Effects of Dietary Supplementation with Chitosan on the Muscle Composition, Digestion, Lipid Metabolism, and Stress Resistance of Juvenile Tilapia (Oreochromis niloticus) Exposed to Cadmium-Induced Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Diets
2.3. Experimental Fish, Acclimatization, and Culture
2.4. Sampling
2.5. Determination of Digestive Enzyme Activity
2.6. Determination of Muscle Composition
2.7. Determination of Gene Expression
2.8. Data Calculation and Statistics
3. Results
3.1. Effects of Dietary Supplementation with Chitosan on the Muscle Composition of Juvenile GIFT Exposed to Cadmium-Induced Stress
3.2. Effects of Dietary Supplementation with Chitosan on the Intestinal Digestive Enzyme Activities of Juvenile GIFT Exposed to Cadmium-Induced Stress
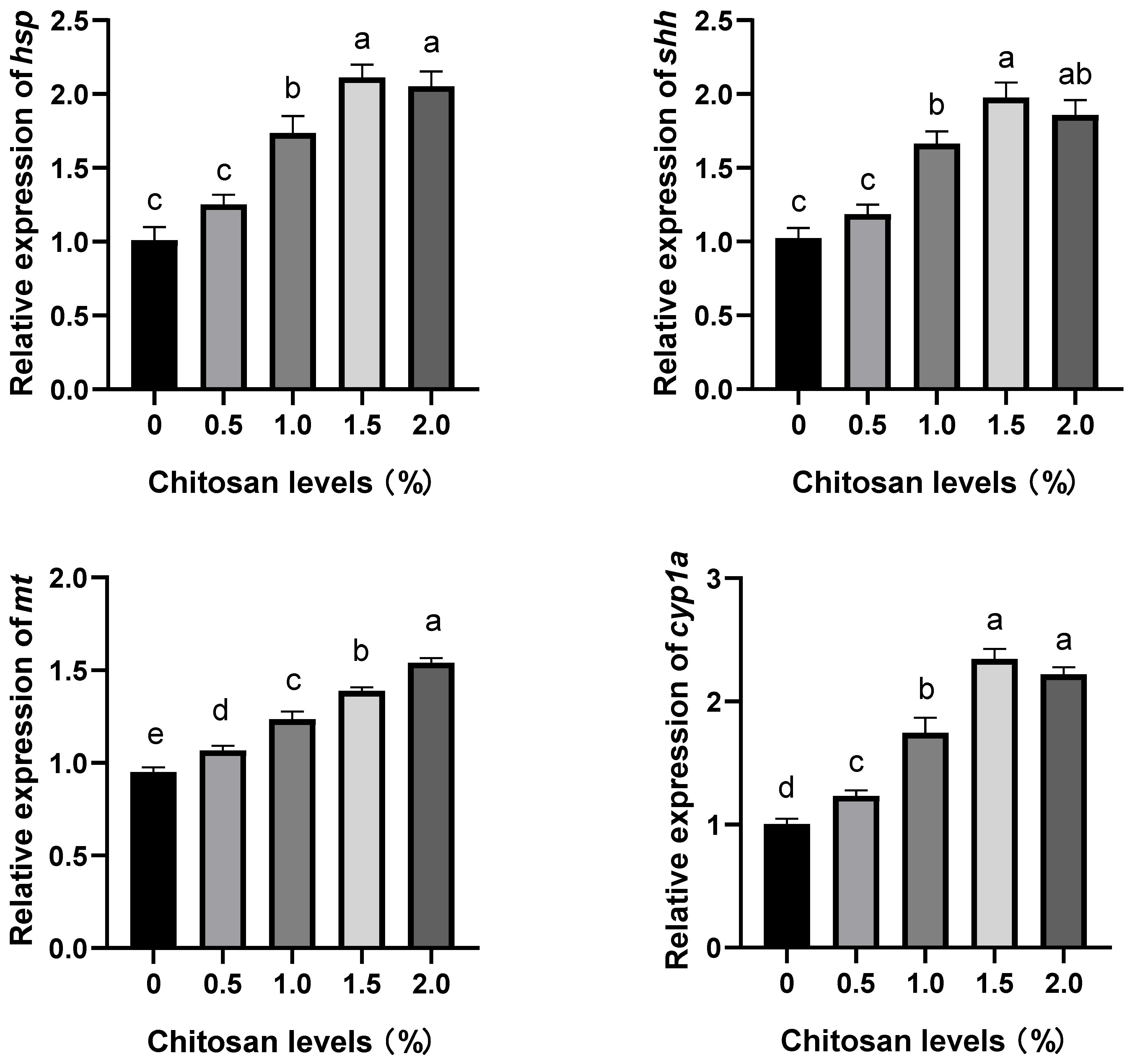
3.3. Effects of Dietary Supplementation with Chitosan on the Relative Expression Levels of Stress-Resistance Genes in the Liver of Juvenile GIFT Exposed to Cadmium-Induced Stress
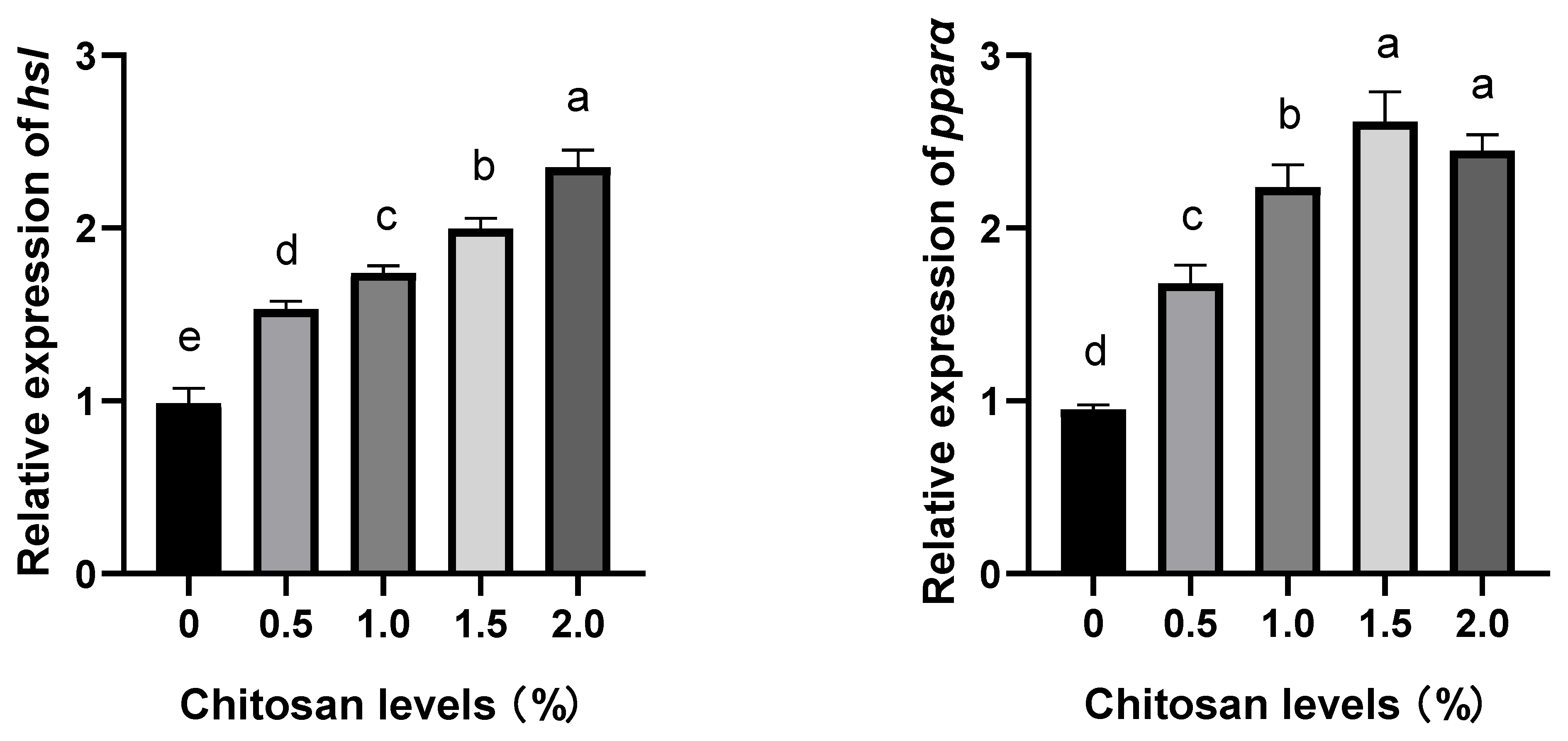
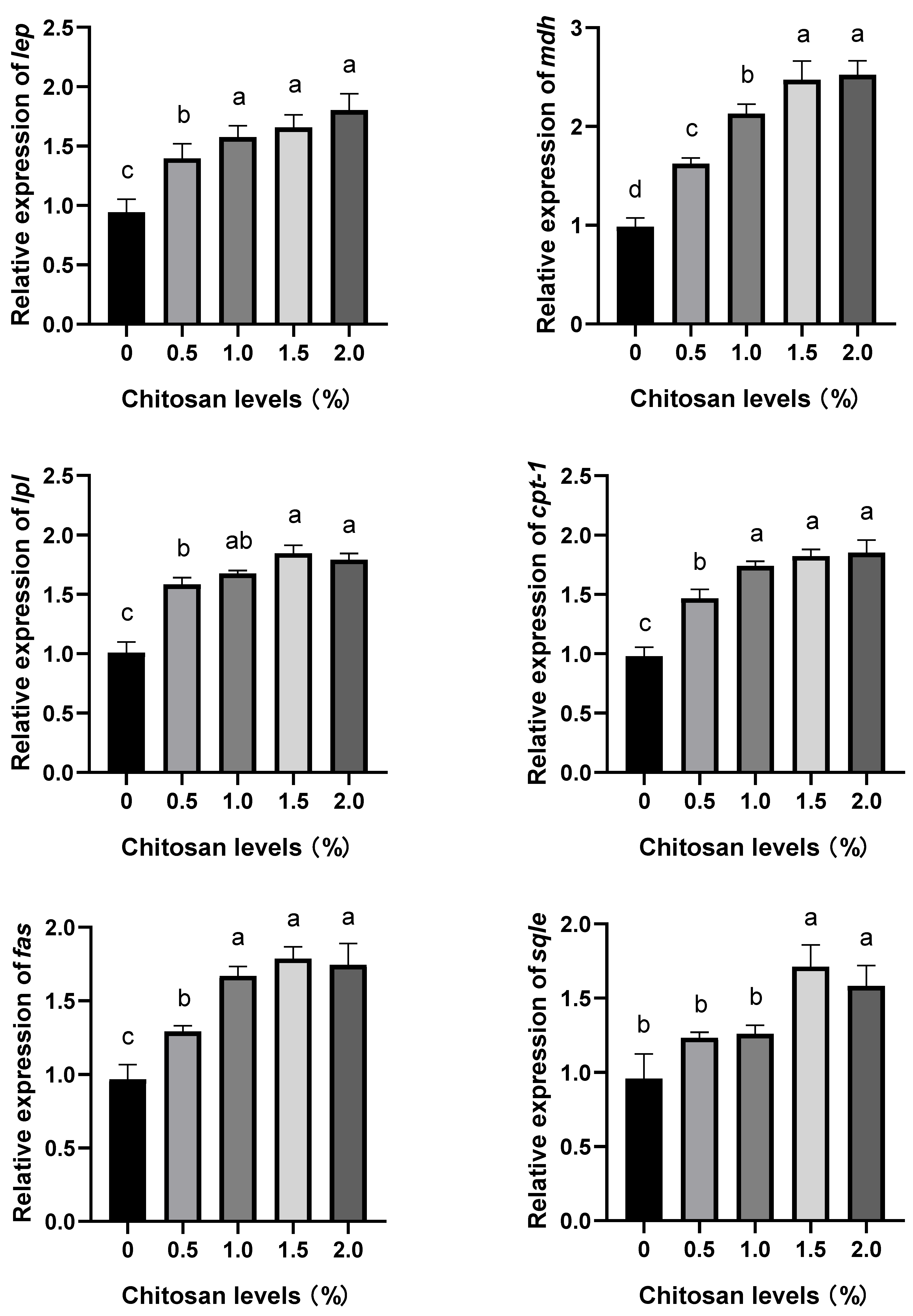
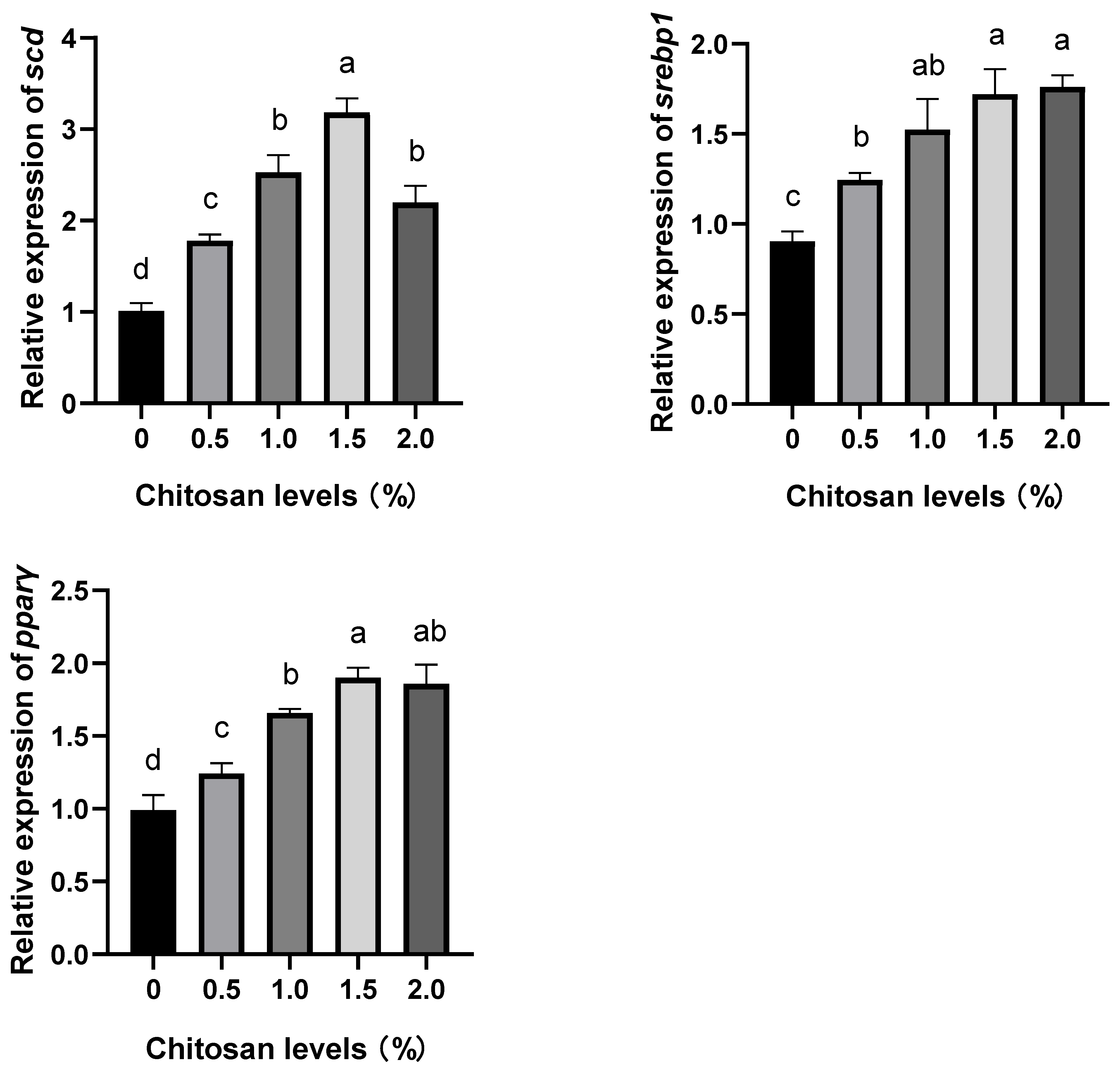
3.4. Effects of Dietary Supplementation with Chitosan on the Relative Expression Levels of Lipid Metabolism Genes in the Liver of Juvenile GIFT Exposed to Cadmium-Induced Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.; Lauria, G.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Paíga, P.; Sousa, S.; Vera, J.; Bitencourt, L.; Vieira, J.; Jorge, S.; Silva, J.; Correia, M.; Domingues, V.; Delerue-Matos, C. Multi-residue analysis of fifty pesticides in river waters and in wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 66787–66803. [Google Scholar] [CrossRef]
- Abou-Kassem, D.; Abd EI-Hack, A.; Taha, A.; Ajarem, J.; Maodaa, S.; Allam, A. Detoxification Impacts of Ascorbic Acid and Clay on Laying Japanese Quail Fed Diets Polluted by Various Levels of Cadmium. Animals 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Ma, H.; Deng, Y.; Feng, J.; Jie, Y.; Guo, Z. Oxidative stress, cell cycle arrest, DNA damage and apoptosis in the mud crab (Scylla paramamosain) induced by cadmium exposure. Chemosphere 2021, 263, 128277. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhiyan, S.; Karthikeyan, P.; Rathinam, K.; Farzana, M.; Park, C. Magnetic kaolinite immobilized chitosan beads for the removal of Pb(II) and Cd(II) ions from an aqueous environment. Carbohydr. Polym. 2021, 261, 117892. [Google Scholar] [CrossRef]
- Godiya, C.; Park, B. Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques. A review. Environ. Chem. Lett. 2022, 20, 1801–1837. [Google Scholar] [CrossRef]
- Othman, Z.; Mackey, H.; Mahmoud, K. MXene/chitosan/lignosulfonate (MCL) nanocomposite for simultaneous removal of Co(II), Cr(VI), Cu(II), Ni(II) and Pb(II) heavy metals from wastewater. 2D Mater. 2023, 10, 020224. [Google Scholar] [CrossRef]
- Lan, Z.; Lin, Y.; Yang, C. Lanthanum-iron incorporated chitosan beads for adsorption of phosphate and cadmium from aqueous solutions. Chem. Eng. J. 2022, 448, 137519. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.; Saleh, N.; Kamarudin, N.; Sulong, A. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2022, 259, 117613. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, K.; Ganesan, R.; Ganesan, A.; Divya, D.; Johansen, J.; Zhang, S. Chitin, chitosan and chitooligosaccharides as potential growth promoters and immunostimulants in aquaculture: A comprehensive review. Int. J. Biol. Macromol. 2023, 251, 126285. [Google Scholar] [CrossRef]
- Bhusare, S.; Sardar, P.; Sahu, P.; Shamna, N.; Kumar, P.; Paul, M.; Jana, P.; Raghuvaran, N.; Bhavatharaniya, U. Bile acid improves growth, lipid utilization and antioxidative status of genetically improved farmed tilapia (Oreochromis niloticus) fed with varying protein-lipid diets reared in inland saline water. Anim. Feed Sci. Technol. 2023, 303, 115677. [Google Scholar] [CrossRef]
- Adene, C. Effects of Pretilachor Pyribenzoxim Pollution on the Water Quality, Serum Biochemical Indices, and Behavioural Response of Oreochromis niloticus Juveniles. Asian J. Res. Agric. For. 2023, 9, 119–127. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.; Civera, M.; Arias, C.; Elorza, B.; Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Ibrahim, R.; Elshopakey, G.; Abdelwarith, A.; Younis, E.; Ismail, S.; Ahmed, A.; El-Saberf, M.; Abdelhamid, A.; El-Murr, A.; Rahman, A. Chitosan neem nanocapsule enhances immunity and disease resistance in nile tilapia (Oreochromis niloticus). Heliyon 2023, 9, e19354. [Google Scholar] [CrossRef] [PubMed]
- GB/T 6432-2018; Determination of Crude Protein in Feeds-Kjeldahl Method. Standardization Administration of the P.R.C: Beijing, China, 2018.
- GB/T 6433-2006; Determination of Crude Fat in Feeds. Standardization Administration of the P.R.C: Beijing, China, 2006.
- GB/T 6435-2014; Determination of Moisture in Feeds. Standardization Administration of the P.R.C: Beijing, China, 2014.
- GB/T 6438-2007; Determination of Ash in Feeds. Standardization Administration of the P.R.C: Beijing, China, 2007.
- Liu, Y.; Huang, E.; Xie, Y.; Meng, L.; Liu, D.; Zhang, Z.; Zhou, J.; Zhang, Q.; Tong, T. The Effect of Dietary Lipid Supplementation on the Serum Biochemistry, Antioxidant Responses, Initial Immunity, and mTOR Pathway of Juvenile Tilapia (Oreochromis niloticus). Fishes 2023, 8, 535. [Google Scholar] [CrossRef]
- Schmittgen, T.; Livak, J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Falahatgar, D.; Javadian, S.; Bahram, S.; Bahrekazemi, M. EDTA detoxifies heavy metals on exposed beluga (Huso huso) with pollution stress: Growth performance, immunohaematology, blood biochemistry and antioxidant activity. Aquac. Res. 2021, 52, 4336–4349. [Google Scholar] [CrossRef]
- Lee, J.; Jo, A.; Lee, D.; Choi, C.; Kang, J.; Kim, J. Review of cadmium toxicity effects on fish: Oxidative stress and immune responses. Environ. Res. 2023, 236, 116600. [Google Scholar] [CrossRef]
- Bacou, E.; Walk, C.; Rider, S.; Litta, G.; Perez-Calvo, E. Dietary Oxidative Distress: A Review of Nutritional Challenges as Models for Poultry, Swine and Fish. Antioxidants 2021, 10, 525. [Google Scholar] [CrossRef]
- Lall, S.; Kaushik, S. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 3510. [Google Scholar] [CrossRef]
- El-Naggar, M.; Salaah, S.; El-Shabaka, H.; El-Rahman, F.; Khalil, M.; Suloma, A. Efficacy of dietary chitosan and chitosan nanoparticles supplementation on health status of Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2021, 19, 100628. [Google Scholar] [CrossRef]
- Mushawwir, A.; Permana, R.; Darwis, D.; Puspitasari, T.; Pengerteni, D.; Nuryanthi, N.; Suwarno, N. Enhancement of the liver histologic of broiler induced by irradiated chitosan (IC). Proc. Int. Conf. Phys. Nucl. Sci. Technol. 2021, 2381, 020046. [Google Scholar]
- Hilițanu, L.; MititeluTarțău, L.; Bogdan, M.; Buca, B.; Păuna, A.; Pavel, L.; Pelin, A.; Meca, A.; Popa, G. The Use of Chitosan-Coated Nanovesicles in Repairing Alcohol-Induced Damage of Liver Cells in Mice. Medicina 2022, 58, 762. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Arora, S.; Singh, J. Smart thermosensitive copolymer incorporating chitosan–zinc–insulin electrostatic complexes for controlled delivery of insulin: Effect of chitosan chain length. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Khan, M.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, C.; Wu, S. Dietary chitosan modulates the growth performance, body composition and nonspecific immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Int. J. Biol. Macromol. 2022, 217, 188–192. [Google Scholar] [CrossRef]
- Gheytasi, A.; Shekarabi, S.; Islami, H.; Mehrgan, M. Feeding rainbow trout, Oncorhynchus mykiss, with lemon essential oil loaded in chitosan nanoparticles: Effect on growth performance, serum hemato-immunological parameters, and body composition. Aquac. Int. 2021, 29, 2207–2221. [Google Scholar] [CrossRef]
- Assan, D.; Kuebutornye, F.; Hlordzi, V.; Chen, H.; Mraz, J.; Mustapha, U.; Abarike, E. Effects of probiotics on digestive enzymes of fish (finfish and shellfish); status and prospects: A mini review. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 257, 110653. [Google Scholar] [CrossRef]
- Kakade, A.; Sharma, M.; Salama, E.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Su, Y.; et al. Heavy metals (HMs) pollution in the aquatic environment: Role of probiotics and gut microbiota in HMs remediation. Environ. Res. 2023, 223, 115186. [Google Scholar] [CrossRef]
- Cheaib, B.; Seghouani, H.; Llewellyn, M.; Vandal-Lenghan, K.; Mercier, P.; Derome, N. The yellow perch (Perca flavescens) microbiome revealed resistance to colonisation mostly associated with neutralism driven by rare taxa under cadmium disturbance. Anim. Microbiome 2021, 3, 3. [Google Scholar] [CrossRef]
- Rahman, A.; Shin, J.; Whang, C.; Jung, W.; Yoo, D.; Seo, C.; Cho, B.; Jon, S. Bilirubin Nanomedicine Rescues Intestinal Barrier Destruction and Restores Mucosal Immunity in Colitis. ACS Nano 2023, 17, 10996–11013. [Google Scholar] [CrossRef]
- Asmaa, S.; Adham, A.; Samar, S.; Naiel, M. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Hendawy, O.; AlSanea, M.; Mohammed, E.; Rahman, H.; Hassan, Y.; Elshaarawy, R.; Khedr, A. Alginate-chitosan-microencapsulated tyrosols/oleuropein-rich olive mill waste extract for lipopolysaccharide-induced skin fibroblast inflammation treatment. Int. J. Pharm. 2023, 643, 123260. [Google Scholar] [CrossRef]
- Xiong, M.; Li, Y.; He, H.; Hao, S.; Fang, P.; Xu, M.; Chen, Y.; Chen, Y.; Yu, S.; Hu, H. Cyclosporine A-loaded colon-targeted oral nanomicelles self-assembly by galactosylated carboxymethyl chitosan for efficient ulcerative colitis therapy. Eur. J. Pharm. Biopharm. 2023, 189, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chang, X.; Zhang, H.; Wang, J.; Qiu, K.; Wu, S. Effects of Dietary Rare Earth Chitosan Chelate on Performance, Egg Quality, Immune and Antioxidant Capacity, and Intestinal Digestive Enzyme Activity of Laying Hens. Polymers 2023, 15, 1600. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, W.; Liu, Y.; Wang, Y.; Zhang, J.; Wang, Z.; Mai, K.; Ai, Q. Effects of Chitosan-Coated Microdiet on Dietary Physical Properties, Growth Performance, Digestive Enzyme Activities, Antioxidant Capacity, and Inflammation Response of Large Yellow Croaker (Larimichthys crocea) Larvae. Aquac. Nutr. 2022, 2022, 4355132. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Tamuli, R. Heat shock proteins and the calcineurin-crz1 signaling regulate stress responses in fungi. Arch. Microbiol. 2022, 204, 240. [Google Scholar] [CrossRef] [PubMed]
- Velikic, G.; Maric, D.; Maric, D.; Supic, G.; Puletic, M.; Dulic, O.; Vojvodic, D. Harnessing the Stem Cell Niche in Regenerative Medicine: Innovative Avenue to Combat Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 993. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Jorge, D.; Lilia, L.; Miguel, A.; Méndez-Estrada, R.; Felix-Portillo, M.; Yepiz-Plascencia, G. Cloning, expression, purification and biochemical characterization of recombinant metallothionein from the white shrimp Litopenaeus vannamei. Protein Expr. Purif. 2020, 166, 105511. [Google Scholar]
- Choudhury, C.; Mazumder, R.; Biswas, R.; Sengupta, M. Cadmium exposure induces inflammation through the canonical NF-κΒ pathway in monocytes/macrophages of Channa punctatus Bloch. Fish Shellfish Immunol. 2021, 110, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, R.; Gupta, R.; Kaur, T.; Kour, A.; Kaur, S.; Rajput, A. Heavy metal toxicity in earthworms and its environmental implications: A Review. Environ. Adv. 2023, 12, 100374. [Google Scholar] [CrossRef]
- Bailei, L.; Jeevithan, E.; Wenhui, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719. [Google Scholar]
- Dornjak, L.; Ostojić, K.; Klaser, T.; Urlić, I.; Rogina, A. Boric Acid Modified Chitosan Scaffolds Chemically Crosslinked by Genipin. Kem. U Ind. Časopis Kemičara I Kem. Inženjera Hrvat. 2022, 71, 691–698. [Google Scholar]
- Alamrani, N.; Almutairi, F.; Alotaibi, F.; Alenazi, D.; Monier, M.; Abdel-Latif, D.; Elsayed, N. Developing thiosemicarbazide-modified/ion-imprinted chitosan for selective cadmium ion biosorption. Mater. Today Chem. 2023, 30, 101547. [Google Scholar] [CrossRef]
- Mukarram, M.; Ali, J.; Dadkhah, A.; Kurjak, D.; Kačík, F.; Ďurkovič, J. Chitosan-induced biotic stress tolerance and crosstalk with phytohormones, antioxidants, and other signalling molecules. Front. Recent Dev. Plant Sci. 2023, 14, 1217822. [Google Scholar] [CrossRef]
- Varghese, L.; Thomas, G. Chitosan triggers tolerance to Pythium myriotylum infection in ginger (Zingiber officinale) by modulating multiple defense signaling pathways. Physiol. Mol. Plant Pathol. 2023, 125, 101985. [Google Scholar] [CrossRef]
- Araújo, L.; Matos, H.; Facchi, D.; Almeida, D.; Gonçalves, B.; Monteiro, J.; Martins, A.; Bonafé, E. Natural carbohydrate-based thermosensitive chitosan/pectin adsorbent for removal of Pb(II) from aqueous solutions. Int. J. Biol. Macromol. 2021, 193, 1813–1822. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, T.; Fu, X.; Zhu, C.; Mou, H. Partially degraded chitosan-based flocculation to achieve effective deodorization of oyster (Crassostrea gigas) hydrolysates. Carbohydr. Polym. 2020, 234, 115948. [Google Scholar] [CrossRef]
- Le, L.; Giang, N.; Chien, P.; Trinh, X.; Long, N.; Anh, L.; Nga, P.; Zhang, X.; Nam, S.; Heo, C. Enhancement of Wound Healing Efficacy by Chitosan-based Hydrocolloid on Sprague Dawley Rats. Int. Inst. Anticancer. Res. 2023, 37, 1052–1064. [Google Scholar] [CrossRef]
- Brol, J.; Müller, L.; Prates, E.; Farias, B.; Pedrosa, V.; Pinto, L.; Cadaval, T.; Tesser, M.; Wasielesky, W.; Ventura-Lima, J. Dietary chitosan supplementation in Litopenaeus vannamei reared in a biofloc system: Effect on antioxidant status facing saline stress. Aquaculture 2021, 544, 737034. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Tang, J.; Liu, J.; Yin, H.; Li, R.; Ye, S. Dietary effect chitosan nanoparticles on growth performance, immunity and resistance against Vibrio splendidus in the sea cucumber Apostichopus japonicas. Aquac. Rep. 2023, 30, 101625. [Google Scholar] [CrossRef]
- Grabner, G.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Mu, H.; Wan, W.; Song, J.; Kuang, R.; Deng, T. Mitochondrial Lipid Peroxidation and Microsomal Drug-metabolizing Enzyme Activity of Rat Hepatotoxicity under Heavy Metals from Slag Waste Exposure. Cell Biochem. Biophys. 2023, 81, 285–298. [Google Scholar] [CrossRef]
- Jie, J.; Debora, T.; Àngels, R.; Roher, N. Nanodelivery Systems as New Tools for Immunostimulant or Vaccine Administration: Targeting the Fish Immune System. Biology 2015, 4, 664–696. [Google Scholar] [CrossRef]
- Rosidah, Y. Mini-Review: The Role of Chitosan in Aquaculture Fish Health Management. Asian J. Fish. Aquat. Res. 2022, 9, 18197. [Google Scholar] [CrossRef]
- Stanek, M.; Mazurkiewicz, J.; Rawski, M.; Bogucka, J.; Ziółkowska, E.; Dankowiakowska, A.; Kierończyk, B. Effect of chitosan on common carp (Cyprinus carpio) fry growth performance, feed utilization and nutriphysiological status. Aquac. Rep. 2023, 30, 101622. [Google Scholar] [CrossRef]
- Mei, Q.; Hu, J.; Huang, Z.; Fan, J.; Huang, C.; Lu, Y.; Wang, X.; Zeng, Y. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol. Sin. 2021, 42, 942–953. [Google Scholar] [CrossRef]
- Tzeng, H.; Liu, S.; Chiang, M. Antidiabetic Properties of Chitosan and Its Derivatives. Mar. Drugs 2022, 20, 784. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, S.; Gao, X.; Huang, H.; Lao, F.; Dai, X. Protective Effect of Chitosan Oligosaccharide against Hydrogen Peroxide-Mediated Oxidative Damage and Cell Apoptosis via Activating Nrf2/ARE Signaling Pathway. Neurotox. Res. 2021, 39, 1708–1720. [Google Scholar] [CrossRef]
- Koochacksaraei, R.; Dastar, B.; Samadi, F.; Ebrahimi, P. Investigating of Antioxidant Protective Effects of Shrimp Shells Extracted Chitosan in Broiler Chickens. World’s Poult. Sci. J. 2020, 8, 77–81. [Google Scholar]
| Ingredients | Chitosan Levels (%) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Chitosan | 0.00 | 0.50 | 1.00 | 1.50 | 2.00 |
| Soybean oil | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Fish oil | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Fish meal | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Rapeseed meal | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 |
| Soybean meal | 33.00 | 33.00 | 33.00 | 33.00 | 33.00 |
| Dextrin | 24.39 | 23.89 | 23.39 | 22.89 | 22.39 |
| Gelatin | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Vitamins mixture 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Minerals mixture 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Sodium chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Adhesive 3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Attractant 4 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Preservative 5 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Proximate composition (%) | |||||
| Crude protein | 33.88 | 33.88 | 33.88 | 33.88 | 33.88 |
| Crude fat | 7.35 | 7.35 | 7.35 | 7.35 | 7.35 |
| Ash | 6.96 | 6.96 | 6.96 | 6.96 | 6.96 |
| Moisture | 9.36 | 9.36 | 9.36 | 9.36 | 9.36 |
| Crude fiber | 5.56 | 5.56 | 5.56 | 5.56 | 5.56 |
| Gross energy (Mcal/kg) | 3.83 | 3.83 | 3.83 | 3.83 | 3.83 |
| Gene | Primer Sequence (5′→3′) | Amplicon Size (bp) | Gene Bank |
|---|---|---|---|
| β-actin 1 | F: TGACCCAGATCATGTTTGAGACC | 146 | XM_031811226.1 |
| R: CTCGTAGATGGGTACTGTGTGGG | |||
| shh 2 | F: GGGAGAGGCAGACTGTAGAGATAGC | 125 | XM_003439222.5 |
| R: GACAAGCAGATGAGACCGACCAAC | |||
| hsp 3 | F: CAAGGTGATTTCAGACGGAGGGAAG | 123 | XM_003442456.5 |
| R: GCCTCTGCGATCTCCTTCATCTTC | |||
| mt 4 | F: AACGCCAGCATCACTCGGAAC | 84 | YP_003587621.1 |
| R: GCGGCAGGAACACTCACTCTTG | |||
| cyp1a 5 | F: AGAGTCAGTAGGCACAGTGTCCATC | 129 | NM_001279489.1 |
| R: GGGGCAAGTTGTTCCGATCAGAG | |||
| cpt-1 6 | F: ATTGGCAGGACAGCGACTACATTG | 143 | XM_019362661.2 |
| R: GGAAGGAGGTGAAGGGTCATCTAGG | |||
| pparα 7 | F: GTGGCTGCTATTATCTGCTGTGGAG | 140 | XM_019346353.2 |
| R: CTGGGGAAAAGGAAGGTGTCATCTG | |||
| hsl 8 | F: CAAGCGGCATCAGTCAGGAATAGG | 80 | XM_005463937.4 |
| R: CTCAACTCGGGGTCAATGGCATAC | |||
| lpl 9 | F: CTTCAGCCAGAACCAGCAGAGC | 142 | NM_001279753.1 |
| R: GTCGGTGGTGATGAGGAAGGATTG | |||
| mdh 10 | F: GGTGCTCGCTTCTTGTGGACAG | 121 | XM_005450070.4 |
| R: GACGGCCTCATTCTCATCTTCTTCC | |||
| lep 11 | F: GAAGTGGATCGCTGAGCATCTGG | 129 | XM_005449522.4 |
| R: CCATCCAAGCAGACCGTGACTATG | |||
| pparγ 12 | F: GTACACGGAGGCTACACGGAAAC | 139 | XM_019358463.2 |
| R: CTGCTTCTGCTGAACGAGACTGAC | |||
| fas 13 | F: AAGCCTTGTGTGCCTTCATCCAG | 133 | XM_003454056.5 |
| R: TCCCTGTGAGCGGAGGTGATTAG | |||
| srebp1 14 | F: GAACAGCAGCCGACAGATCACTC | 116 | XM_005473610.4 |
| R: TACAGCAGCCATTAACGAGCAAGTC | |||
| sqle 15 | F: CTGACGGGAGGAGGGATGAGTG | 82 | XM_003453510.5 |
| R: CATACAGGTCGGGAATGCTCTTGAG | |||
| scd 16 | F: ACAAGCTCTCCGTGCTGGTCAT | 102 | XM_005471382.2 |
| R: GCAGAGTTGGGACGAAGTAGGC |
| Index | Chitosan Levels (%) | p-Values | ||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | ||
| Moisture (%) | 73.17 ± 1.23 | 72.67 ± 1.11 | 72.42 ± 1.37 | 72.48 ± 0.74 | 73.02 ± 0.63 | 0.401 |
| Crude protein (%) | 22.72 ± 0.25 b | 23.49 ± 0.30 a | 23.42 ± 0.37 a | 23.71 ± 0.43 a | 23.69 ± 0.23 a | 0.000 |
| Crude fat (%) | 6.97 ± 0.16 b | 7.38 ± 0.12 a | 7.43 ± 0.10 a | 7.58 ± 0.08 a | 7.50 ± 0.18 a | 0.000 |
| Ash (%) | 1.40 ± 0.02 | 1.40 ± 0.01 | 1.37 ± 0.01 | 1.37 ± 0.01 | 1.39 ± 0.01 | 0.297 |
| Index | Chitosan Levels (%) | p-Values | ||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | ||
| Lipase (U/mgprot) 1 | 0.12 ± 0.01 c | 0.27 ± 0.01 b | 0.28 ± 0.01 b | 0.33 ± 0.02 a | 0.32 ± 0.01 a | 0.000 |
| Trypsin (U/mgprot) 2 | 8.21 ± 1.39 b | 18.31 ± 1.55 a | 18.58 ± 0.94 a | 19.24 ± 0.58 a | 18.24 ± 1.69 a | 0.000 |
| α-amylase (U/mgprot) 3 | 1592.97 ± 100.05 b | 2764.49 ± 322.01 a | 2815.82 ± 185.95 a | 2912.66 ± 367.67 a | 2995.89 ± 249.55 a | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Xie, Y.; Zhang, Y.; Huang, E.; Meng, L.; Liu, Y.; Tong, T. Effects of Dietary Supplementation with Chitosan on the Muscle Composition, Digestion, Lipid Metabolism, and Stress Resistance of Juvenile Tilapia (Oreochromis niloticus) Exposed to Cadmium-Induced Stress. Animals 2024, 14, 541. https://doi.org/10.3390/ani14040541
Zhang Q, Xie Y, Zhang Y, Huang E, Meng L, Liu Y, Tong T. Effects of Dietary Supplementation with Chitosan on the Muscle Composition, Digestion, Lipid Metabolism, and Stress Resistance of Juvenile Tilapia (Oreochromis niloticus) Exposed to Cadmium-Induced Stress. Animals. 2024; 14(4):541. https://doi.org/10.3390/ani14040541
Chicago/Turabian StyleZhang, Qin, Yi Xie, Yuanhui Zhang, Enhao Huang, Liuqing Meng, Yongqiang Liu, and Tong Tong. 2024. "Effects of Dietary Supplementation with Chitosan on the Muscle Composition, Digestion, Lipid Metabolism, and Stress Resistance of Juvenile Tilapia (Oreochromis niloticus) Exposed to Cadmium-Induced Stress" Animals 14, no. 4: 541. https://doi.org/10.3390/ani14040541
APA StyleZhang, Q., Xie, Y., Zhang, Y., Huang, E., Meng, L., Liu, Y., & Tong, T. (2024). Effects of Dietary Supplementation with Chitosan on the Muscle Composition, Digestion, Lipid Metabolism, and Stress Resistance of Juvenile Tilapia (Oreochromis niloticus) Exposed to Cadmium-Induced Stress. Animals, 14(4), 541. https://doi.org/10.3390/ani14040541







