Increased Dietary Trp, Thr, and Met Supplementation Improves Performance, Health, and Protein Metabolism of Weaned Piglets under Mixed Management and Poor Housing Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
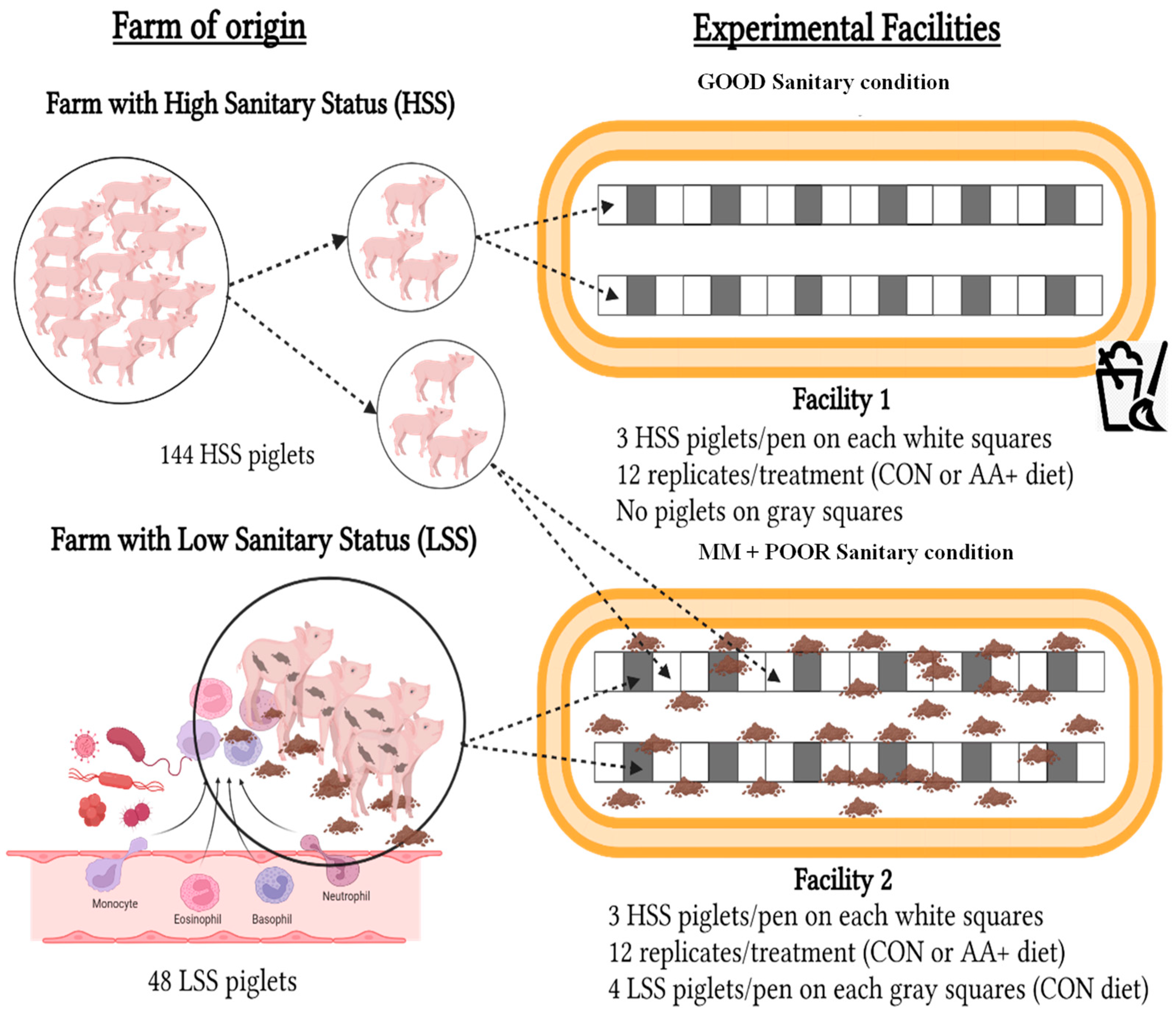
2.1. Housing, Animals, and Experimental Design
2.2. Experimental Diets
2.3. Data Collection
2.3.1. Performance
2.3.2. Blood Sampling
2.3.3. Analysis of Acute Phase Proteins and Biochemical and Redox Parameters
2.3.4. Fecal Sampling and DNA Extraction
2.3.5. 16S rRNA Amplicon Sequencing and Bioinformatic Processing
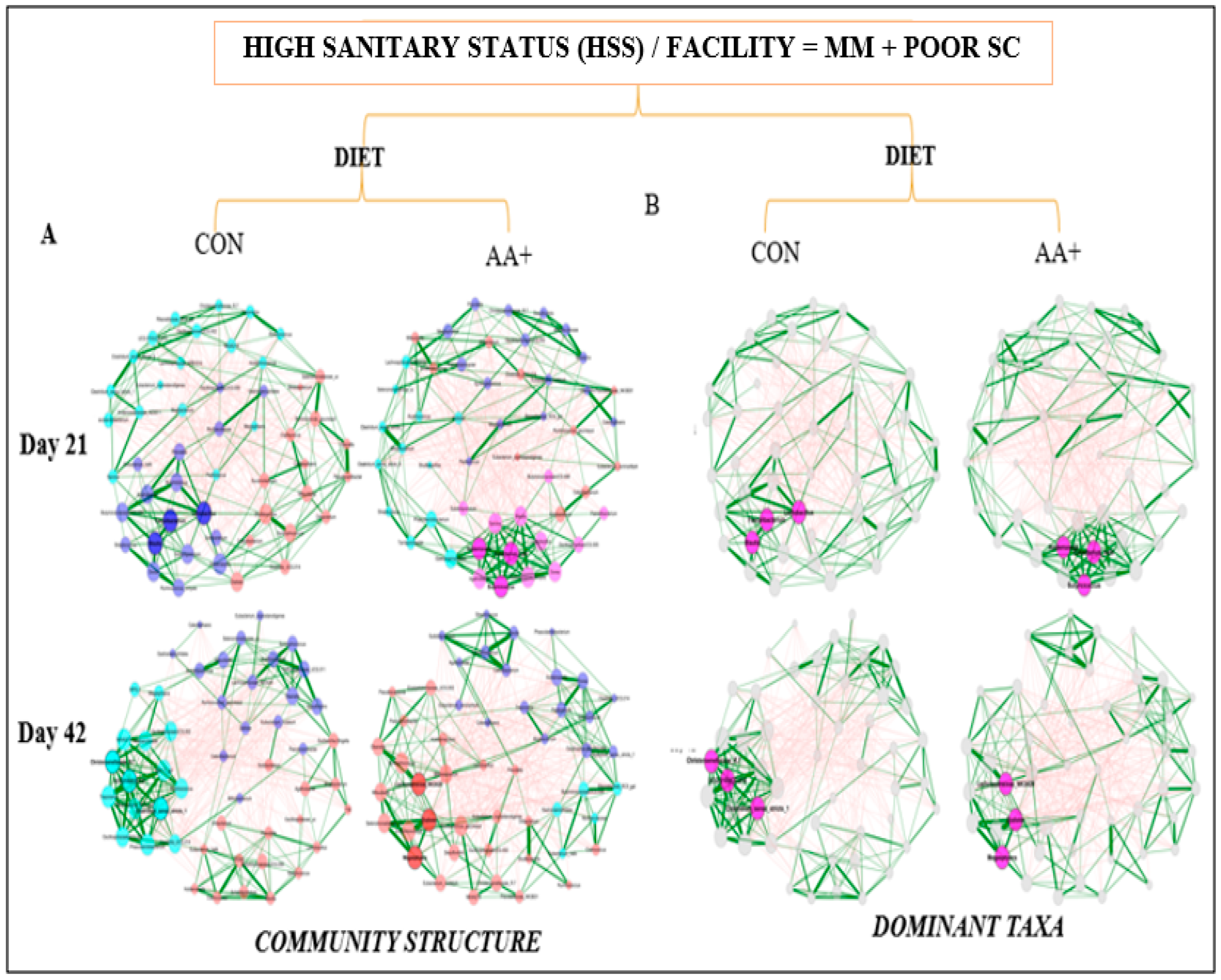
2.4. Network Analysis of the Fecal Microbiome Data
2.5. Statistical Analyses
3. Results
3.1. Characterization of the Health and GI Microbiome Status
3.2. Growth Performance
3.3. Acute-Phase Proteins, Biochemical and Redox Parameters
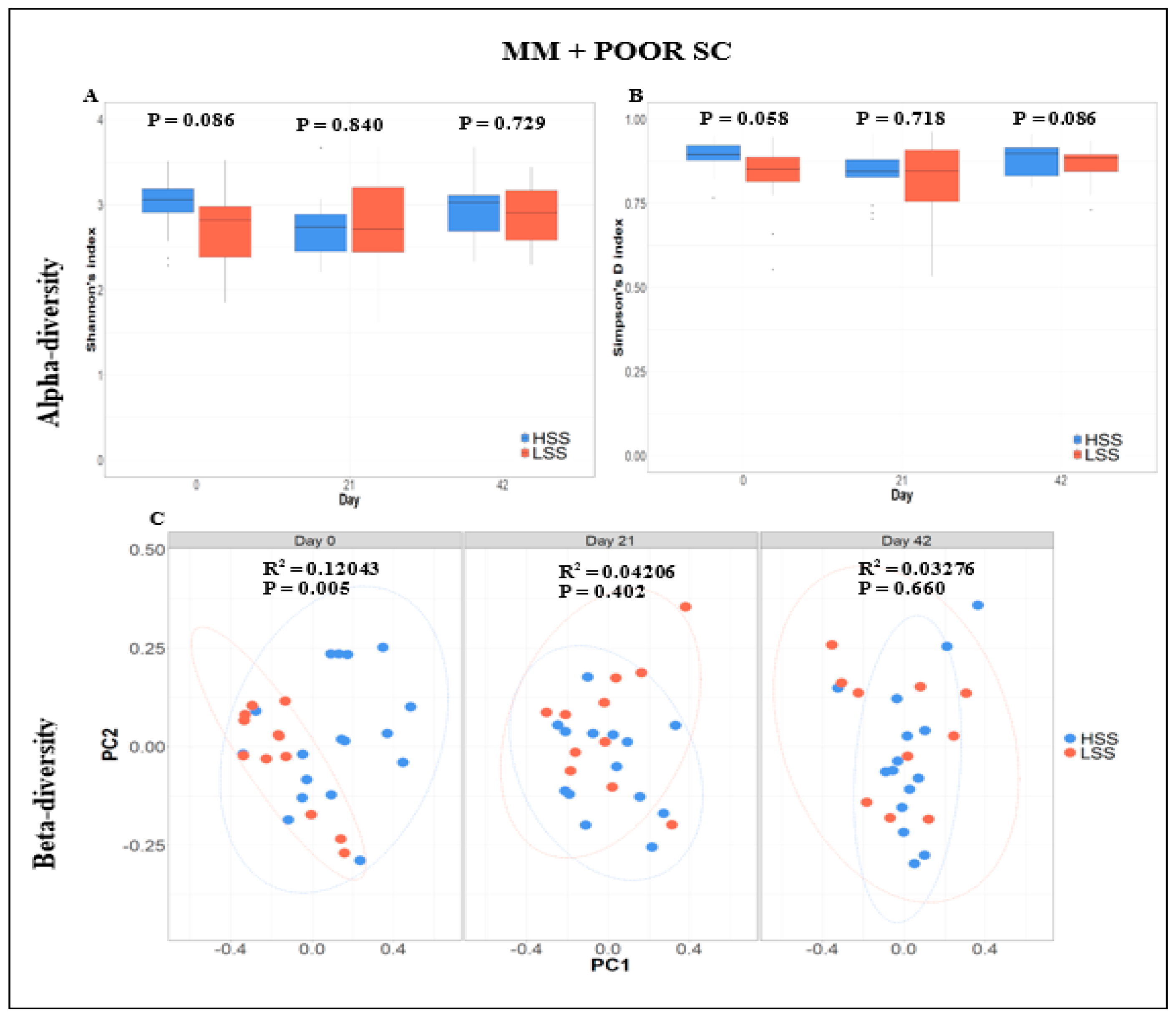
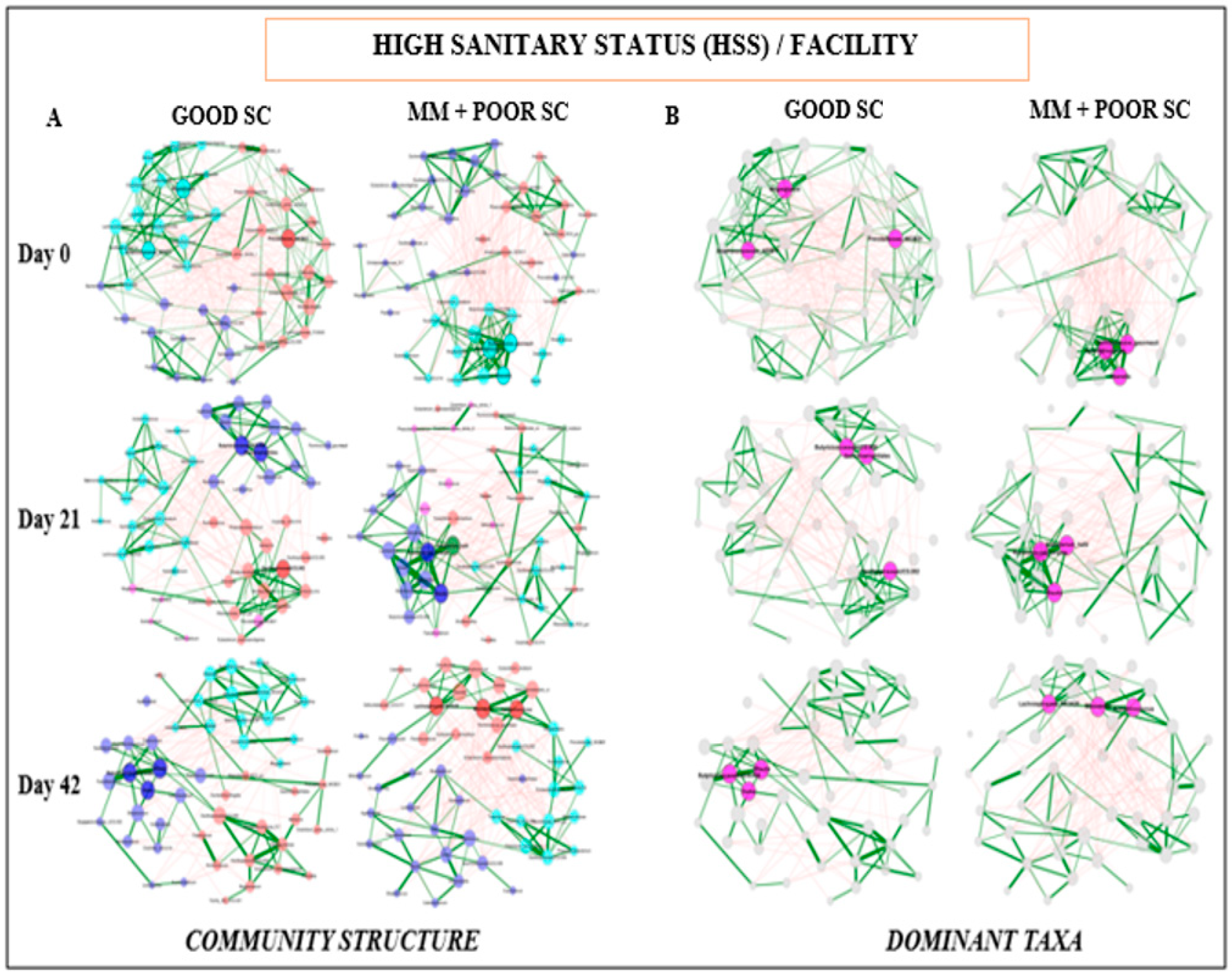
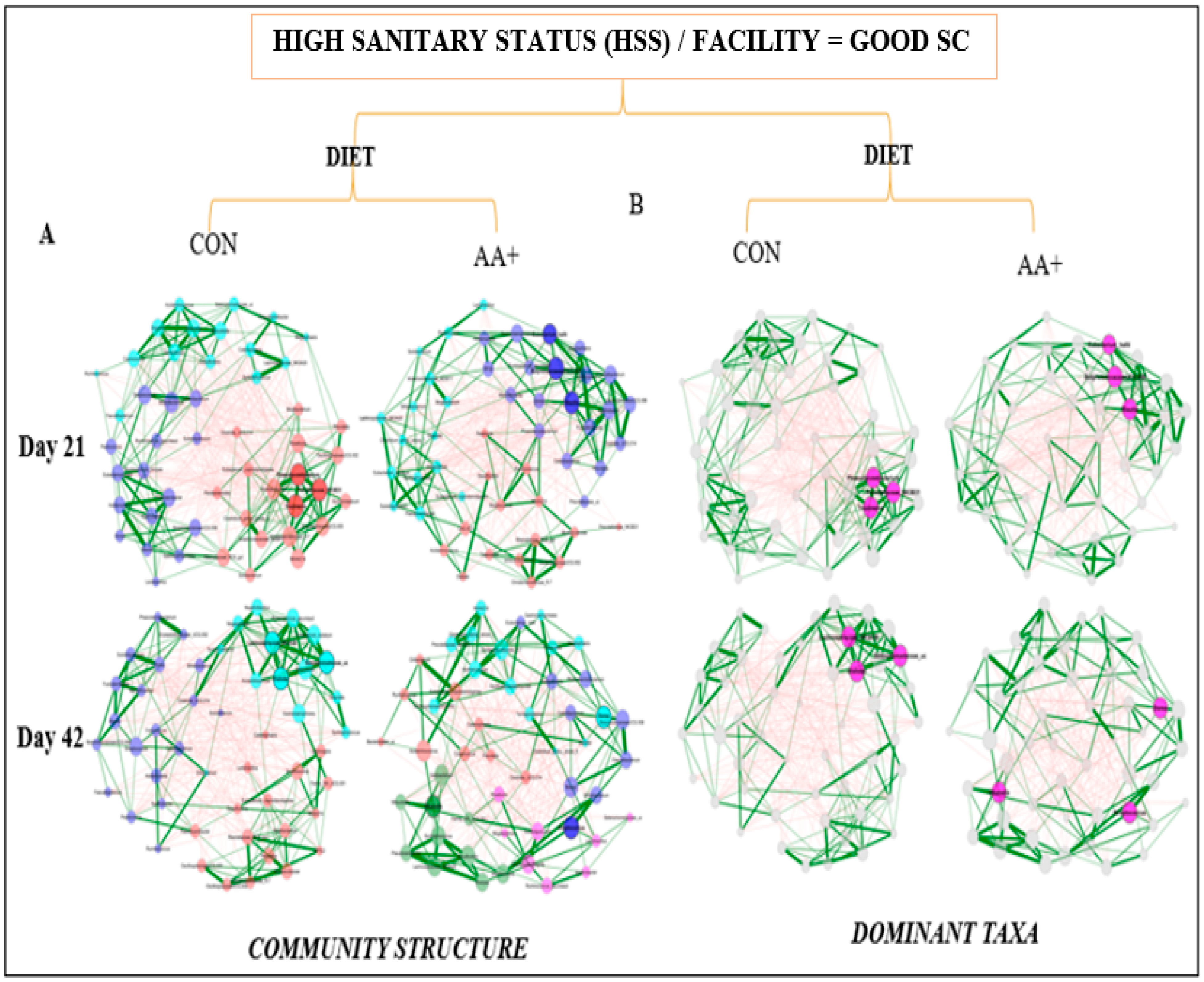
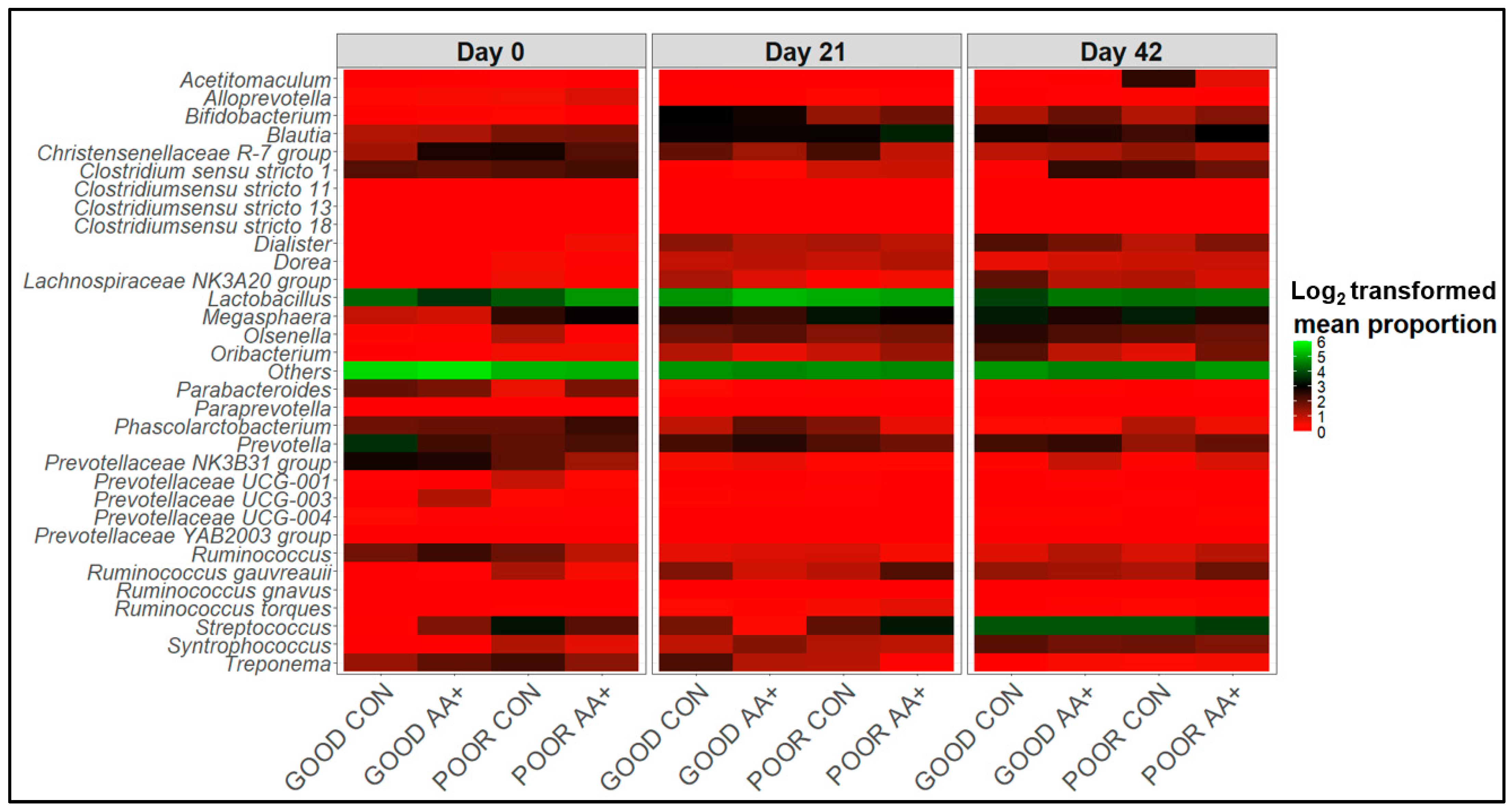
3.4. Fecal Microbiome Compositional Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayaraman, B.; Nyachoti, C.M. Husbandry practices and gut health outcomes in weaned piglets: A review. Anim Nutr 2017, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Scollo, A.; Levallois, P.; Fourichon, C.; Motta, A.; Mannelli, A.; Lombardo, F.; Ferrari, P. Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest. Animals 2022, 12, 2655. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.G.D.; Dewulf, J.; Piñeiro, C.; Edwards, S.; Kyriazakis, I. A critical reflection on intensive pork production with an emphasis on animal health and welfare. J. Anim. Sci. 2020, 18, 98. [Google Scholar] [CrossRef]
- Passafaro, T.L.; Fernandes, A.F.A.; Valente, B.D.; Williams, N.H.; Rosa, G.J.M. Network analysis of swine movements in a multi-site pig production system in Iowa, USA. Prev. Vet. Med. 2020, 174, 104856. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Martín-Valls, G.E.; Tello, M.; Mateu, E.; Martín, M.; Darwich, L. Prevalence of enteric pathogens in diarrheic and non-diarrheic samples from pig farms with neonatal diarrhea in the North East of Spain. Vet. Microbiol. 2019, 237, 108419. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Giménez-Lirola, L.G.; Halbur, P.G. Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 2011, 12, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Wessels, A.; Corrent, E.; Wu, G.; Bosi, P. The relevance of functional amino acids to support the health of growing pigs. Anim. Feed Sci. Technol. 2018, 245, 104–116. [Google Scholar] [CrossRef]
- Van der Meer, Y.; Gerrits, W.J.J.; Jansman, A.J.M.; Kemp, B.; Bolhuis, J.E. A link between damaging behavior in pigs, sanitary conditions, and dietary protein and amino acid supply. PLoS ONE 2017, 12, e0174688. [Google Scholar] [CrossRef]
- Niemi, J.; Bennett, R.; Clark, B.; Frewer, L.; Jones, P.; Rimmler, T.; Tranter, R. A value chain analysis of interventions to control production diseases in the intensive pig production sector. PLoS ONE 2020, 15, e0231338. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Gondret, F.; Resmond, R. Identification of blood immune and metabolic indicators explaining the variability of growth of pigs under contrasted sanitary conditions. BMC Vet. Res. 2021, 17, 166–175. [Google Scholar] [CrossRef]
- Van der Meer, Y.; Lammers, A.; Jansman, A.J.; Rijnen, M.M.; Hendriks, W.H.; Gerrits, W.J. Performance of pigs kept under different sanitary conditions affected by protein intake and amino acid supplementation. J. Anim. Sci. 2016, 94, 4704–4719. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Lebellego, L.; Matte, J.J.; Melchior, D.; Sève, B. The effect of sanitary status degradation and dietary tryptophan content on growth rate and tryptophan metabolism in weaning pigs. J. Anim. Sci. 2009, 87, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H.; Frost, R.A.; Vary, T.C. Regulation of muscle protein synthesis during sepsis and inflammation. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Melchior, D.; Obled, C. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livest. Prod. Sci. 2004, 87, 37–45. [Google Scholar] [CrossRef]
- Buchet, A.; Belloc, C.; Leblanc-Maridor, M.; Merlot, E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS ONE 2017, 12, e0178487. [Google Scholar] [CrossRef]
- Pastorelli, H.J.; van Milgen, J.; Lovatto, P.; Montagne, L. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 2012, 6, 952–961. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Jondreville, C.; Matte, J.J.; Seve, B. Importance of sanitary environment for growth performance and plasma nutrient homeostasis during the post-weaning period in piglets. Arch. Anim. Nutr. 2006, 60, 23–34. [Google Scholar] [CrossRef]
- Morales, A.; Sánchez, V.; Pérez, B.; Camacho, R.L.; Arce, N.; Avelar, E.; González-Vega, J.C.; Htoo, J.K.; Cervantes, M. Effect of DL-methionine supplementation above requirement on performance; intestinal morphology, antioxidant activity, and gene expression; and serum concentration of amino acids in heat stressed pigs. J. Anim. Sci. 2023, 101, skac379. [Google Scholar] [CrossRef] [PubMed]
- Chalvon-Demersay, T.; Luise, D.; Le Floc’h, N.; Tesseraud, S.; Lambert, W.; Bosi, P.; Trevisi, P.; Beaumont, M.; Corrent, E. Functional Amino Acids in Pigs and Chickens: Implication for Gut Health. Front. Vet. Sci. 2021, 8, 663727. [Google Scholar] [CrossRef]
- Wellington, M.O.; Htoo, J.K.; van Kessel, A.G.; Columbus, D.A. Impact of dietary fiber and immune system stimulation on threonine requirement for protein deposition in growing pigs. J. Anim. Sci. 2018, 96, 5222–5232. [Google Scholar] [CrossRef]
- Mao, X.; Lv, M.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Wang, Q.; Chen, D. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J. Anim. Sci. Biotechnol. 2014, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Litvak, N.; Rakhshandeh, A.; Htoo, J.K.; de Lange, C.F. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 2013, 91, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, K.; Levesque, C.L.; Htoo, J.K.; de Lange, C.F.M. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 2012, 90, 3485–3491. [Google Scholar] [CrossRef] [PubMed]
- Valini, G.A.C.; Arnaut, P.R.; França, I.; Ortiz, M.T.; de Oliveira, M.J.K.; Melo, A.D.B.; Marçal, D.A.; Campos, P.H.R.F.; Htoo, J.K.; Brand, H.G.; et al. Increased dietary Trp, Thr, and Met supplementation improves growth performance and protein deposition of Salmonella-challenged growing pigs under poor housing conditions. J. Anim. Sci. 2023, 101, skad141. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Wellington, M.O.; González-Vega, J.C.; Htoo, J.K.; Kessel, A.G.; Columbus, D.A. Functional amino acid supplementation, regardless of dietary protein content, improves growth performance and immune status of weaned pigs challenged with Salmonella Typhimurium. J. Anim. Sci. 2021, 99, skaa365. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Roura, E.; Lambert, W.; Turni, C.; Michiels, J.; Chalvon-Demersay, T. Selective nourishing of gut microbiota with amino acids: A novel prebiotic approach? Front. Nutr. 2022, 9, 1066898. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- AminoDat® 6.0 Animal Nutritionist’s Information Edge; Evonik Nutrition and Care GmbH: Essen, Germany, 2021.
- Weber, K.; Osborn, M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 1969, 244, 4406–4412. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Keston, A.S.; Brandt, R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal. Biochem. 1965, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S- transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Van Haute, M.; Korth, N.; Sattler, S.E.; Toy, J.; Rose, D.J.; Schnable, J.C.; Benson, A.K. Genetic analysis of seed traits in Sorghum bicolor that affect the human gut microbiome. Nat. Commun. 2022, 13, 5641. [Google Scholar] [CrossRef]
- Yang, Q.; Van Haute, M.; Korth, N.; Sattlerd, S.; Rose, D.; Juritsch, A.; Shaoa, J.; Beede, K.; Schmaltz, R.; Price, J.; et al. The waxy mutation in sorghum and other cereal grains reshapes the gut microbiome by reducing levels of multiple beneficial species. Gut Microbes 2023, 15, 2178799. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Michel, V.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; Vanderplas, J.; Passos, A.; Cournapeau, D.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. Available online: http://scikit-learn.sourceforge.net (accessed on 19 May 2021).
- Peschel, S.; Müller, C.L.; Mutius Ev Boulesteix, A.L.; Depner, M. NetCoMi: Network construction and comparison for microbiome data in R. Brief. Bioinform. 2021, 22, bbaa290. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS, 9.4, SAS Institute: Cary, NC, USA, 2013.
- Luo, Y.; Ren, W.; Smidt, H.; Wright, A.G.; Yu, B.; Schyns, G.; McCormack, U.M.; Cowieson, A.J.; Yu, J.; He, J.; et al. Dynamic distribution of gut microbiota in pigs at different growth stages: Composition and contribution. Microbiol. Spectr. 2022, 10, e00688-21. [Google Scholar] [CrossRef]
- Menegat, M.B.; Goodband, R.D.; DeRouchey, J.M.; Tokach, M.D.; Woodworth, J.C.; Dritz, S.S. Kansas State University Swine Nutrition Guide: Economics in Swine Nutrition; Kansas State University: Manhattan, KS, USA, 2019. [Google Scholar]
- Patil, Y.; Gooneratne, R.; Ju, X.H. Interactions between host and gut microbiota in domestic pigs: A review. Gut Microbes 2020, 11, 310–334. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, J.; Li, D.; Zhou, X.; Li, X. Tryptophan enhances ghrelin expression and secretion associated with increased food intake and weight gain in weanling pigs. Domest. Anim. Endocrinol. 2007, 33, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Melchior, D.; Sève, B. Dietary tryptophan helps to preserve tryptophan homeostasis in pigs suffering from lung inflammation. J. Anim. Sci. 2008, 86, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Melchior, D.; Séve, B.; Le Floc’h, N. Chronic lung inflammation affects amino acid concentrations in pigs. J. Anim. Sci. 2004, 82, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, P.; Melchior, D.; Mazzoni, M.; Casini, L.; De Filippi, S.; Minieri, L.; Lalatta-Costerbosa, G.; Bosi, P. A tryptophan-enriched diet improves feed intake and growth performance of susceptible weanling pigs orally challenged with Escherichia coli K88. J. Anim. Sci. 2009, 87, 148–156. [Google Scholar] [CrossRef]
- Capozzalo, M.M.; Resink, J.W.; Htoo, J.K.; Kim, J.C.; de Lange, F.M.; Mullan, B.P.; Hansen, C.F.; Pluske, J.R. Determination of the optimum standardized ileal digestible sulphur amino acids to lysine ratio in weaned pigs challenged with enterotoxigenic Escherichia coli. Anim. Feed Sci. Technol. 2017, 227, 118–130. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Wellington, M.O.; González-Vega, J.C.; Htoo, J.K.; Kessel, A.G.; Columbus, D.A. A longer adaptation period to a functional amino acid-supplemented diet improves growth performance and immune status of Salmonella Typhimurium-challenged pigs. J. Anim. Sci. 2021, 99, skab146. [Google Scholar] [CrossRef]
- Fraga, A.Z.; Campos, P.H.R.F.; Hauschild, L.; Chalvon-Demersay, T.; Beaumont, M.; Le Floc’h, N. A blend of functional amino acids and grape polyphenols improves the pig capacity to cope with an inflammatory challenge caused by poor hygiene of housing conditions. BMC Vet. Res. 2023, 19, 25. [Google Scholar] [CrossRef]
- Zem Fraga, A.; Louveau, I.; Campos, P.H.R.F.; Hauschild, L.; Le Floc’h, N. Selection for feed efficiency elicits different postprandial plasma metabolite profiles in response to poor hygiene of housing conditions in growing pigs. PLoS ONE 2021, 16, e0246216. [Google Scholar] [CrossRef] [PubMed]
- Pas, M.F.W.; Jansman, A.J.M.; Kruijt, L.; van der Meer, Y.; Vervoort, J.J.M.; Schokker, D. Sanitary conditions affect the colonic microbiome and the colonic and systemic metabolome of female pigs. Front. Vet. Sci. 2020, 7, 585730. [Google Scholar] [CrossRef] [PubMed]
- Kampman-van de Hoek, E.; Jansman, A.J.; van den Borne, J.J.; van der Peet-Schwering, C.M.; van Beers-Schreurs, H.; Gerrits, W.J. Dietary amino acid deficiency reduces the utilization of amino acids for growth in growing pigs after a period of poor health. J. Nutr. 2016, 146, 51–58. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Knudsen, C.; Gidenne, T.; Montagne, L.; Zemb, O. Impact of feed restriction on health, digestion and faecal microbiota of growing pigs housed in good or poor hygiene conditions. Animal 2014, 8, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Knura-Deszczk, S.; Lipperheide, C.; Pettersen, C.; Jobert, J.L.; Berthelot-Hérault, F.; Kobisch, M.; Madec, F. Plasma haptoglobin concentration in swine after challenge with Streptococcus suis. J. Vet. Med. B Infect. Dis. Vet. Public. Health 2002, 49, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Onur, S.; Ayhanci, A. “LIPID PEROXIDATION”. Accenting Lipid Peroxidation; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; de Lange, C.F.M. Evaluation of chronic immune system stimulation models in growing pigs. Animal 2012, 6, 305–310. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; Htoo, J.K.; Karrow, N.; Miller, S.P.; de Lange, C.F.M. Impact of immune system stimulation on the ileal nutrient digestibility and utilization of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 2014, 111, 101–110. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Hidalgo, A.I.; Alarcón, P.; Anseoleaga, N.; Hidalgo, M.A.; Burgos, R.A. Role of Lactate in Inflammatory Processes: Friend or Foe. Front. Immunol. 2022, 12, 808799. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; de Wiele, T.V.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Dai, Z.; Kou, J.; Sun, K.; Chen, J.; Yang, Y.; Wu, G.; Wu, Z. Dietary L-Tryptophan supplementation enhances the intestinal mucosal barrier function in weaned piglets: Implication of tryptophan-metabolizing microbiota. Int. J. Mol. Sci. 2019, 20, 20. [Google Scholar] [CrossRef]
- Liang, H.; Dai, Z.; Liu, N.; Ji, Y.; Chen, J.; Zhang, Y.; Yang, Y.; Li, J.; Wu, Z.; Wu, G. Dietary L-Tryptophan modulates the structural and functional composition of the intestinal microbiome in weaned piglets. Front. Microbiol. 2018, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; Brunelle, B.W.; Trachsel, J.; Allen, H.K. Meta-analysis to define a core microbiota in the swine gut. mSistems 2017, 23, e00004-17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, Q.; Van Haute, M.J.S.; Kok, C.R.; Gomes-Neto, J.C.; Pavlovikj, N.; Pillai, R.; Sinha, R.; Hassenstab, H.; Mustoe, A.; et al. Captive common marmosets (Callithrix jacchus) are colonized throughout their lives by a community of Bifidobacterium species with species-specific genomic content that can support adaptation to distinct metabolic niches. mBio 2021, 31, e0115321. [Google Scholar] [CrossRef] [PubMed]
- Wylensek, D.; Hitch, T.C.; Riedel, T.; Afrizal, A.; Kumar, N.; Wortmann, E.; Liu, T.; Devendran, S.; Lesker, T.R.; Hernández, S.B.; et al. A collection of bacterial isolates from the pig intestine reveals functional and taxonomic diversity. Nat. Commun. 2020, 11, 6389. [Google Scholar] [CrossRef] [PubMed]
- Arfken, A.M.; Frey, J.F.; Summers, K.L. Temporal dynamics of the gut bacteriome and mycobiome in the weanling pig. Microorganisms 2020, 8, 868. [Google Scholar] [CrossRef]
- Summers, K.L.; Frey, J.F.; Ramsay, T.G.; Arfken, A.M. The piglet mycobiome during the weaning transition: A pilot study. J. Anim. Sci. 2019, 12, 2889–2900. [Google Scholar] [CrossRef]

| Ingredients, % | Phase 1 (d 0 to 14) | Phase 2 (d 15 to 28) | Phase 3 (d 29 to 42) | |||
|---|---|---|---|---|---|---|
| CON 1 | AA+ 1 | CON | AA+ | CON | AA+ | |
| Corn | 49.41 | 49.41 | 58.85 | 58.85 | 69.64 | 69.64 |
| Soybean meal | 15.00 | 15.00 | 20.00 | 20.00 | 26.00 | 26.00 |
| Whey protein | 21.42 | 21.42 | 12.14 | 12.14 | - | - |
| Soy protein concentrated (60% CP) | 5.23 | 5.23 | 2.55 | 2.55 | - | - |
| Spray-dried blood plasma | 5.00 | 5.00 | 2.50 | 2.50 | - | - |
| Dicalcium phosphate | 0.870 | 0.870 | 0.655 | 0.655 | 0.512 | 0.512 |
| Limestone | 0.795 | 0.795 | 0.884 | 0.884 | 0.819 | 0.819 |
| Soybean oil | - | - | 0.100 | 0.100 | 0.125 | 0.125 |
| Salt | 0.330 | 0.330 | 0.445 | 0.445 | 0.450 | 0.450 |
| Mineral premix 2 | 0.110 | 0.110 | 0.100 | 0.100 | 0.090 | 0.090 |
| Vitamin premix 3 | 0.060 | 0.060 | 0.050 | 0.050 | 0.050 | 0.050 |
| Choline chloride | 0.060 | 0.060 | 0.060 | 0.060 | 0.060 | 0.060 |
| Corn starch | 0.500 | 0.105 | 0.500 | 0.146 | 1.000 | 0.665 |
| Sodium bicarbonate | - | - | - | - | 0.053 | 0.053 |
| Enzae® (phytase) 4 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 |
| Biolys (54.6%) 5 | 0.66 | 0.66 | 0.68 | 0.68 | 0.77 | 0.77 |
| DL-Met (99%) 5 | 0.23 | 0.39 | 0.19 | 0.34 | 0.16 | 0.30 |
| L-Thr (98.5%) 5 | 0.14 | 0.32 | 0.13 | 0.30 | 0.15 | 0.30 |
| L-Trp (98%) 5 | 0.001 | 0.05 | 0.008 | 0.04 | 0.008 | 0.04 |
| L-Val (98%) 5 | 0.11 | 0.11 | 0.11 | 0.11 | 0.09 | 0.09 |
| L-Ile | 0.04 | 0.04 | 0.01 | 0.01 | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutrients, calculated values | ||||||
| ME, kcal/kg | 3386 | 3391 | 3354 | 3358 | 3331 | 3335 |
| NE, kcal/kg | 2521 | 2524 | 2516 | 2518 | 2470 | 2473 |
| CP, % | 21.2 | 21.5 | 19.5 | 19.9 | 18.8 | 18.98 |
| SID Lys, % | 1.50 | 1.50 | 1.35 | 1.35 | 1.23 | 1.23 |
| SID Met + Cys, % | 0.82 | 0.98 | 0.74 | 0.89 | 0.68 | 0.82 |
| SID Met, % | 0.48 | 0.64 | 0.44 | 0.59 | 0.41 | 0.55 |
| SID Thr, % | 0.88 | 1.06 | 0.79 | 0.95 | 0.73 | 0.88 |
| SID Trp, % | 0.25 | 0.30 | 0.22 | 0.26 | 0.20 | 0.24 |
| SID Val, % | 1.02 | 1.02 | 0.92 | 0.92 | 0.84 | 0.84 |
| SID Arg, % | 1.11 | 1.11 | 1.05 | 1.05 | 1.08 | 1.08 |
| SID Ile, % | 0.80 | 0.80 | 0.72 | 0.72 | 0.69 | 0.69 |
| SID Leu, % | 1.58 | 1.58 | 1.46 | 1.46 | 1.42 | 1.42 |
| SID His, % | 0.52 | 0.52 | 0.46 | 0.46 | 0.44 | 0.44 |
| SID Phe + Tyr, % | 1.61 | 1.61 | 1.45 | 1.45 | 1.38 | 1.38 |
| SID Phe, % | 0.94 | 0.94 | 0.85 | 0.85 | 0.81 | 0.81 |
| STTD P, % | 0.45 | 0.45 | 0.40 | 0.40 | 0.33 | 0.33 |
| Ca, % | 0.85 | 0.85 | 0.80 | 0.80 | 0.70 | 0.70 |
| Na, % | 0.40 | 0.40 | 0.35 | 0.35 | 0.20 | 0.20 |
| Lactose, % | 15.00 | 15.00 | 8.50 | 8.50 | - | - |
| Nutrients, total analyzed values | ||||||
| CP, % | 21.6 | 22.0 | 20.7 | 21.0 | 19.2 | 19.3 |
| Lys, % | 1.58 | 1.59 | 1.50 | 1.58 | 1.36 | 1.38 |
| Met + Cys, % | 0.89 | 0.94 | 0.85 | 0.99 | 0.74 | 0.81 |
| Met, % | 0.48 | 0.53 | 0.48 | 0.63 | 0.43 | 0.51 |
| Thr, % | 1.02 | 1.08 | 0.91 | 1.05 | 0.84 | 0.90 |
| Val, % | 1.16 | 1.20 | 1.10 | 1.09 | 0.96 | 0.94 |
| Arg, % | 1.23 | 1.25 | 1.20 | 1.22 | 1.15 | 1.13 |
| Ile, % | 0.84 | 0.85 | 0.80 | 0.82 | 0.77 | 0.76 |
| Leu, % | 1.79 | 1.81 | 1.72 | 1.73 | 1.66 | 1.70 |
| His, % | 0.56 | 0.56 | 0.53 | 0.51 | 0.50 | 0.50 |
| Phe, % | 1.01 | 1.03 | 0.95 | 0.96 | 0.89 | 0.90 |
| Item (no. × 109/L) | HSS Piglets | LSS Piglets | RSD 1 | p-Value |
|---|---|---|---|---|
| Leukocytes | 17.2 | 20.6 | 0.349 | <0.01 |
| Granulocytes 2 | 7.7 | 9.9 | 0.749 | <0.01 |
| Lymphocytes | 8.5 | 9.4 | 0.422 | 0.19 |
| Monocytes | 1.0 | 1.3 | 0.053 | <0.01 |
| GOOD SC 1 | MM + POOR SC 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | CON 1 | AA+ 1 | p Value | CON | AA+ | p Value | RSD 2 | SC |
| Initial BW, kg | 6.35 | 6.26 | 0.64 | 6.26 | 6.16 | 0.57 | 0.215 | 0.51 |
| Phase 1 (d 0 to 14) | ||||||||
| Final BW, kg | 8.91 | 8.85 | 0.83 | 8.24 | 8.61 | 0.17 | 0.313 | 0.03 |
| ADFI, g | 282 | 256 | 0.19 | 245 | 223 | 0.45 | 32 | 0.03 |
| ADG, g | 182 | 185 | 0.89 | 123 | 154 | 0.32 | 37 | 0.02 |
| G:F | 0.67 | 0.72 | 0.70 | 0.49 | 0.65 | 0.19 | 0.138 | 0.15 |
| Phase 2 (d 15 to 28) | ||||||||
| Final BW, kg | 15.60 | 16.12 | 0.45 | 12.76 | 13.86 | 0.09 | 0.723 | <0.01 |
| ADFI, g | 595 | 646 | 0.35 | 434 | 523 | 0.06 | 55 | <0.01 |
| ADG, g | 482 | 524 | 0.22 | 325 | 373 | 0.10 | 46 | <0.01 |
| G:F | 0.84 | 0.83 | 0.91 | 0.77 | 0.77 | 1.00 | 0.078 | 0.16 |
| Phase 3 (d 29 to 42) | ||||||||
| Final BW, kg | 22.71 | 23.85 | 0.26 | 18.20 | 20.95 | <0.01 | 1.053 | <0.01 |
| ADFI, g | 834 | 953 | 0.02 | 652 | 824 | <0.01 | 64 | <0.01 |
| ADG, g | 503 | 555 | 0.24 | 395 | 512 | <0.01 | 46 | <0.01 |
| G:F | 0.61 | 0.58 | 0.34 | 0.61 | 0.61 | 0.87 | 0.031 | 0.68 |
| Overall (d 0 to 42) | ||||||||
| ADFI, g | 573 | 614 | 0.17 | 441 | 523 | 0.01 | 38 | <0.01 |
| ADG, g | 394 | 425 | 0.32 | 282 | 345 | 0.01 | 37 | <0.01 |
| G:F | 0.69 | 0.69 | 0.86 | 0.63 | 0.65 | 0.34 | 0.034 | 0.01 |
| Item | GOOD SC 1 | MM + POOR SC 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON 1 | AA+ 1 | p-Value | CON | AA+ | p-Value | RSD 2 | SC | |
| Day 0 | ||||||||
| IgA, mg/mL | 1.50 | 1.40 | 0.51 | 1.30 | 1.50 | 0.36 | 0.024 | 0.68 |
| IgG, mg/mL | 5.90 | 6.10 | 0.76 | 5.10 | 6.40 | 0.13 | 0.096 | 0.62 |
| Ceruloplasmin, mg/mL | 0.90 | 0.90 | 0.95 | 0.80 | 1.00 | 0.15 | 0.013 | 0.45 |
| Transferrin, mg/mL | 3.60 | 3.90 | 0.29 | 3.50 | 3.50 | 0.99 | 0.031 | 0.15 |
| Albumin, g/L | 35.3 | 35.1 | 0.94 | 34.9 | 34.5 | 0.85 | 0.238 | 0.75 |
| Haptoglobin, mg/mL | 0.50 | 0.60 | 0.73 | 0.60 | 0.70 | 0.22 | 0.014 | 0.19 |
| α-1 acid glycoprotein, µg/mL | 38.6 | 48.6 | 0.84 | 45.7 | 51.4 | 0.69 | 0.013 | 0.53 |
| Day 21 | ||||||||
| IgA, mg/mL | 1.30 | 1.20 | 0.29 | 1.40 | 1.20 | 0.17 | 0.025 | 0.73 |
| IgG, mg/mL | 6.90 | 6.60 | 0.82 | 8.40 | 7.20 | 0.24 | 0.106 | 0.16 |
| Ceruloplasmin, mg/mL | 0.60 | 0.60 | 0.54 | 0.70 | 0.70 | 0.77 | 0.014 | 0.31 |
| Transferrin, mg/mL | 3.80 | 3.90 | 0.84 | 3.70 | 4.10 | 0.26 | 0.044 | 0.91 |
| Albumin, g/L | 28.8 | 28.8 | 0.99 | 28.4 | 29.5 | 0.53 | 0.197 | 0.92 |
| Haptoglobin, mg/mL | 0.40 | 0.30 | 0.52 | 1.00 | 0.60 | 0.07 | 0.025 | 0.002 |
| α-1 acid glycoprotein, µg/mL | 45.0 | 38.3 | 0.44 | 48.6 | 51.4 | 0.29 | 0.019 | 0.13 |
| Day 42 | ||||||||
| IgA, mg/mL | 1.50 | 1.30 | 0.41 | 1.40 | 1.40 | 0.80 | 0.025 | 0.89 |
| IgG, mg/mL | 9.50 | 9.80 | 0.87 | 10.40 | 12.30 | 0.29 | 0.185 | 0.18 |
| Ceruloplasmin, mg/mL | 1.10 | 0.90 | 0.20 | 0.80 | 1.00 | 0.18 | 0.014 | 0.13 |
| Transferrin, mg/mL | 4.30 | 4.60 | 0.21 | 4.20 | 4.30 | 0.82 | 0.026 | 0.44 |
| Albumin, g/L | 33.5 | 34.1 | 0.78 | 31.3 | 31.1 | 0.91 | 0.187 | 0.05 |
| Haptoglobin, mg/mL | 1.00 | 0.70 | 0.16 | 0.60 | 0.80 | 0.35 | 0.022 | 0.43 |
| α-1 acid glycoprotein, µg/mL | 48.3 | 48.6 | 0.94 | 50.0 | 34.3 | 0.14 | 0.014 | 0.38 |
| Item | GOOD SC 1 | MM + POOR SC 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON 1 | AA+ 1 | p-Value | CON | AA+ | p-Value | RSD 2 | SC | |
| Day 0 | ||||||||
| Total protein, g/dL | 5.22 | 5.21 | 0.95 | 5.01 | 5.09 | 0.76 | 0.277 | 0.39 |
| Urea, mg/dL | 17.96 | 19.44 | 0.66 | 24.77 | 18.60 | 0.10 | 3.520 | 0.24 |
| Creatinine, mg/dL | 1.59 | 1.44 | 0.10 | 1.65 | 1.46 | 0.05 | 0.090 | 0.51 |
| Lactate, mg/dL | 50.57 | 59.38 | 0.26 | 43.07 | 31.13 | 0.13 | 7.710 | 0.003 |
| Lactate dehydrogenase, U/L | 1415 | 1345 | 0.33 | 1485 | 1303 | 0.01 | 69.980 | 0.78 |
| Day 21 | ||||||||
| Total protein, g/dL | 4.68 | 4.65 | 0.91 | 4.78 | 4.84 | 0.824 | 0.272 | 0.45 |
| Urea, mg/dL | 19.35 | 19.18 | 0.96 | 27.13 | 16.35 | 0.003 | 3.420 | 0.30 |
| Creatinine, mg/dL | 1.08 | 1.08 | 0.97 | 1.19 | 1.04 | 0.04 | 0.070 | 0.42 |
| Lactate, mg/dL | 48.79 | 51.25 | 0.75 | 41.21 | 44.19 | 0.70 | 7.720 | 0.18 |
| Lactate dehydrogenase, U/L | 1333 | 1314 | 0.80 | 1227 | 1279 | 0.49 | 76.810 | 0.18 |
| Day 42 | ||||||||
| Total protein, g/dL | 5.59 | 5.69 | 0.69 | 5.45 | 5.63 | 0.43 | 0.235 | 0.54 |
| Urea, mg/dL | 23.38 | 18.67 | 0.16 | 26.06 | 19.21 | 0.04 | 3.310 | 0.49 |
| Creatinine, mg/dL | 1.31 | 1.34 | 0.65 | 1.27 | 1.22 | 0.47 | 0.080 | 0.14 |
| Lactate, mg/dL | 67.28 | 71.09 | 0.81 | 85.75 | 48.71 | 0.03 | 15.990 | 0.86 |
| Lactate dehydrogenase, U/L | 1256 | 1403 | 0.09 | 1281 | 1253 | 0.75 | 87.250 | 0.31 |
| Item 3 | GOOD SC 1 | MM + POOR SC 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON 1 | AA+ 1 | p Value | CON | AA+ | p Value | RSD 2 | SC | |
| GSH, pmol.mL−1 | 20.27 | 22.47 | 0.46 | 19.57 | 22.99 | 0.31 | 2.32 | 0.96 |
| GSSG, pmol.mL−1 | 8.02 | 5.92 | 0.16 | 5.94 | 6.89 | 0.62 | 1.24 | 0.64 |
| GSH:GSSG Eh, mV 4 | −316.9 | −324.3 | 0.05 | −320.5 | −321.4 | 0.55 | 3.139 | 0.89 |
| GST, mU.mL−1 | 6.82 | 6.94 | 0.82 | 7.21 | 7.30 | 0.85 | 0.42 | 0.32 |
| LPO, mmol.mL−1 | 38.75 | 25.17 | 0.18 | 32.79 | 28.64 | 0.69 | 7.28 | 0.70 |
| SOD, U.mL−1 | 47.02 | 46.79 | 0.91 | 44.04 | 44.16 | 0.90 | 1.15 | 0.01 |
| ROS, DCFHA-DA 5 | 458.8 | 336.9 | 0.21 | 601.7 | 440.6 | 0.27 | 88.26 | 0.08 |
| T-AOC, µM.L−1 | 2.99 | 3.51 | 0.80 | 1.55 | 2.61 | 0.49 | 1.97 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.P.R.; Melo, A.D.B.; Yang, Q.; de Oliveira, M.J.K.; Marçal, D.A.; Ortiz, M.T.; Righetti Arnaut, P.; França, I.; Alves da Cunha Valini, G.; Silva, C.A.; et al. Increased Dietary Trp, Thr, and Met Supplementation Improves Performance, Health, and Protein Metabolism of Weaned Piglets under Mixed Management and Poor Housing Conditions. Animals 2024, 14, 1143. https://doi.org/10.3390/ani14081143
Gonçalves JPR, Melo ADB, Yang Q, de Oliveira MJK, Marçal DA, Ortiz MT, Righetti Arnaut P, França I, Alves da Cunha Valini G, Silva CA, et al. Increased Dietary Trp, Thr, and Met Supplementation Improves Performance, Health, and Protein Metabolism of Weaned Piglets under Mixed Management and Poor Housing Conditions. Animals. 2024; 14(8):1143. https://doi.org/10.3390/ani14081143
Chicago/Turabian StyleGonçalves, Joseane Penteado Rosa, Antonio Diego Brandão Melo, Qinnan Yang, Marllon José Karpeggiane de Oliveira, Danilo Alves Marçal, Manoela Trevisan Ortiz, Pedro Righetti Arnaut, Ismael França, Graziela Alves da Cunha Valini, Cleslei Alisson Silva, and et al. 2024. "Increased Dietary Trp, Thr, and Met Supplementation Improves Performance, Health, and Protein Metabolism of Weaned Piglets under Mixed Management and Poor Housing Conditions" Animals 14, no. 8: 1143. https://doi.org/10.3390/ani14081143
APA StyleGonçalves, J. P. R., Melo, A. D. B., Yang, Q., de Oliveira, M. J. K., Marçal, D. A., Ortiz, M. T., Righetti Arnaut, P., França, I., Alves da Cunha Valini, G., Silva, C. A., Korth, N., Pavlovikj, N., Campos, P. H. R. F., Brand, H. G., Htoo, J. K., Gomes-Neto, J. C., Benson, A. K., & Hauschild, L. (2024). Increased Dietary Trp, Thr, and Met Supplementation Improves Performance, Health, and Protein Metabolism of Weaned Piglets under Mixed Management and Poor Housing Conditions. Animals, 14(8), 1143. https://doi.org/10.3390/ani14081143






