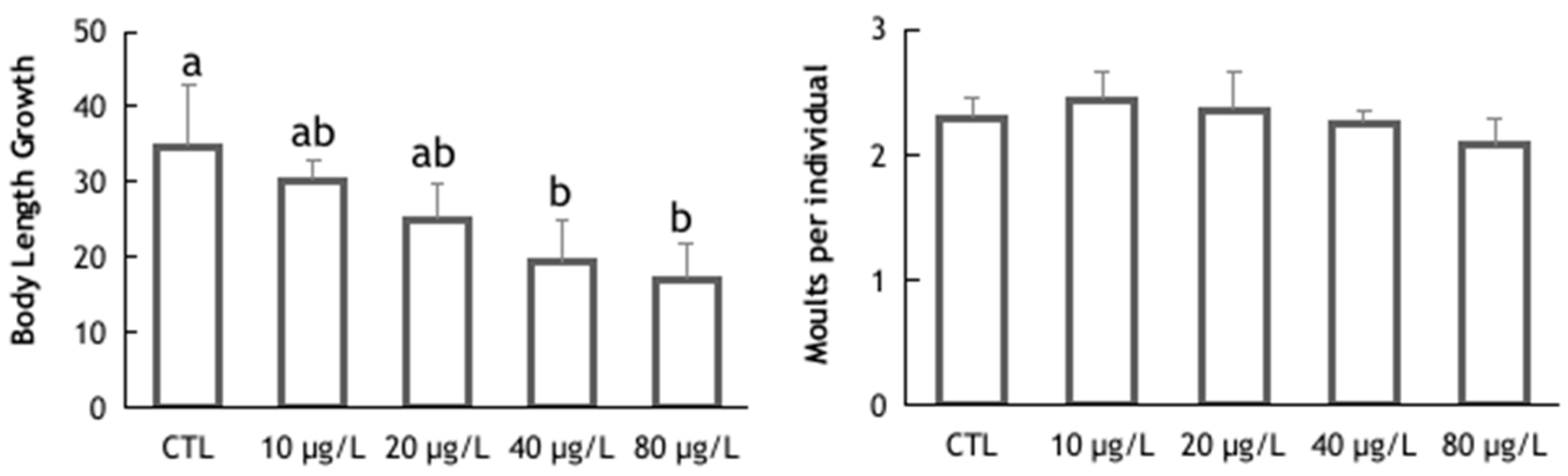
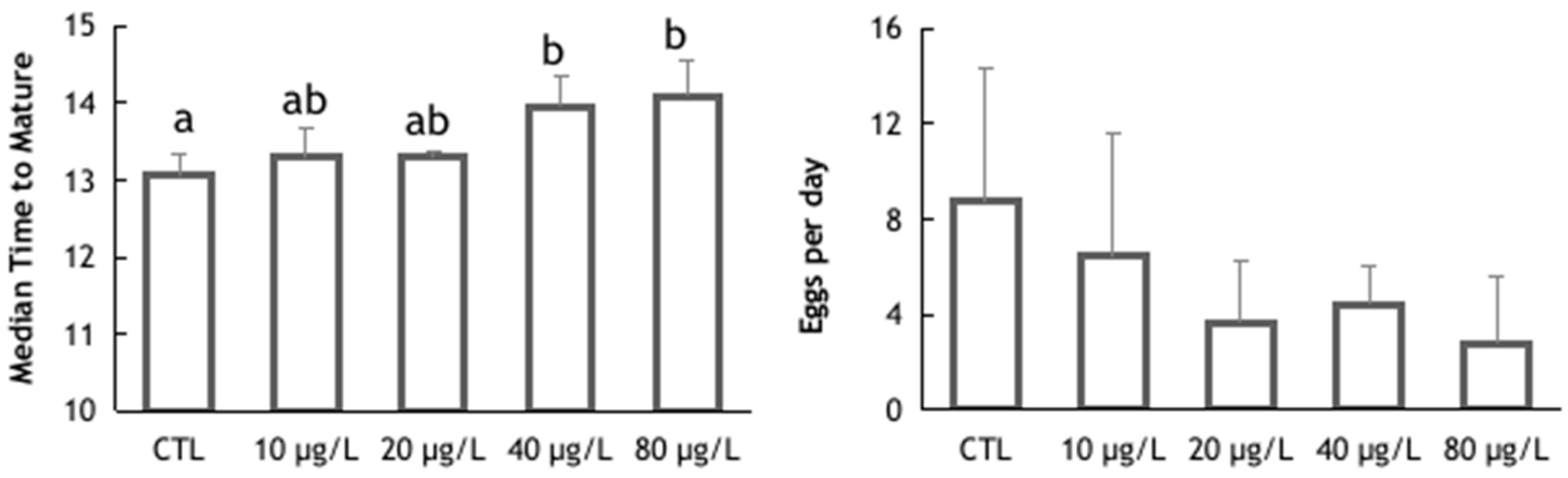
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sassaman, C. Sex ratio variation in female-biased populations of Notostracans. Hydrobiologia 1991, 212, 169–179. [Google Scholar] [CrossRef]
- Takahashi, F. Pioneer life of the tadpole shrimps, Triops spp. (Notostraca:Triopsidae). Appl. Entomol. Zool. 1977, 12, 104–117. [Google Scholar] [CrossRef]
- Meintjes, S. Observations on some reproductive traits of Triops granarius (Lucas) (Crustacea: Notostraca). Hydrobiologia 1996, 325, 213–218. [Google Scholar] [CrossRef]
- Longhurst, A.R. A review of the Notostraca. Bull. Br. Mus. Nat. Hist. Zool. 1955, 3, 1–57. [Google Scholar] [CrossRef]
- Su, T.; Mulla, M.S. Factors affecting egg hatch of the tadpole shrimp, Triops newberryi, a potential biological control agent of immature mosquitoes. Biol. Control 2002, 23, 18–26. [Google Scholar] [CrossRef]
- Fry-O’Brien, L.; Mulla, M. Optimal conditions for rearing the tadpole shrimp, Triops longicaudatus (Notostraca:Triopsidae), a biological control agent against mosquitoes. J. Am. Mosq. Control Assoc. 1996, 12, 446–453. [Google Scholar]
- Davis, J.; Madison, D. The ontogeny of light-dark response in Triops Longicaudatus as a response to changing selective pressures. Crustaceana 2000, 73, 283–288. [Google Scholar] [CrossRef]
- Grigarick, A.A.; Lange, W.H.; Finfrock, D.C. Control of the tadpole shrimp, Triops longicaudatus, in California rice fields. J. Econ. Entomol. 1961, 54, 36–40. [Google Scholar] [CrossRef]
- Walton, W.E.; Darwazeh, H.A.; Mulla, M.S.; Schreiber, E.T. Impact of selected synthetic pyrethroids and organophosphorous pesticides on the tadpole shrimp, Triops longicaudatus (Le Conte) (Notostraca:Triopsidae). Bull. Environ. Contam. Toxicol. 1990, 45, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tsukimura, B.; Nelson, W.K.; Linder, C.J. Inhibition of ovarian development by methyl farnesoate in the tadpole shrimp, Triops longicaudatus. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2006, 144, 135–144. [Google Scholar] [CrossRef]
- Su, T.; Mulla, M.S. Toxicity and effects of microbial mosquito larvicides and larvicidal oil on the development and fecundity of the tadpole shrimp Triops newberryi (Packard) (Notostraca:Triopsidae). J. Vector Ecol. 2005, 30, 107–114. [Google Scholar]
- Su, T.; Jiang, Y.; Mulla, M.S. Toxicity and effects of mosquito larvicides methoprene and surface film (Agnique(R) MMF) on the development and fecundity of the tadpole shrimp Triops newberryi (Packard) (Notostraca:Triopsidae). J. Vector Ecol. 2014, 39, 340–346. [Google Scholar] [CrossRef]
- Ferreira, N.G.C.; Chessa, A.; Abreu, I.O.; Teles, L.O.; Kille, P.; Carvalho, A.P.; Guimarães, L. Toxic relationships: Prediction of TBT’s affinity to the ecdysteroid receptor of Triops longicaudatus. Toxics 2023, 11, 937. [Google Scholar] [CrossRef]
- Guimarães, L.; Carvalho, A.P.; Ribeiro, P.; Teixeira, C.; Silva, N.; Pereira, A.; Amorim, J.; Oliva-Teles, L. Sensitivity of Triops longicaudatus locomotor behaviour to detect short low-level exposure to pollutants. Water 2024, 16, 126. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD: Paris, France, 2004.
- ISO 6341: 2012; Water Quality–Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)-Acute Toxicity Test. International Organisation for Standardisation: Geneva, Switzerland, 2012.
- Barceloux, D.G. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef]
- Velma, V.; Vutukuru, S.S.; Tchounwou, P.B. Ecotoxicology of hexavalent chromium in freshwater fish: A critical review. Rev. Environ. Health 2009, 24, 129–145. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.; Tian, T.; Lin, S.; Xu, P.; Zhou, L.; Dai, C.; Hao, Q.; Wu, Y.; Zhai, Z.; et al. The effect of hexavalent chromium on the incidence and mortality of human cancers: A meta-analysis based on published epidemiological cohort studies. Front. Oncol. 2019, 9, 24. [Google Scholar] [CrossRef]
- Rao, K.R.; Doughtie, D.G. Histopathological changes in grass shrimp exposed to chromium, pentachlorophenol and dithiocarbamates. Mar. Environ. Res. 1984, 14, 371–395. [Google Scholar] [CrossRef]
- Yang, G.; Chen, C.; Yu, Y.; Zhao, H.; Wang, W.; Wang, Y.; Cai, L.; He, Y.; Wang, X. Combined effects of four pesticides and heavy metal chromium (VI) on the earthworm using avoidance behavior as an endpoint. Ecotoxicol. Environ. Saf. 2018, 157, 191–200. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mohanty, B. Acute toxicity impacts of hexavalent chromium on behavior and histopathology of gill, kidney and liver of the freshwater fish, Channa punctatus (Bloch). Environ. Toxicol. Pharmacol. 2008, 26, 136–141. [Google Scholar] [CrossRef]
- Yan, T.; Xu, Y.; Zhu, Y.; Jiang, P.; Zhang, Z.; Li, L.; Wu, Q. Chromium exposure altered metabolome and microbiome-associated with neurotoxicity in zebrafish. J. Appl. Toxicol. 2023, 43, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Chu, K.H.; Tang, K.W.; Tam, T.W.; Wong, L.J. Effects of chromium, copper and nickel on survival and feeding behaviour of Metapenaeus ensis larvae and postlarvae (Decapoda: Penaeidae). Mar. Environ. Res. 1993, 36, 63–78. [Google Scholar] [CrossRef]
- Teles, M.; Pacheco, M.; Santos, M.A. Physiological and genetic responses of European eel (Anguilla anguilla L.) to short-term chromium or copper exposure–Influence of preexposure to a PAH-like compound. Environ. Toxicol. 2005, 20, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, L.d.F.; Borrely, S.I.; Hamada, N.; Grazeffe, V.S.; Ohlweiler, F.P.; Okazaki, K.; Granatelli, A.T.; Pereira, I.W.; Pereira, C.A.; Nakano, E. Developmental toxicity, acute toxicity and mutagenicity testing in freshwater snails Biomphalaria glabrata (Mollusca: Gastropoda) exposed to chromium and water samples. Ecotoxicol. Environ. Saf. 2014, 110, 208–215. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Kundu, G.K.; Al-Mamun, M.H.; Sarkar, S.K.; Akter, M.S.; Khan, M.S. Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging catfish, Heteropneustes fossilis. Ecotoxicol. Environ. Saf. 2013, 92, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Heffern, K.; Tierney, K.; Gallagher, E.P. Comparative effects of cadmium, zinc, arsenic and chromium on olfactory-mediated neurobehavior and gene expression in larval zebrafish (Danio rerio). Aquat. Toxicol. 2018, 201, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Zhu, J.; Jiang, P.; Zhang, Z.; Li, L.; Wu, Q. Chromium induced neurotoxicity by altering metabolism in zebrafish larvae. Ecotoxicol. Environ. Saf. 2021, 228, 112983. [Google Scholar] [CrossRef]
- Wise, J.P., Jr.; Young, J.L.; Cai, J.; Cai, L. Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ. Int. 2022, 158, 106877. [Google Scholar] [CrossRef]
- Hellou, J. Behavioural ecotoxicology, an “early warning” signal to assess environmental quality. Environ. Sci. Pollut. Res. Int. 2011, 18, 1–11. [Google Scholar] [CrossRef]
- Oliva Teles, L.; Fernandes, M.; Amorim, J.; Vasconcelos, V. Video-tracking of zebrafish (Danio rerio) as a biological early warning system using two distinct artificial neural networks: Probabilistic neural network (PNN) and self-organizing map (SOM). Aquat. Toxicol. 2015, 165, 241–248. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Oliva-Teles, T.; Mesquita, S.R.; Delerue-Matos, C.; Guimaraes, L. Integrated biomarker responses of an estuarine invertebrate to high abiotic stress and decreased metal contamination. Mar. Environ. Res. 2014, 101, 101–114. [Google Scholar] [CrossRef]
- Abreu, I.O.; Monteiro, C.; Rocha, A.C.S.; Reis-Henriques, M.A.; Teixeira, C.; Basto, M.C.P.; Ferreira, M.; Almeida, C.M.R.; Oliva-Teles, L.; Guimaraes, L. Multibiomarker interactions to diagnose and follow-up chronic exposure of a marine crustacean to Hazardous and Noxious Substances (HNS). Environ. Pollut. 2018, 242, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.; Fernandes, M.; Abreu, I.; Tavares, F.; Oliva-Teles, L. Escherichia coli’s water load affects zebrafish (Danio rerio) behavior. Sci. Total Environ. 2018, 636, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Román, A.C.; Vicente-Page, J.; Pérez-Escudero, A.; Carvajal-González, J.M.; Fernández-Salguero, P.M.; de Polavieja, G.G. Histone H4 acetylation regulates behavioral inter-individual variability in zebrafish. Genome Biol. 2018, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Lehtonen, K.K.; Guilhermino, L.; Guimaraes, L. Exposure of Carcinus maenas to waterborne fluoranthene: Accumulation and multibiomarker responses. Sci. Total Environ. 2013, 443, 454–463. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ball, J.W.; Izbicki, J.A. Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Appl. Geochem. 2004, 19, 1123–1135. [Google Scholar] [CrossRef]
- Bourotte, C.; Bertolo, R.; Almodovar, M.; Hirata, R. Natural occurrence of hexavalent chromium in a sedimentary aquifer in Urânia, State of São Paulo, Brazil. An. Acad. Bras. Ciênc 2009, 81, 227–242. [Google Scholar] [CrossRef]
- Tziritis, E.; Kelepertzis, E.; Korres, G.; Perivolaris, D.; Repani, S. Hexavalent chromium contamination in groundwaters of Thiva Basin, central Greece. Bull. Environ. Contam. Toxicol. 2012, 89, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003.
- Moreira, R.A.; da Silva Mansano, A.; Rocha, O. The toxicity of carbofuran to the freshwater rotifer, Philodina roseola. Ecotoxicology 2015, 24, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, C.; Yuan, L. Characteristics of six cladocerans in relation to ecotoxicity testing. Ecol. Indic. 2007, 7, 768–775. [Google Scholar] [CrossRef]
- Castro, B.B.; Freches, A.R.; Rodrigues, M.; Nunes, B.; Antunes, S.C. Transgenerational effects of toxicants: An extension of the Daphnia 21-day Chronic Assay? Arch. Environ. Contam. Toxicol. 2018, 74, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Peluso, L.; Giusto, A.; Rossini, G.D.B.; Ferrari, L.; Salibian, A.; Ronco, A.E. Hyalella curvispina (AMPHIPODA) as a test organism in laboratory toxicity testing of environmental samples. Fresenius Environ. Bull. 2011, 20, 372–376. [Google Scholar]
- Obregón-Barboza, H.; Maeda-Martínez, A.M.; Murugan, G. Reproduction, molting, and growth of two Mexican uniparental forms of the tadpole shrimp Triops (Branchiopoda:Notostraca) under a recirculating culture system. Hydrobiologia 2001, 462, 173–184. [Google Scholar] [CrossRef]
- Arzate-Cardenas, M.A.; Martinez-Jeronimo, F. Energy resource reallocation in Daphnia schodleri (Anomopoda: Daphniidae) reproduction induced by exposure to hexavalent chromium. Chemosphere 2012, 87, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.; Guilhermino, L.; Afonso, M.J.; Marques, J.M.; Chaminé, H.I. Assessment of urban groundwater: Towards integrated hydrogeological and effects-based monitoring. Sustain. Water Resour. Manag. 2019, 5, 217–233. [Google Scholar] [CrossRef]
- Ribeiro, S.; Guilhermino, L.; Sousa, J.P.; Soares, A.M. Novel bioassay based on acetylcholinesterase and lactate dehydrogenase activities to evaluate the toxicity of chemicals to soil isopods. Ecotoxicol. Environ. Saf. 1999, 44, 287–293. [Google Scholar] [CrossRef]
- Diamantino, T.C.; Guilhermino, L.; Almeida, E.; Soares, A.M. Toxicity of sodium molybdate and sodium dichromate to Daphnia magna straus evaluated in acute, chronic, and acetylcholinesterase inhibition tests. Ecotoxicol. Environ. Saf. 2000, 45, 253–259. [Google Scholar] [CrossRef][Green Version]
- Gutierrez, M.F.; Gagneten, A.M.; Paggi, J.C. Exposure to sublethal chromium and endosulfan alter the diel vertical migration (DVM) in freshwater zooplankton crustaceans. Ecotoxicology 2012, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.F.; Paggi, J.C.; Gagneten, A.M. Infodisruptions in predator-prey interactions: Xenobiotics alter microcrustaceans responses to fish infochemicals. Ecotoxicol. Environ. Saf. 2012, 81, 11–16. [Google Scholar] [CrossRef] [PubMed]
| Hours | LC50 (µg/L) | CI 95% (µg/L) | LC10 (µg/L) | CI 95% (µg/L) |
|---|---|---|---|---|
| 24 | 181.4 | 154.2–222.0 | 95.0 | 65.7–116.1 |
| 48 | 81.4 | 70.3–94.3 | 46.9 | 34.9–56.2 |
| 96 | 65.3 | 57.6–74.9 | 43.4 | 33.8–50.2 |
| Species | Hours | LC50/EC50 (µg/L) | CI 95% (µg/L) |
|---|---|---|---|
| Philodina roseola | 48 | 47,100 * | 29,520–64,670 [45] |
| Daphnia pulex | 48 | 509.2 | 340–734 [46] |
| 96 | 198.1 | 85–283 [46] | |
| Daphnia carinata | 48 | 396.1 | 255–594 [46] |
| 96 | 198.1 | 85–283 [46] | |
| Daphnia magna ** | 48 | 3451.3 | 2263–6224 [46] |
| 96 | 1725.6 | 1075–2546 [46] | |
| 48 | 494 * | 457–532 [47] | |
| Ceriodaphnia quadrangular | 48 | 537.5 | 368–764 [46] |
| 96 | 254.6 | 170–368 [46] | |
| Bosmina longirostris | 48 | 480.9 | 311–736 [46] |
| 96 | 169.7 | 85–283 [46] | |
| Simocephalus vetulus | 48 | 763.8 | 509–1216 [46] |
| 96 | 396.1 | 226–622 [46] | |
| Hyalella curvispina | 96 | 1555.9 | 1330–1839 [48] |
| 96 | 565.8 | 424–792 [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.C.; Saraiva, A.; Oliva-Teles, L.; Guimarães, L.; Carvalho, A.P. Ecotoxicological Effects of Potassium Dichromate on the Tadpole Shrimp Triops longicaudatus. Animals 2024, 14, 358. https://doi.org/10.3390/ani14030358
Pereira AC, Saraiva A, Oliva-Teles L, Guimarães L, Carvalho AP. Ecotoxicological Effects of Potassium Dichromate on the Tadpole Shrimp Triops longicaudatus. Animals. 2024; 14(3):358. https://doi.org/10.3390/ani14030358
Chicago/Turabian StylePereira, André Carido, Aurélia Saraiva, Luís Oliva-Teles, Laura Guimarães, and António Paulo Carvalho. 2024. "Ecotoxicological Effects of Potassium Dichromate on the Tadpole Shrimp Triops longicaudatus" Animals 14, no. 3: 358. https://doi.org/10.3390/ani14030358
APA StylePereira, A. C., Saraiva, A., Oliva-Teles, L., Guimarães, L., & Carvalho, A. P. (2024). Ecotoxicological Effects of Potassium Dichromate on the Tadpole Shrimp Triops longicaudatus. Animals, 14(3), 358. https://doi.org/10.3390/ani14030358








