Impact of Climate Change on the Distribution of Three Rare Salamanders (Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis) in Chongqing, China, and Their Conservation Implications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Occurrence Data Collection
2.2.1. Field Surveys
2.2.2. Museum and Literature-Based Distribution Data Collection
2.2.3. Occurrence Data Processing
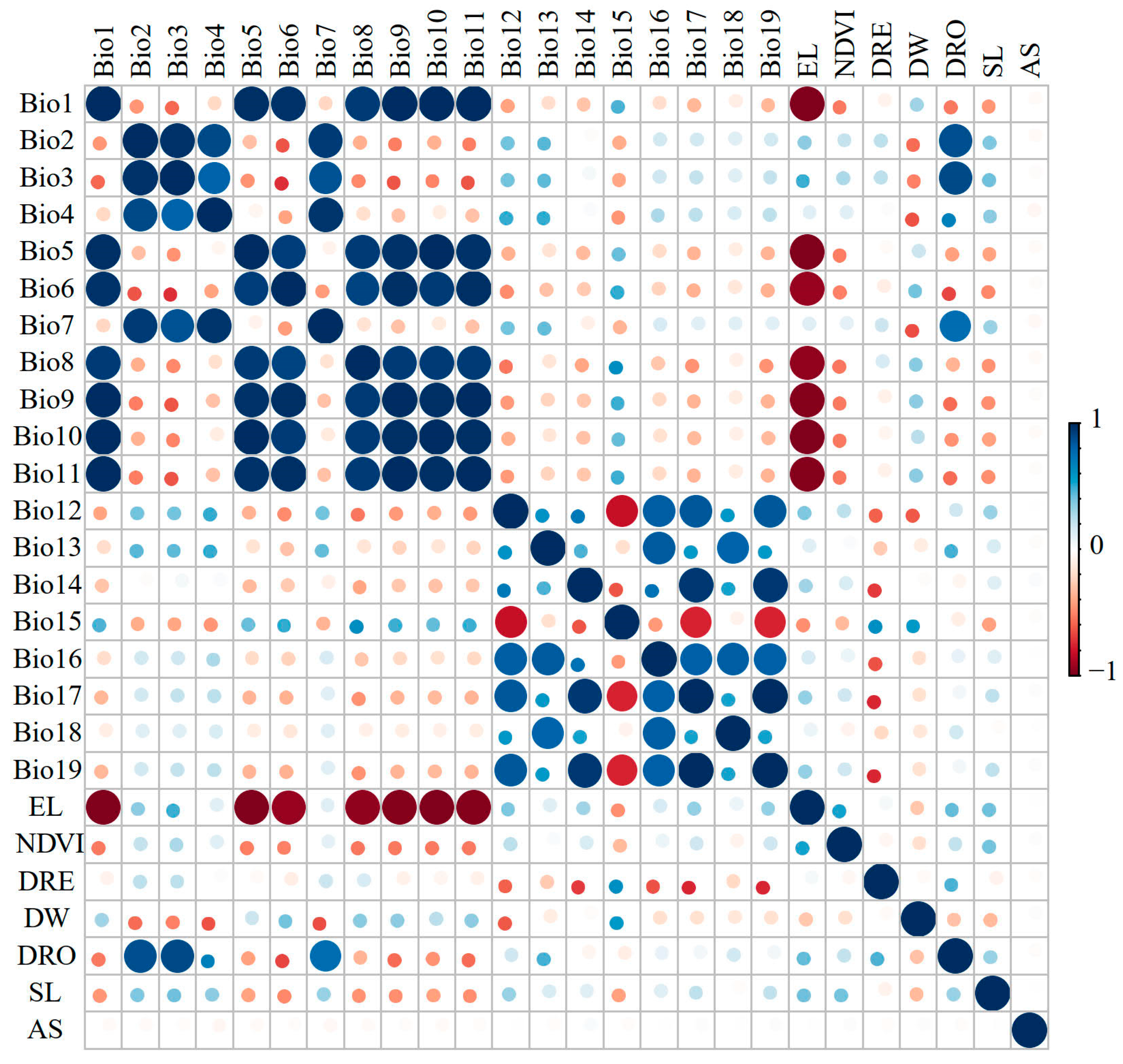
2.3. Environmental Variables
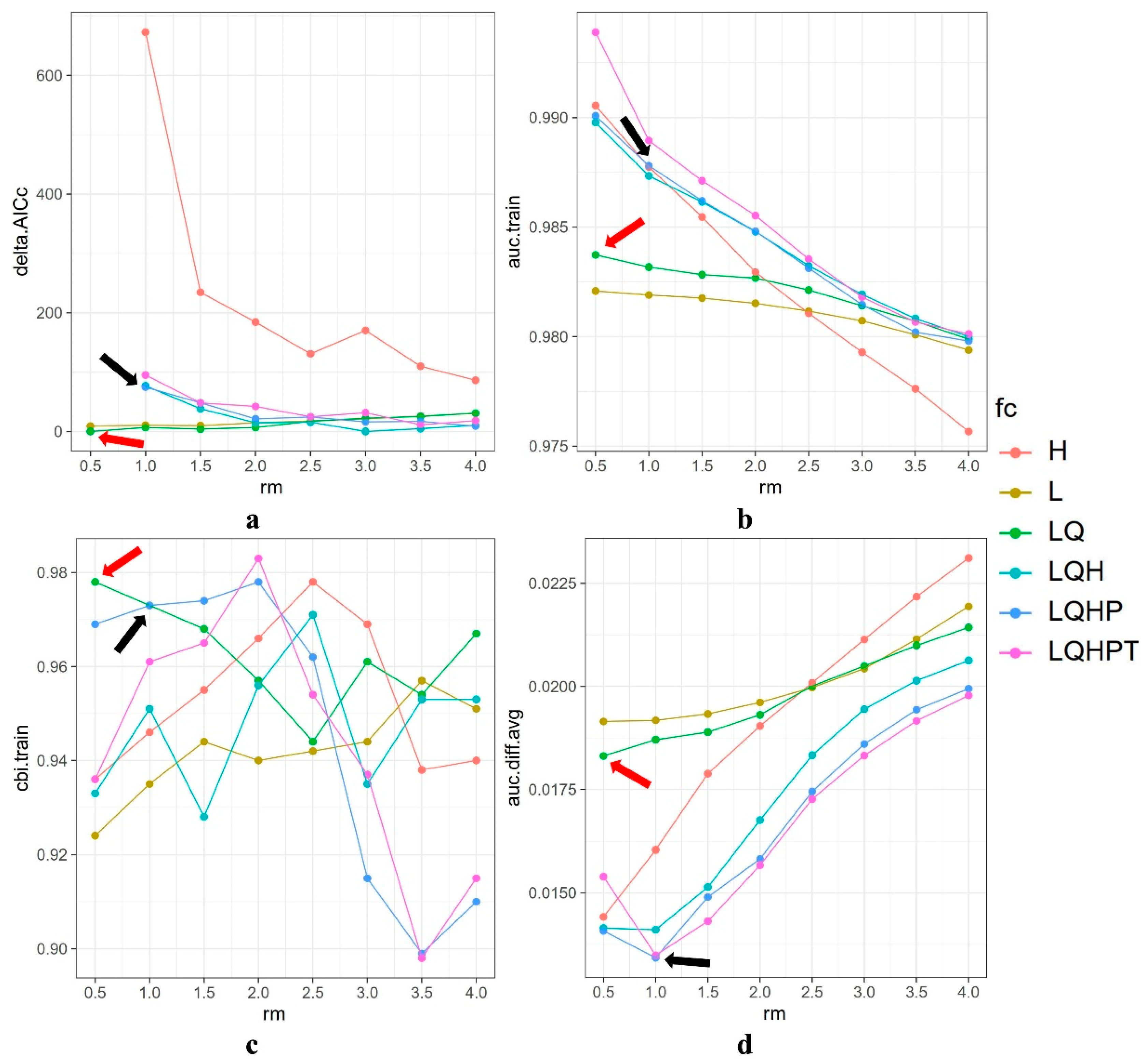
2.4. Model Parameter Optimization
2.5. Species Distribution Models
2.6. Changes in Suitable Area under Different Climatic Conditions
3. Results
3.1. Optimal Model and Model Accuracy Evaluation
3.2. Key Environmental Factors of the Predicted Distribution of Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis
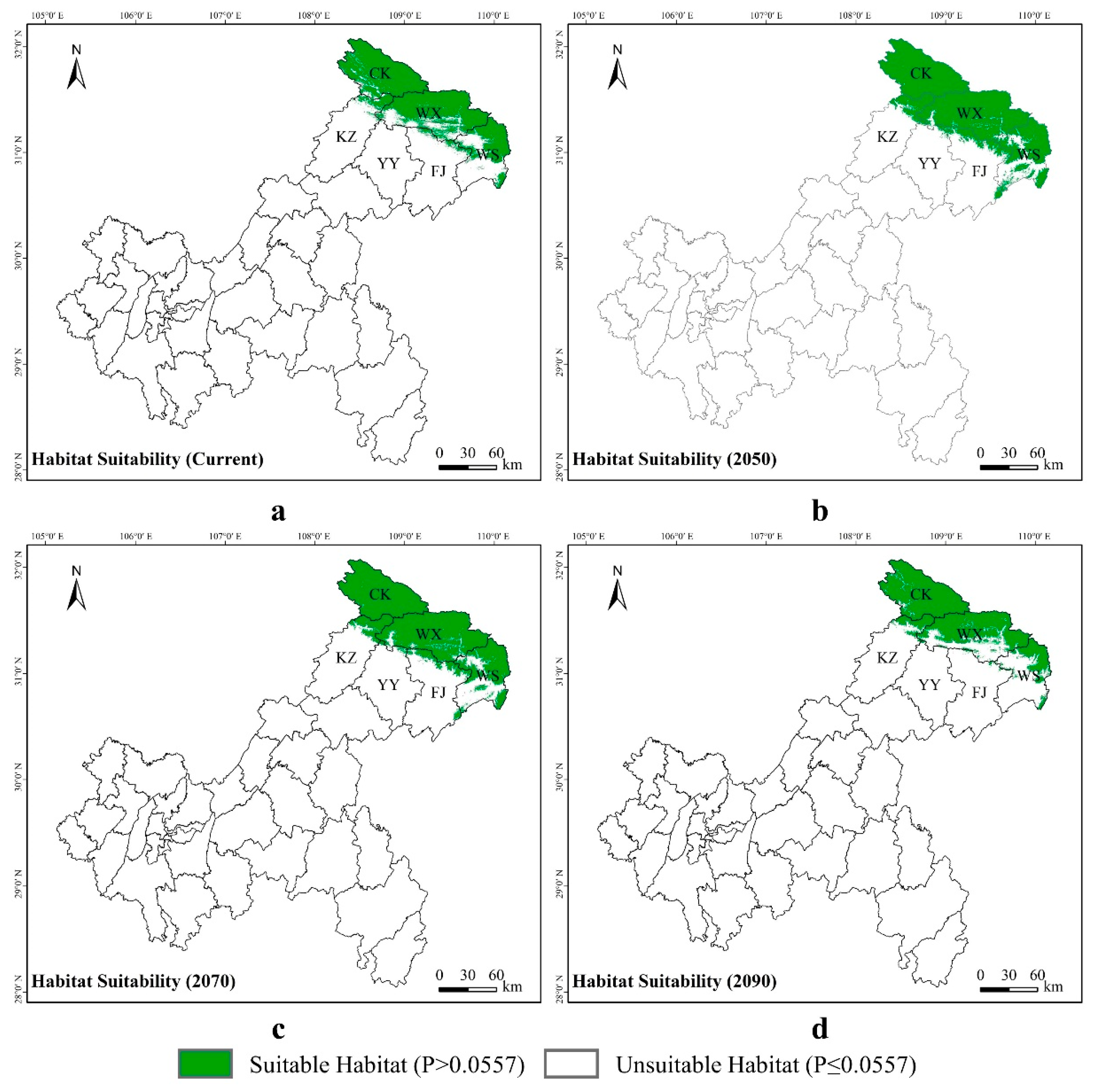
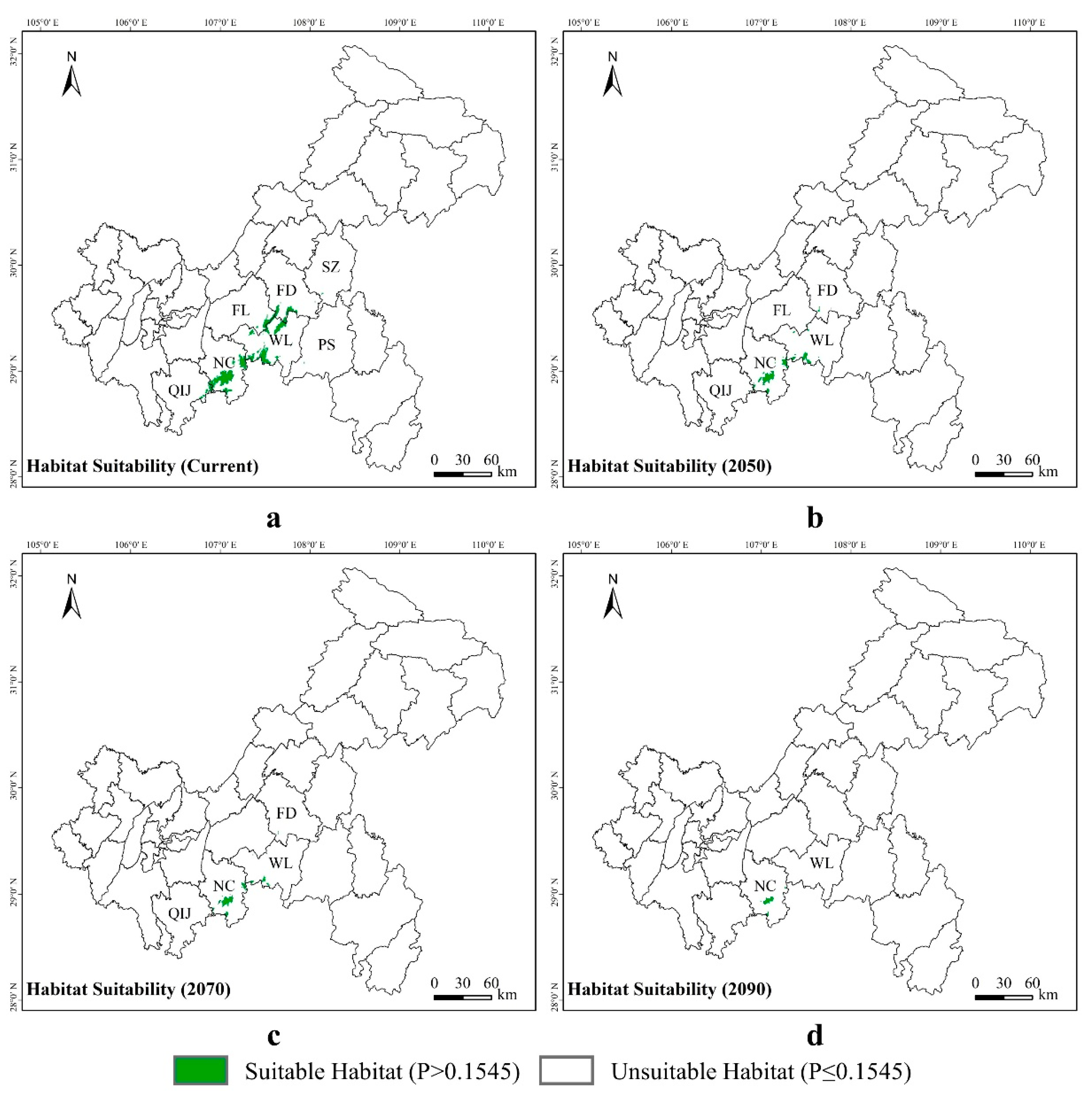
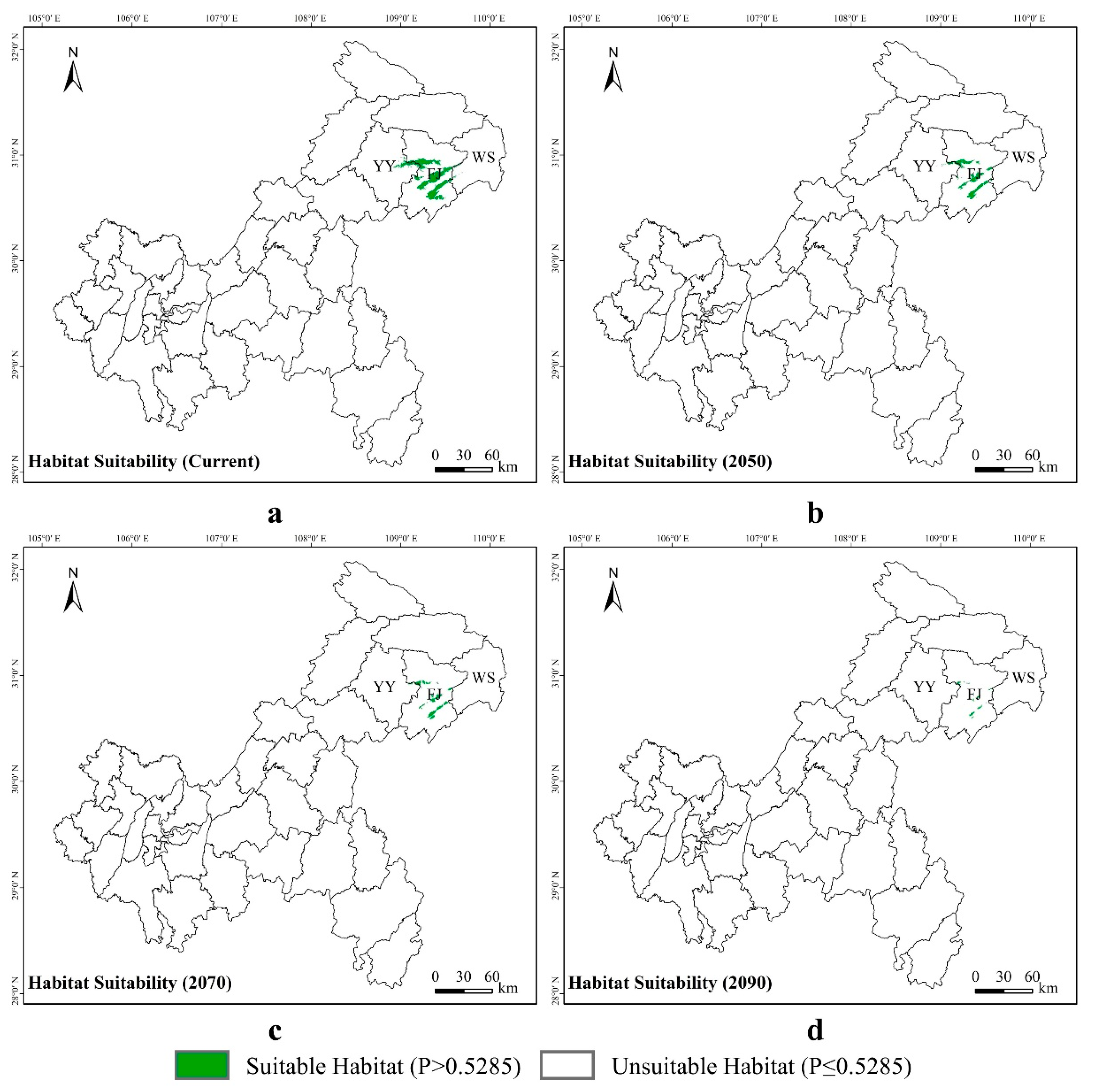
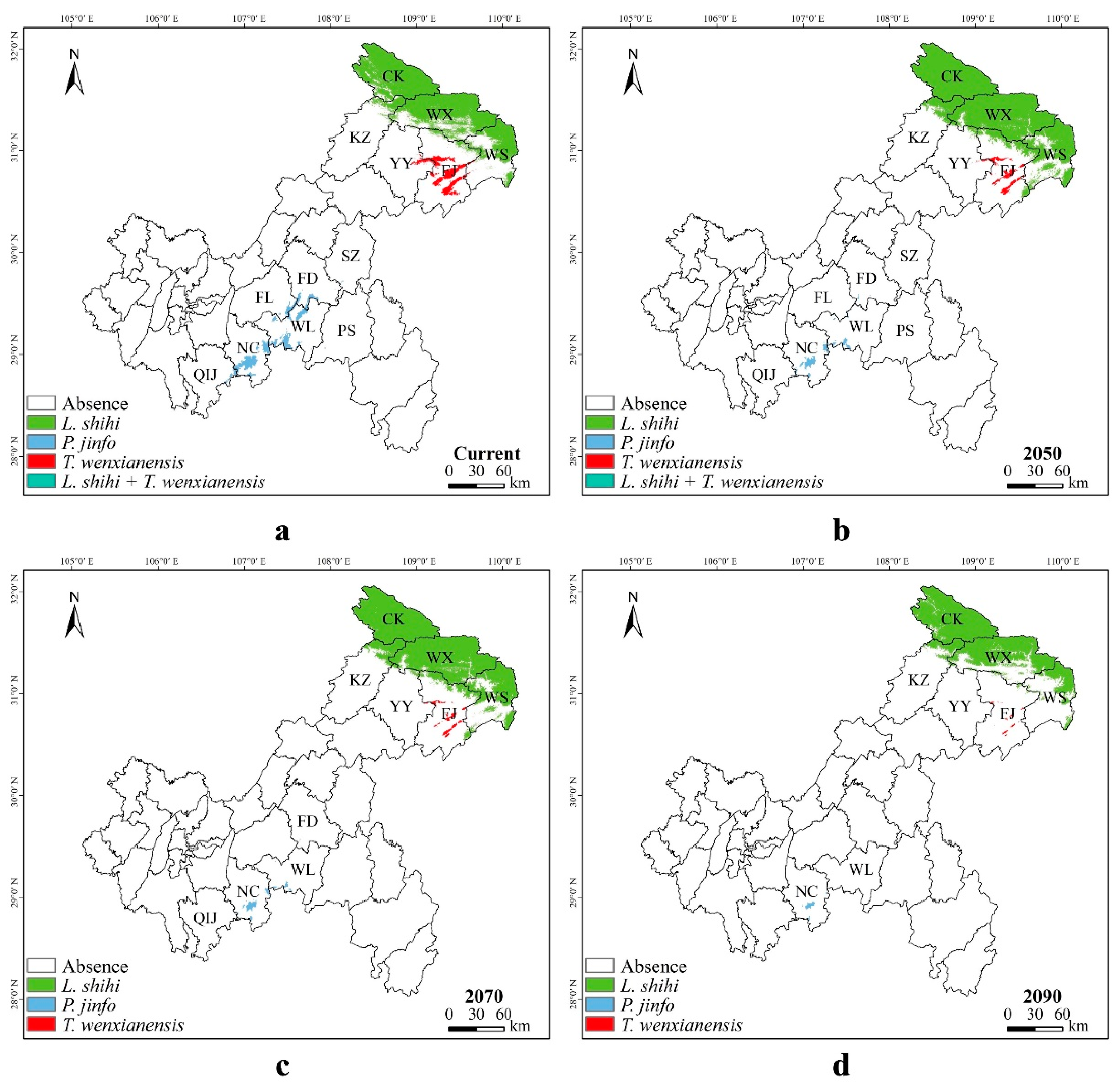
3.3. Potential Suitable Areas for Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis under Current and Future Climate Conditions
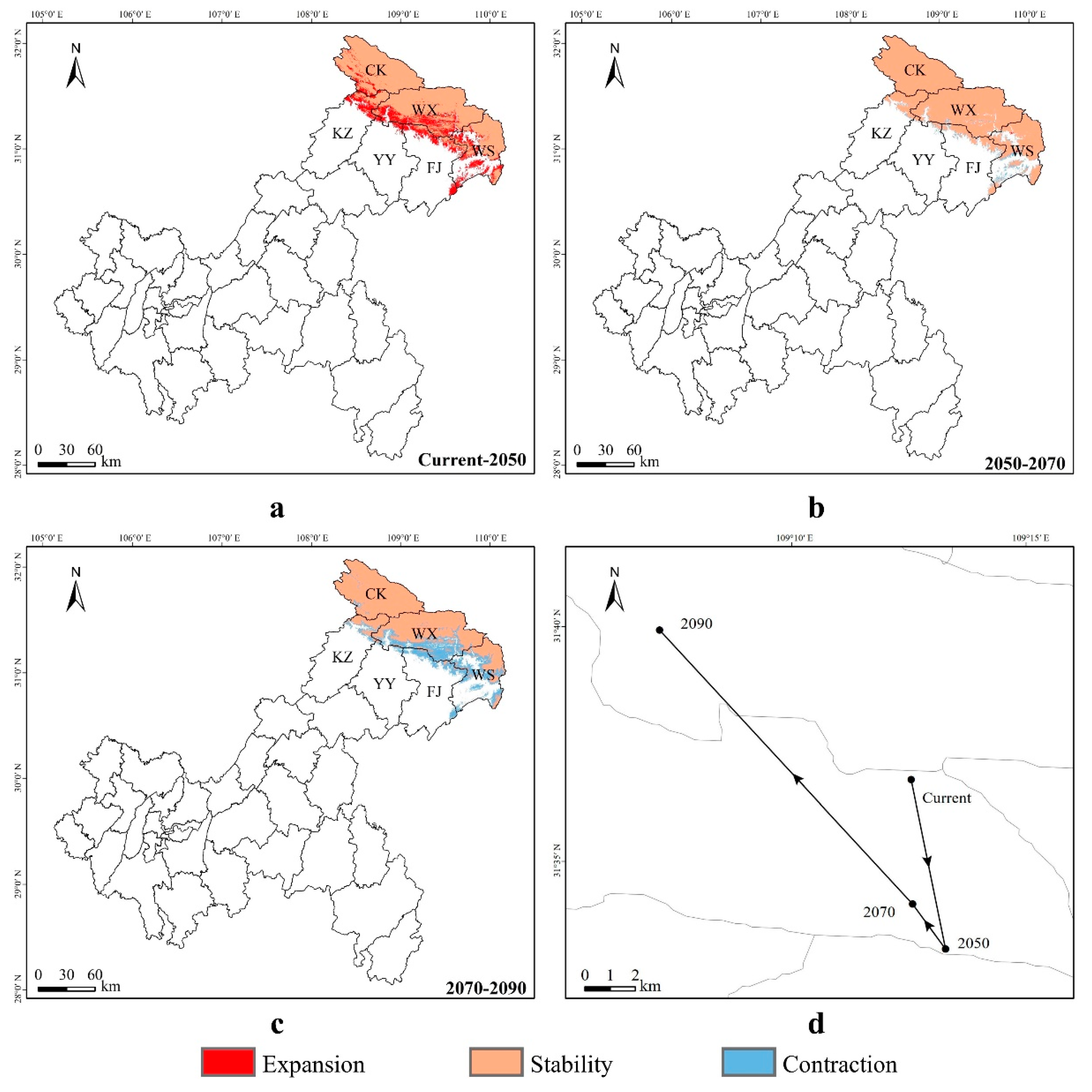
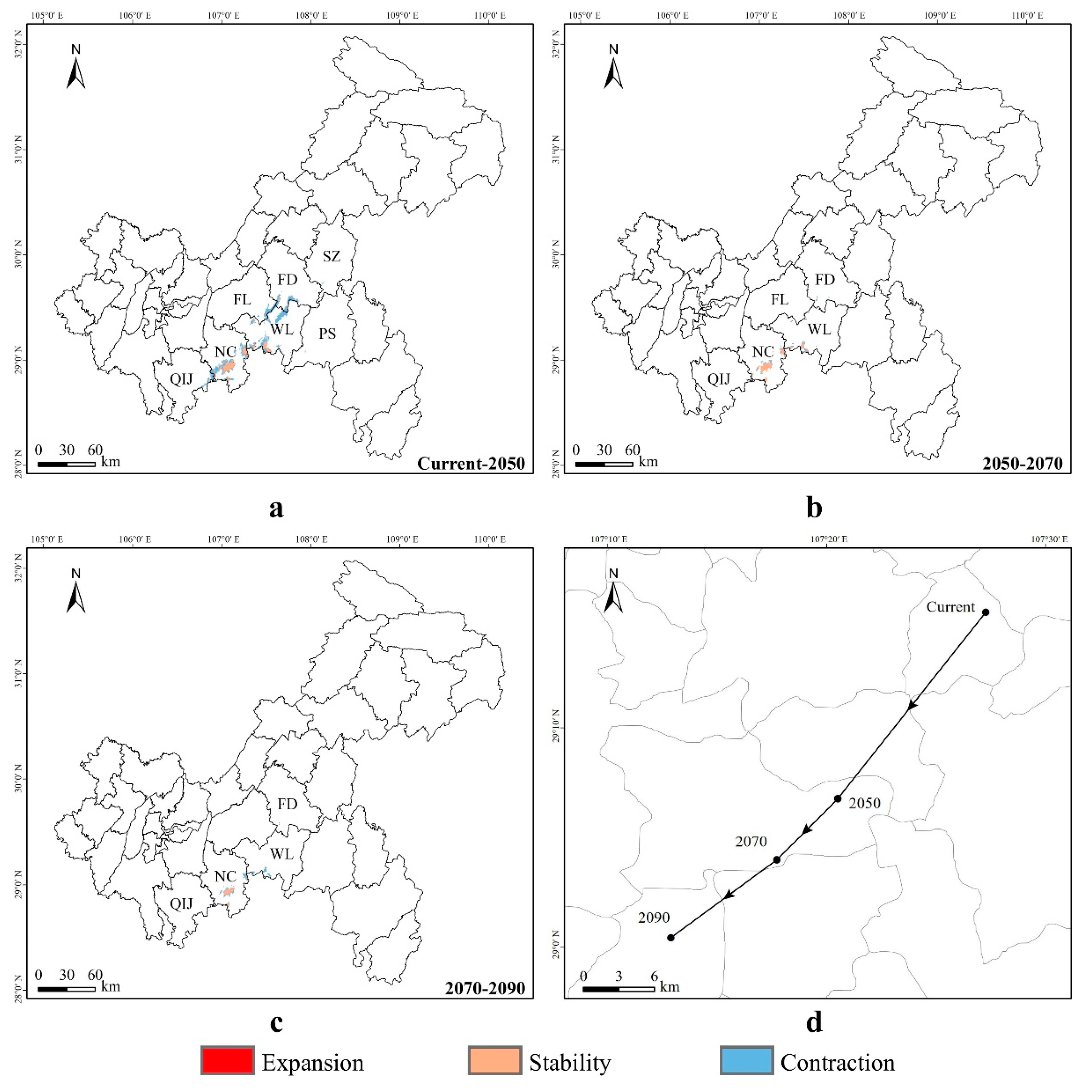
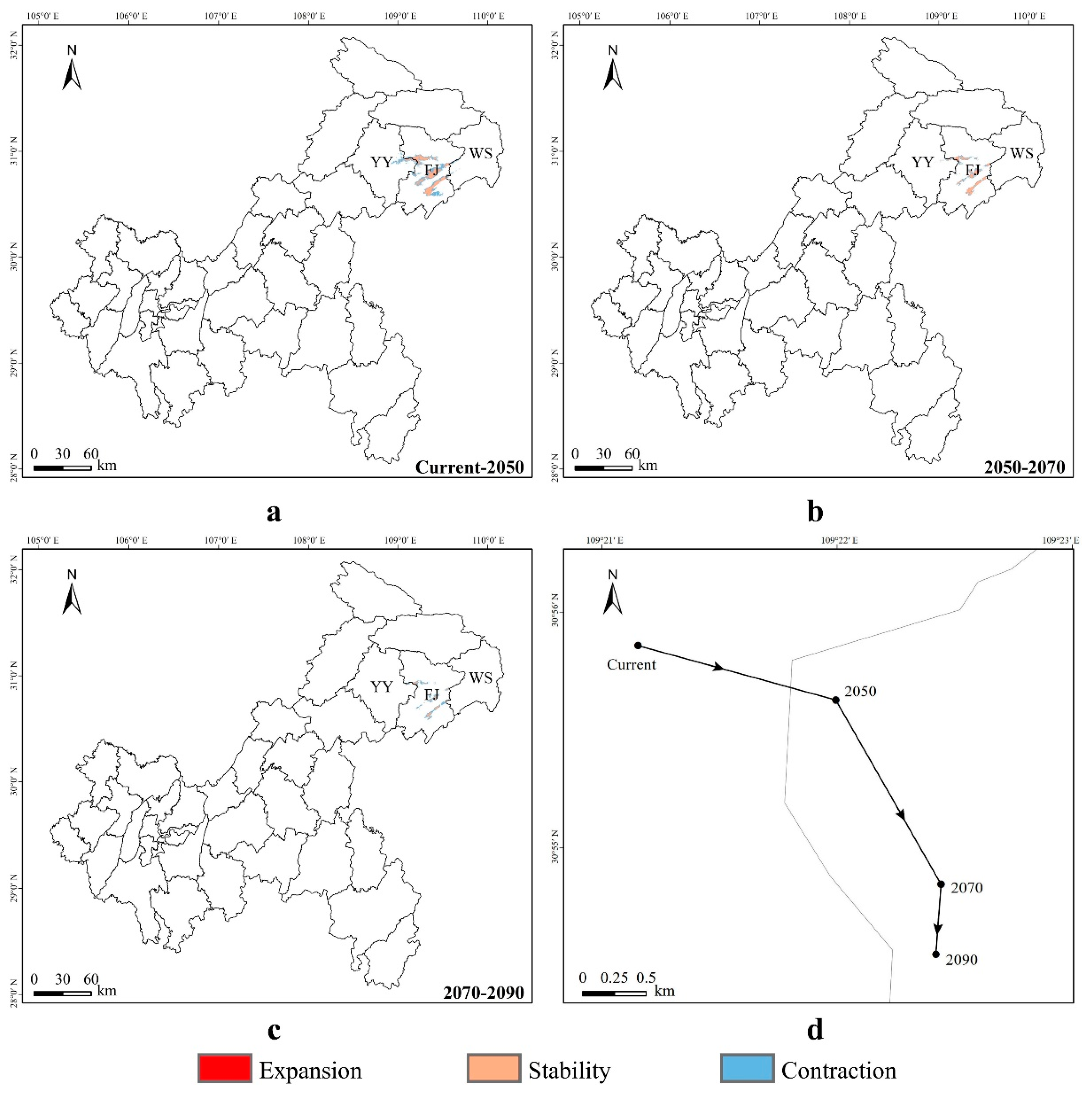
3.4. Spatial Transfer Characteristics of Suitable Areas for Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis
3.5. Overlapping Distribution Regions of Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis during Various Periods
4. Discussion
4.1. Impact of Anthropogenic Disturbances, Bioclimatic Variables, and Water Sources on the Predicted Distribution Areas of Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis
4.2. Key Factors Influencing the Predicted Distributional Shifts of Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis under Climate Change and Implications for Conservation
4.3. Changes in the Predicted Distribution Areas of Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis in the Mountainous Regions of Chongqing under Climate Change Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panetta, A.M.; Stanton, M.L.; Harte, J. Climate warming drives local extinction: Evidence from observation and experimentation. Sci. Adv. 2018, 4, eaaq1819. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Tape, K.D.; Christie, K.; Carroll, G.; O’Donnell, J.A. Novel wildlife in the Arctic: The influence of changing riparian ecosystems and shrub habitat expansion on snowshoe hares. Glob. Chang. Biol. 2015, 22, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Houlahan, J.E.; Findlay, C.S.; Schmidt, B.R.; Meyer, A.H.; Kuzmin, S.L. Quantitative evidence for global amphibian population declines. Nature 2000, 404, 752–755. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Han, B.A.; Relyea, R.A.; Johnson, P.T.J.; Buck, J.C.; Gervasi, S.S.; Kats, L.B. The complexity of amphibian population declines: Understanding the role of cofactors in driving amphibian losses. Ann. N. Y. Acad. Sci. 2011, 1223, 108–119. [Google Scholar] [CrossRef]
- Button, S.; Borzée, A. An integrative synthesis to global amphibian conservation priorities. Glob. Chang. Biol. 2021, 27, 4516–4529. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.; Van Der Wal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E.; et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Chang. 2013, 3, 678–682. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Varela, S.; Ji, X. The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ 2016, 4, e2185. [Google Scholar] [CrossRef]
- Jiang, J.P.; Xie, F.; Li, C.; Wang, B. China’s Red List of Biodiversity: Vertebrates Volume IV, Amphibians (I); Science Press: Beijing, China, 2021; p. 413. (In Chinese) [Google Scholar]
- Jiang, J.P.; Xie, F.; Li, C.; Wang, B. China’s Red List of Biodiversity: Vertebrates Volume IV, Amphibians (II); Science Press: Beijing, China, 2021; pp. 414–1052. (In Chinese) [Google Scholar]
- IUCN 2024. The IUCN Red List of Threatened Species. Version 2023-1. Available online: https://www.iucnredlist.org/en (accessed on 17 January 2024).
- National Forestry and Grassland Administration Ministry of Agriculture and Rural Affairs. List of National Key Protected Wild Animals (Revised on 1 February 2021). Chin. J. Wildl. 2021, 42, 605–640. (In Chinese) [Google Scholar] [CrossRef]
- Zank, C.; Becker, F.; Abadie, M.; Baldo, D.; Maneyro, R.; Borges-Martins, M. Climate change and the Distribution of Neotropical Red-Bellied Toads (Melanophryniscus, Anura, Amphibia): How to prioritize species and populations? PLoS ONE 2014, 9, e94625. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, S.; Schefczyk, L.; Wagner, N.; Heinemann, G.; Veith, M.; Lötters, S. Exploring the Distribution of the spreading Lethal salamander chytrid fungus in its invasive range in Europe—A macroecological approach. PLoS ONE 2016, 11, e0165682. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ellepola, G.; Herath, J.; Meegaskumbura, M. The two chytrid pathogens of amphibians in Eurasia—Climatic niches and future expansion. BMC Ecol. Evol. 2023, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.R. Overview of Chytrid Emergence and Impacts on Amphibians. Philos. Trans. R. Soc. B 2016, 371, 20150465. [Google Scholar] [CrossRef]
- Groff, L.A.; Marks, S.B.; Hayes, M.P. Using Ecological niche models to direct rare amphibian surveys: A case study using the Oregon Spotted Frog (Rana pretiosa). Herpetol. Conserv. Biol. 2014, 9, 354–368. [Google Scholar]
- Maryam, P.; Mohamad, D.D.; Eskandar, R.P.; Haji, G.K.; Mahsa, H.; Mahsa, H. Species distribution modeling and environmental suitability of the southern crested newt, Triturus karelinii (Strauch, 1870) (Amphibia: Caudata) in Iran. J. Wildl. Biodivers. 2021, 5, 44–52. [Google Scholar] [CrossRef]
- Kim, H.W.; Adhikari, P.; Chang, M.H.; Seo, C. Potential distribution of amphibians with different habitat characteristics in response to climate change in South Korea. Animals 2021, 11, 2185. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cunningham, A.A.; Wei, G.; Yang, J.; Liang, Z.; Wang, J.; Wu, M.; Yan, F.; Xiao, H.; Harrison, X.A.; et al. Determining threatened species distributions in the face of limited data: Spatial conservation prioritization for the Chinese giant salamander (Andrias davidianus). Ecol. Evol. 2018, 8, 3098–3108. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Willard, S.; Wang, Q.; Jiang, W.; Zhang, H.-X.; Kouba, A. Spatial distribution and seasonal movement patterns of reintroduced Chinese giant salamanders. BMC Zool. 2019, 4, 7. [Google Scholar] [CrossRef]
- Zhang, P.; Grenouillet, G.; Dong, X.; Zheng, Y.; Lek, S.; Chang, J. Capturing response differences of species distribution to climate and human pressures by incorporating local adaptation: Implications for the conservation of a critically endangered species. J. Environ. Manag. 2021, 284, 111998. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, W.; Zhou, J.; Zhao, C.; Liu, X.; Liu, Z.; Shu, G.; Wang, S.; Li, C.; Xie, F.; et al. Niche divergence of evolutionarily significant units with implications for repopulation programs of the world’s largest amphibians. Sci. Total Environ. 2020, 738, 140269. [Google Scholar] [CrossRef] [PubMed]
- IUCN SSC Amphibian Specialist Group. Liua shihi. The IUCN Red List of Threatened Species 2020: E.T59112A63859805. Available online: https://www.iucnredlist.org/species/59112/63859805 (accessed on 17 January 2024).
- IUCN SSC Amphibian Specialist Group. Pseudohynobius jinfo. The IUCN Red List of Threatened Species 2020: E.T79123553A122172427. Available online: https://www.iucnredlist.org/species/79123553/122172427 (accessed on 17 January 2024).
- IUCN SSC Amphibian Specialist Group. Tylototriton wenxianensis. The IUCN Red List of Threatened Species 2020: E.T145401116A63871774. Available online: https://www.iucnredlist.org/species/145401116/63871774 (accessed on 17 January 2024).
- Endangered Species Scientific Commission of the People’s Republic of China. Convention on International Trade in Endangered Species of Wild Fauna and Flora, Appendices I, II, and III. Available online: http://www.cites.org.cn/citesgy/fl/202302/t20230227_734178.html (accessed on 17 January 2024).
- Fei, L. Atlas of Amphibians in China (Field Edition); Henan Science and Technology Press: Zhengzhou, China, 2020; p. 837. (In Chinese) [Google Scholar]
- He, J.K.; Gao, E.H.; Xu, Y.; Wang, Z.C.; Lin, S.L.; Tang, X.P.; Yin, M.G.; Ma, G.Z.; Jiang, H.S. Study on the Ecological and Geographical Zonation of Terrestrial Wildlife in China; Science Press: Beijing, China, 2018; p. 298. (In Chinese) [Google Scholar]
- Wei, G.; Xiong, J.; HOU, M.; Zeng, X. A new species of hynobiid salamander (Urodela: Hynobiidae: Pseudohynobius) from Southwestern China. Zootaxa 2009, 2149, 62–68. [Google Scholar] [CrossRef]
- Li, S.Z.; Xu, N.; Liu, J.; Lyu, J.C.; Wang, B.; Wei, G. A revised species list of amphibians in Guizhou Province. Sichuan J. Zool. 2020, 39, 694–710, (In Chinese with English Abstract). [Google Scholar]
- Luo, T.; Yan, S.; Ning, X.; Li, W.; Deng, H.; Zhou, J. A new species of the genus Tylototriton (Amphibia: Urodela: Salamandridae) from the eastern Dalou mountains in Guizhou, China. Zool. Syst. 2022, 47, 66–88. [Google Scholar] [CrossRef]
- Li, S.Z.; Liu, J.; Shi, S.C.; Wei, G.; Wang, B. Description of a new species of the newt genus Tylototriton sensu lato (Amphibia: Urodela: Salamandridae) from southwestern China. Zootaxa 2022, 5128, 248–268. [Google Scholar] [CrossRef]
- Jiang, J.P.; Li, X.; Xie, F.; Wang, B. Supplemental survey on the distribution of Tylototriton wenxianensis in Chongqing, China. Sichuan J. Zool. 2009, 28, 569–571, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y.X.; Xu, Y.Y.; Yang, J.J.; Chen, Y.X.; Wei, J.X.; Zhou, J.; Zhang, W.L.; Cheng, W.X. Dynamic monitoring and spatio-temporal pattern evolution analysis of eco-environmental quality in Chongqing based on remote sensing. Acta Ecol. Sin. 2023, 43, 6278–6292. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, M.; Tong, S.; Liu, Y.; Zhang, D.; Zhu, G.; Lyu, X. Evolution of potential spatial distribution patterns of Carex tussock wetlands under climate change scenarios, Northeast China. Chin. Geogr. Sci. 2022, 32, 142–154. [Google Scholar] [CrossRef]
- Chongqing Municipal Bureau of Statistics NBS Survey Office in Chongqing. Chongoing Statistical Yearbook 2022; China Statistics Press: Beijing, China, 2022; p. 705.
- Chongqing Local Chronicles Office. General Introduction to Natural and Cultural Geography of Chongqing; Chongqing University Press: Chongqing, China, 2021; p. 307.
- Wang, J.X.; Lan, X.Y.; Luo, Q.H.; Deng, K.G.; Liang, Z.Q.; Jiang, W.S. Comparative study on the methods of non-disruptive dna sampling and extraction in Chinese giant salamander (Andrias davidianus). Chin. J. Zool. 2022, 57, 641–652, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Pawar, S.; Koo, M.S.; Kelley, C.; Ahmed, M.F.; Chaudhuri, S.; Sarkar, S. Conservation Assessment and prioritization of areas in Northeast India: Priorities for amphibians and reptiles. Biol. Conserv. 2007, 136, 346–361. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Xiao, N.W.; Liu, G.H.; Li, J.S. Prediction of the potential geographical distribution of five species of Scutiger in the south of Hengduan mountains biodiversity conservation priority zone. Acta Ecol. Sin. 2022, 42, 2636–2647, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Xin, X.G.; Wu, T.W.; Li, J.L.; Wang, Z.Z.; Li, W.P.; Wu, F.H. How well does BCC_CSM1.1 reproduce the 20th century climate change over China? Atmos. Ocean. Sci. Lett. 2013, 6, 21–26. [Google Scholar] [CrossRef]
- Yang, J.; Dong, J.; Xiao, X.; Dai, J.; Wu, C.; Xia, J.; Zhao, G.; Zhao, M.; Li, Z.; Zhang, Y.; et al. Divergent shifts in peak photosynthesis timing of temperate and alpine grasslands in China. Remote Sens. Environ. 2019, 233, 111395. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. MaxEnt is not a presence–absence method: A comment on Thibaud et al. Methods Ecol. Evol. 2014, 5, 1192–1197. [Google Scholar] [CrossRef]
- Dai, Y.; Peng, G.; Wen, C.; Zahoor, B.; Ma, X.; Hacker, C.E.; Xue, Y. Climate and land use changes shift the distribution and dispersal of two umbrella species in the Hindu Kush Himalayan region. Sci. Total Environ. 2021, 777, 146207. [Google Scholar] [CrossRef]
- Zahoor, B.; Liu, X.; Kumar, L.; Dai, Y.; Tripathy, B.R.; Songer, M. Projected shifts in the distribution range of Asiatic black bear (Ursus thibetanus) in the Hindu Kush Himalaya due to climate change. Ecol. Inform. 2021, 63, 101312. [Google Scholar] [CrossRef]
- Aryal, A.; Shrestha, U.B.; Ji, W.; Ale, S.B.; Shrestha, S.; Ingty, T.; Maraseni, T.; Cockfield, G.; Raubenheimer, D. Predicting the distributions of predator (snow leopard) and prey (blue sheep) under climate change in the Himalaya. Ecol. Evol. 2016, 6, 4065–4075. [Google Scholar] [CrossRef]
- Zahoor, B.; Songer, M.; Liu, X.; Huang, Q.; Dai, Y. Identifying stable and overlapping habitats for a predator (common leopard) and prey species (Himalayan grey goral & Himalayan grey langur) in northern Pakistan. Glob. Ecol. Conserv. 2023, 43, e02418. [Google Scholar] [CrossRef]
- Liu, T.M.; Wang, J.M.; Hu, X.K.; Feng, J.M. Land-use change drives present and future distributions of Fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sci. Total Environ. 2020, 706, 135872. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Huang, H.; Qing, Y.; Li, J.; Li, D. Ecological response of an umbrella species to changing climate and land use: Habitat conservation for Asiatic black bear in the Sichuan-Chongqing Region, Southwestern China. Ecol. Evol. 2023, 13, e10222. [Google Scholar] [CrossRef] [PubMed]
- Goldsmit, J.; McKindsey, C.W.; Schlegel, R.W.; Stewart, D.B.; Archambault, P.; Howland, K.L. What and where? Predicting invasion hotspots in the Arctic marine realm. Glob. Chang. Biol. 2020, 26, 4752–4771. [Google Scholar] [CrossRef]
- Merow, C.; Silander, J.A., Jr. A comparison of Maxlike and MaxEnt for modelling species distributions. Methods Ecol. Evol. 2014, 5, 215–225. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better MaxEnt models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Cui, L.; De Angelis, D.L.; Berger, U.; Cao, M.; Zhang, Y.; Zhang, X.; Jiang, J. Global potential distribution of mangroves: Taking into account salt marsh interactions along latitudinal gradients. J. Environ. Manag. 2024, 351, 119892. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Zeng, Y.; Low, B.W.; Yeo, D.C.J. Novel methods to select environmental variables in MaxEnt: A case study using invasive crayfish. Ecol. Model. 2016, 341, 5–13. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. Anderson ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Zhu, G.; Qiao, H. Effect of the MaxEnt Model’s complexity on the prediction of species potential distributions. Biodivers. Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef]
- Wen, X.; Zhao, G.; Cheng, X.; Chang, G.; Dong, X.; Lin, X. Prediction of the potential distribution pattern of the great gerbil (Rhombomys opimus) under climate change based on ensemble modelling. Pest Manag. Sci. 2022, 78, 3128–3134. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhou, B.; Ma, C.; Gao, C.; Han, D.; Chai, Y. Impacts of climate change on species distribution patterns of Polyspora sweet in China. Ecol. Evol. 2022, 12, e9516. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; da Silva, R.S.; Santana, P.A.; Picanço, M.C.; Kyerematen, R.; Sètamou, M.; Ekesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef]
- Lemke, D.; Hulme, P.E.; Brown, J.A.; Tadesse, W. Distribution modelling of Japanese honeysuckle (Lonicera japonica) invasion in the Cumberland Plateau and Mountain Region, USA. For. Ecol. Manag. 2011, 262, 139–149. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Zang, E.; Qie, Q.; He, S.; Hao, W.; Lan, Y.; Liu, Z.; Sun, G.; Wang, Y. Geographic distribution pattern and ecological niche differentiation of endangered Opisthopappus in Taihang Mountains. Braz. J. Bot 2023, 46, 217–226. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Caruso, N.M.; Sears, M.W.; Adams, D.C.; Lips, K.R. Widespread rapid reductions in body size of adult salamanders in response to climate change. Glob. Chang. Biol. 2014, 20, 1751–1759. [Google Scholar] [CrossRef]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Coffin, A.W. From roadkill to road ecology: A review of the ecological effects of roads. J. Transp. Geogr. 2007, 15, 396–406. [Google Scholar] [CrossRef]
- Forman, R.T. Road ecology: A solution for the giant embracing us. Landsc. Ecol. 1998, 13, III. [Google Scholar] [CrossRef]
- Gong, M.H.; Ouyang, Z.Y.; Xu, W.H.; Song, Y.L.; Dai, B. The location of wildlife corridors under the impact of road disturbance: Case study of a giant panda conservation corridor. Acta Ecol. Sin. 2015, 35, 3447–3453, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Muir, A.P.; Biek, R.; Mable, B.K. Behavioural and physiological adaptations to low-temperature environments in the common frog, Rana temporaria. BMC Evol. Biol. 2014, 14, 110. [Google Scholar] [CrossRef]
- Ruiz-Aravena, M.; Gonzalez-Mendez, A.; Estay, S.A.; Gaitán-Espitia, J.D.; Barria-Oyarzo, I.; Bartheld, J.L.; Bacigalupe, L.D. Impact of global warming at the range margins: Phenotypic plasticity and behavioral thermoregulation will buffer an endemic amphibian. Ecol. Evol. 2014, 4, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Madden, L.V. Evaluating predictive models with application in regulatory policy for invasive weeds. Agric. Syst. 2003, 76, 755–774. [Google Scholar] [CrossRef]
- Guerry, A.D.; Hunter, M.L. Amphibian distributions in a landscape of forests and agriculture: An examination of landscape composition and configuration. Conserv. Biol. 2002, 16, 745–754. [Google Scholar] [CrossRef]
- Van Buskirk, J. Local and landscape influence on amphibian occurrence and abundance. Ecology 2005, 86, 1936–1947. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, L.; Nobis, M.P.; Wu, X.; Pan, K.; Wang, K.; Dakhil, M.A.; Du, M.; Xiong, Q.; Pandey, B.; et al. Climate change jointly with migration ability affect future range shifts of dominant fir species in Southwest China. Divers. Distrib. 2019, 26, 352–367. [Google Scholar] [CrossRef]




| Environment Variable | Variable Type |
|---|---|
| Bio1 | Annual Mean Temperature/°C |
| Bio2 | Mean Diurnal Range/°C |
| Bio3 | Isothermality [(BIO2/BIO7) × 100] |
| Bio4 | Temperature Seasonality |
| Bio5 | Max Temperature of Warmest Month/°C |
| Bio6 | Min Temperature of Coldest Month/°C |
| Bio7 | Temperature Annual Range (BIO5-BIO6)/°C |
| Bio8 | Mean Temperature of Wettest Quarter/°C |
| Bio9 | Mean Temperature of Driest Quarter/°C |
| Bio10 | Mean Temperature of Warmest Quarter/°C |
| Bio11 | Mean Temperature of Coldest Quarter/°C |
| Bio12 | Annual Precipitation/mm |
| Bio13 | Precipitation of Wettest Month/mm |
| Bio14 | Precipitation of Driest Month/mm |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) |
| Bio16 | Precipitation of Wettest Quarter/mm |
| Bio17 | Precipitation of Driest Quarter/mm |
| Bio18 | Precipitation of Warmest Quarter/mm |
| Bio19 | Precipitation of Coldest Quarter/mm |
| AS | Aspect |
| SL | Slope |
| EL | Elevation/m |
| DRO | Distance to the nearest road/m |
| DRE | Distance to the nearest residential area/m |
| DW | Distance to the nearest surface water/m |
| NDVI | Normalized Difference Vegetation Index |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Wan, L.; Shi, S.; Wang, Z. Impact of Climate Change on the Distribution of Three Rare Salamanders (Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis) in Chongqing, China, and Their Conservation Implications. Animals 2024, 14, 672. https://doi.org/10.3390/ani14050672
Ma Q, Wan L, Shi S, Wang Z. Impact of Climate Change on the Distribution of Three Rare Salamanders (Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis) in Chongqing, China, and Their Conservation Implications. Animals. 2024; 14(5):672. https://doi.org/10.3390/ani14050672
Chicago/Turabian StyleMa, Qi, Lipeng Wan, Shengchao Shi, and Zhijian Wang. 2024. "Impact of Climate Change on the Distribution of Three Rare Salamanders (Liua shihi, Pseudohynobius jinfo, and Tylototriton wenxianensis) in Chongqing, China, and Their Conservation Implications" Animals 14, no. 5: 672. https://doi.org/10.3390/ani14050672





