The Influence of Shelter Type and Coverage on Crayfish (Procambarus clarkii) Predation by Catfish (Silurus asotus): A Controlled Environment Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Maintenance of Experimental Animals
2.2. Experimental Design and Procedure
2.3. Data Statistical Analysis
3. Results
3.1. Predation Rhythm
3.2. Predation and Anti-Predation Behavior
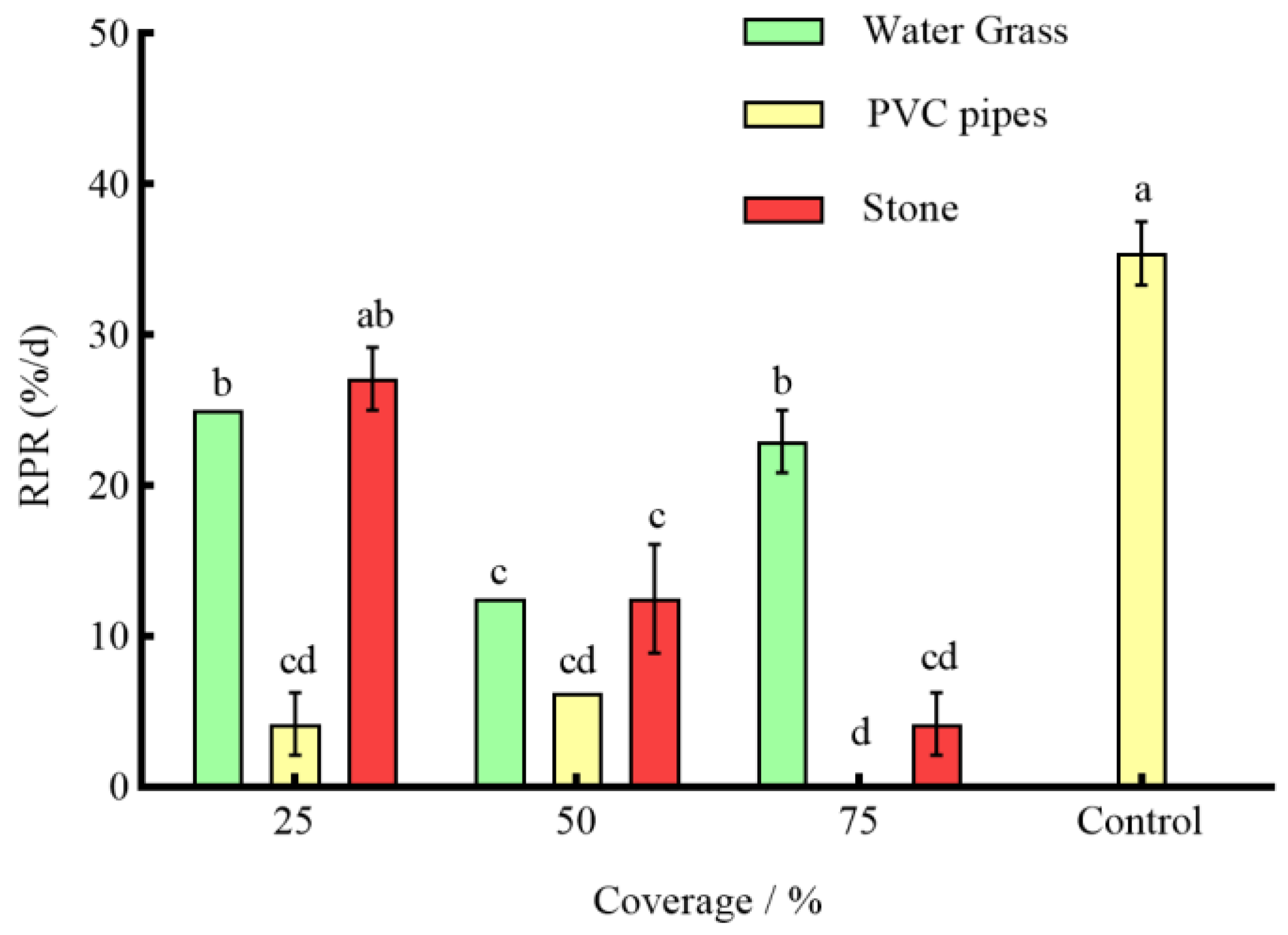
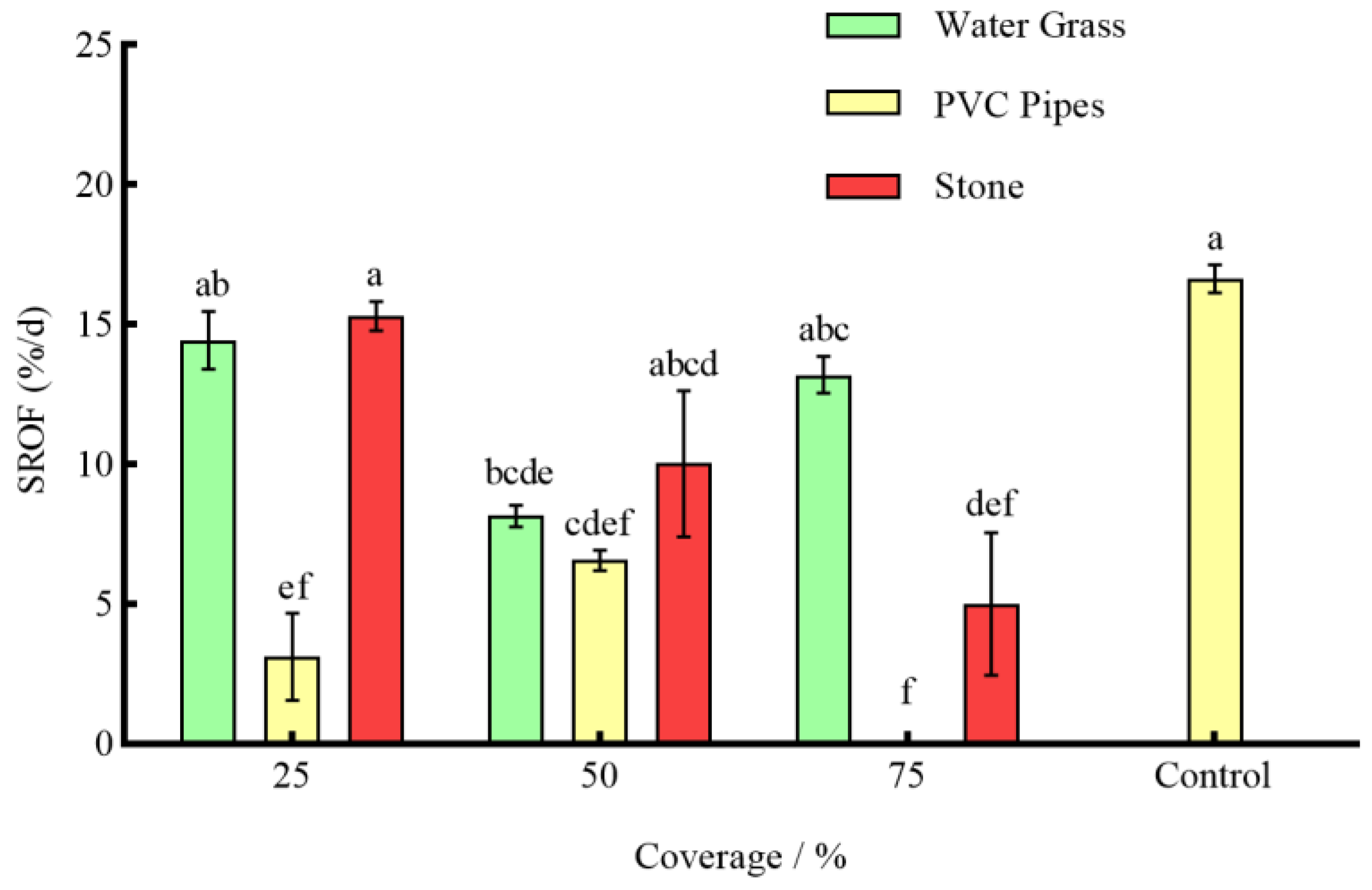
3.3. Effects of Different Coverages of Shelter on the Anti-Predation of P. clarkii
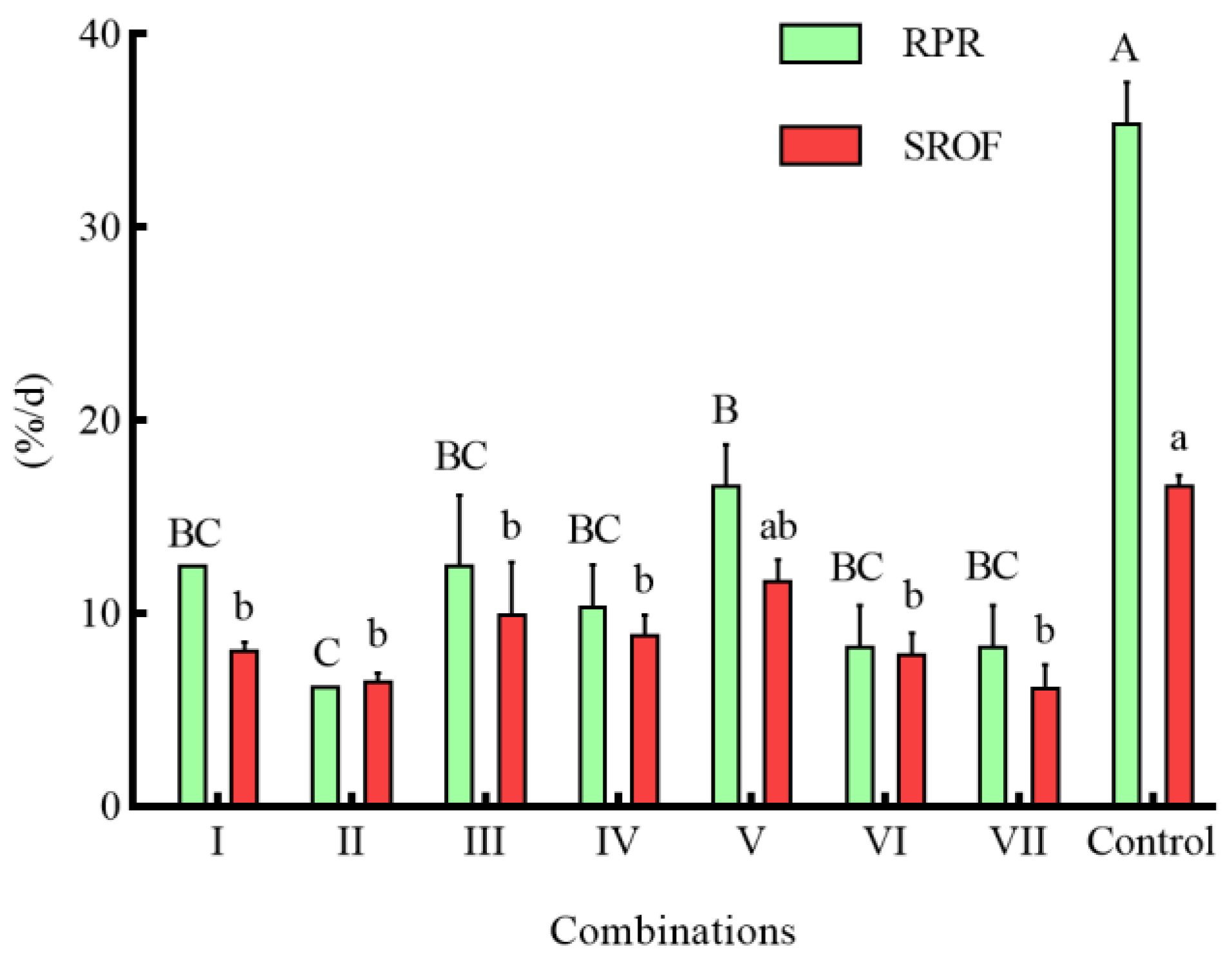
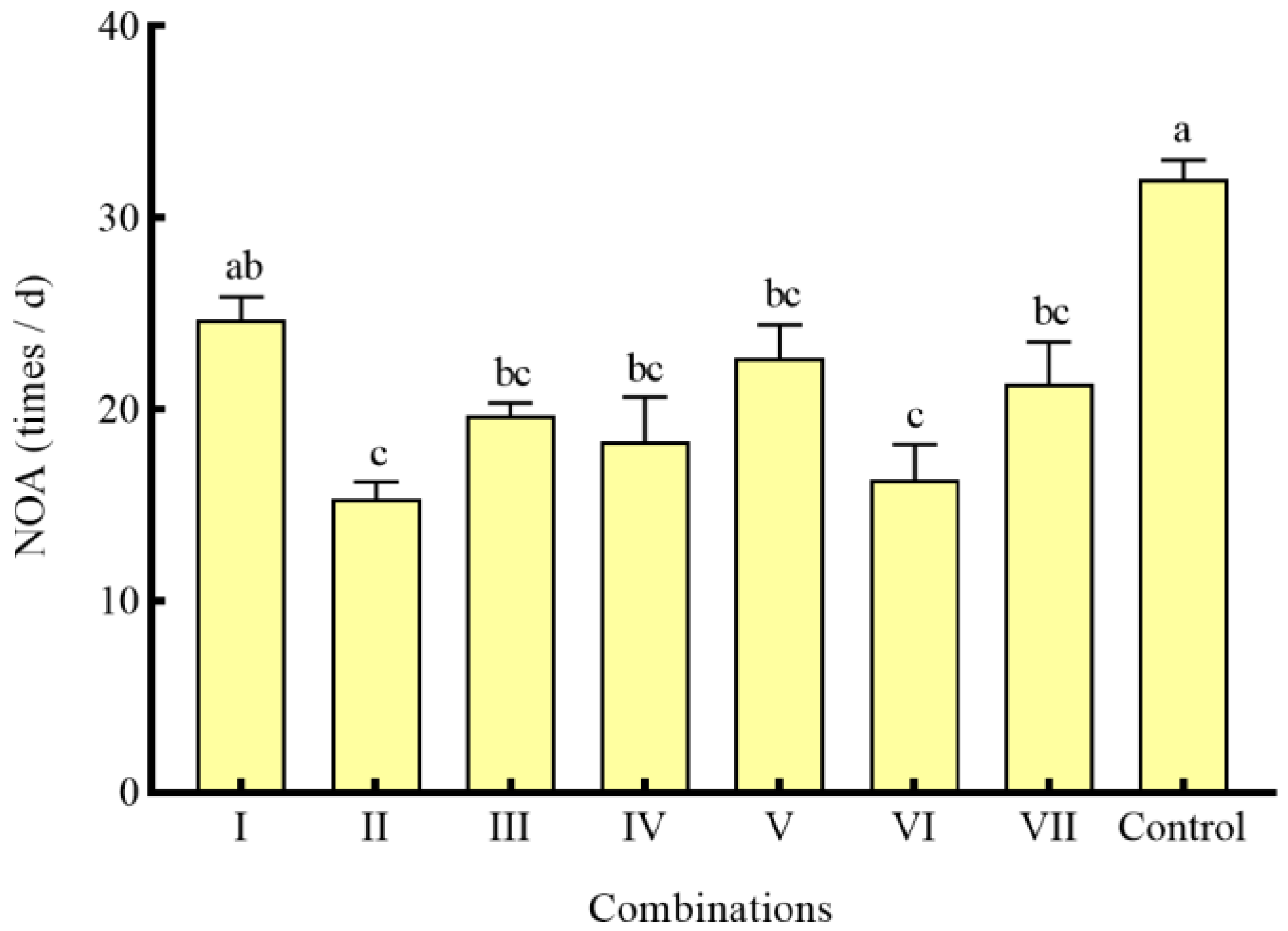
3.4. Effects of Different Combinations of Shelter on the Predation of S. asotus on P. clarkii
4. Discussion
4.1. Predation Rhythm of S. asotus under Different Shelter Conditions
4.2. Effects of Different Coverage of Shelter on the Predation of S. asotus on P. clarkii
4.3. Effects of Different Combinations of Shelter on the Predation of S. asotus on P. clarkii
4.4. Effects of Shelter on the Resources of P. clarkii
4.5. Effects of Environmental Conditions on the Predatory Activities of Fishes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.; Guo, J.Y.; Tang, J.Q.; Huang, C. The differences of growth and living strategies between burrow and corner habitat in Procambarus clarkii. J. Nanjing Univ. 2011, 47, 635–641. [Google Scholar]
- Figler, M.H.; Blank, G.S.; Peeke, H.V.S. Shelter competition between resident male red swamp crayfish Procambarus clarkii (Girard) and conspecific intruders varying by sex and reproductive status. Mar. Freshw. Behav. Physiol. 2005, 38, 237–248. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Y.; Zhong, Y.; Zhu, C.; Zhang, X.; Shi, L.; Zhou, X. Current distribuiton of Procambarus clarkii (Crustacea, Decapoda, Cambaridae) in Poyang Lake and the preliminary morphological cluster analysis of different populaitons. Resour. Environ. Yangtze Basin 2014, 23, 415–421. [Google Scholar]
- Zhang, Y.P.; Chen, W.J.; Fang, C.L. Analysis of the fishing population structure of Procambarus clarkiii in Poyang Lake area. Jiangxi Fish. Sci. Technol. 2014, 2, 6–9. [Google Scholar]
- He, G.; Feng, G.P.; Fu, H.Y.; Wang, H.H.; Zhang, Y.P.; Zhang, A.F.; Ma, B.H.; Yuan, J.X. Distribution characteristics and morphological Differences of the Procambarus Clarkii population in Poyang Lake after the fishing ban. Jiangxi Fish. Sci. Technol. 2024, 1, 1–6. [Google Scholar]
- Wang, C.L.; Qin, J.P.; Huang, C. Behavioral responses of juvenile and subadult crayfishes(Procambarus clarkii) to its predator, the soft-shelled turtle Trionyx sinensis. J. Biosaf. 2013, 22, 169–172. [Google Scholar]
- Song, Z.K. A brief discussion on the methods of preventing natural enemies by cultivating crayfish in paddy field. Henan Agric. 2019, 1, 52. [Google Scholar]
- Blake, M.A.; Hart, P.J.B. The vulnerability of juvenile signal crayfish to perch and eel predation. Freshw. Biol. 1995, 33, 233–244. [Google Scholar] [CrossRef]
- Tierney, A.J.; Godleski, M.S.; Massanari, J.R. Comparative analysis of agonistic behavior in four crayfish species. J. Crustac. Biol. 1992, 20, 54–66. [Google Scholar] [CrossRef]
- Mace, M.M.; Iiirozas, L.P. Fish Predation on Juvenile Penaeid Shrimp: Examining Relative Predator Impact and Size-Selective Predation. Estuaries Coasts 2018, 41, 2128–2134. [Google Scholar] [CrossRef]
- Tyser, A.; Douthwaite, R. Predation on invasive redclaw crayfish Cherax quadricarinatus by native fishes in the Kafue River, Zambia. Afr. J. Aquat. Sci. 2014, 39, 473–477. [Google Scholar] [CrossRef]
- Jones, C.M.; Ruscoe, I.M. Assessment of Five Shelter Types in the Production of Redclaw Crayfish Cherax quadricarinatus (Decapoda: Parastacidae) Under Earthen Pond Conditions. J. World Aquac. Soc. 2001, 32, 41–52. [Google Scholar] [CrossRef]
- Huang, C.; Xiong, Q.H.; Tang, J.Q.; Wu, M. Shadow area and partitioning influencing mortality, healthiness and growth of juvenile Red Swamp Crayfish Procambarus clarkii (Decapoda). Aquac. Res. 2012, 43, 1677–1686. [Google Scholar] [CrossRef]
- Björn, S. Interspecific dominance relationship and aggressive interactions in the freshwater crayfishes Astacus astacus (L.) and Pacifastacus leniusculus (Dana). Can. J. Zool. 1991, 69, 1321–1325. [Google Scholar]
- Sáez-Royuela, M.; Carral, J.M.; Celada, J.D.; Pérez, J.R. Effects of shelter type and food supply frequency on survival and growth of stage-2 juvenile white-clawed crayfish (Austropotamobius pallipes Lereboullet) under laboratory conditions. Aquac. Int. 2001, 9, 489–497. [Google Scholar] [CrossRef]
- Amaral, V.; Paula, J.; Hawkins, S.; Jenkins, S. Cannibalistic interactions in two co-occurring decapod species: Effects of density, food, alternative prey and habitat. J. Exp. Mar. Biol. Ecol. 2009, 368, 88–93. [Google Scholar] [CrossRef]
- Capelli, G.M.; Hamilton, P.A. Effects of food and shelter on aggressive activity in the crayfish Orconectes rusticus (Girard). J. Crustac. Biol. 1984, 4, 252–260. [Google Scholar] [CrossRef]
- Tang, J.Q.; Song, S.L.; Pan, J.L.; Huang, C. The preference to Artificial caves by Procambarus clarkii. Fish. Sci. 2004, 23, 26–28. [Google Scholar]
- Luo, L.; Huang, J.; Liu, D.; Tan, L.; Jiang, S.; Zhou, F.; Jiang, S.; Yang, Q.; Yang, L. Study on selection and spatial distribution of two kinds of caverns for Procambarus clarkii. South China Fish. Sci. 2019, 15, 76–81. [Google Scholar]
- Lodge, D.M. Factors governing species composition, population size, and productivity of cool-water crayfishes. Nord. J. Freshw. Res. 1994, 69, 111–136. [Google Scholar]
- Song, G.; Ding, F.; Chen, J.; Wu, S.; Wang, X. Effects of broodstock sizes, shelter, illumination and stocking density on breeding in red swamp crayfish Procambarus clarkii. Fish. Sci. 2012, 31, 549–553. [Google Scholar]
- Jiang, G.Y.; Yan, W.H. Influence of Different Shroud on the Hatching Rate of the Fertilized Eggs of Procambarus clarkii. Shandong Fish. 2007, 32, 9–10. [Google Scholar]
- Jie, H.; Gao, Y.; Wang, W.; Xie, J.J.; Shi, H.; Wang, G.S.; Xu, W.J. Limb autotomy patterns in the juvenile swimming crab (Portunus trituberculatus) in earth ponds. Aquaculture 2016, 463, 189–192. [Google Scholar]
- Kitagawa, A.T.; Costa, L.S.; Paulino, R.R.; Luz, R.K.; Rosa, P.V.; Guerra-Santos, B.; Fortes-Silva, R. Feeding behavior and the effect of photoperiod on the performance and hematological parameters of the pacamã catfish (Lophiosilurus alexandri). Appl. Anim. Behav. Sci. 2015, 171, 211–218. [Google Scholar] [CrossRef]
- Mattos, B.O.D.; Nascimento-Filho, E.C.T.; Anjos-Santos, A.D.; Sánchez-Vázquez, F.J.; Fortes-Silva, R. Daily self-feeding activity rhythms and dietary self-selection of pirarucu (Arapaima gigas). Aquaculture 2016, 465, 152–157. [Google Scholar] [CrossRef]
- Chen, S.B.; Chen, W.X.; Fang, Z.T. Effect of Water Temperature on Feeding Rhythm in Common Carp (Cyprinus carpio haematopterus Temminck et Schlegel). J. Northeast Agric. Univ. 2012, 19, 57–61. [Google Scholar]
- Gao, X.; Pang, G.; Luo, X.; You, W.; Ke, C. Effects of light cycle on circadian feeding activity and digestive physiology in Haliotis discus hannai. Aquaculture 2021, 539, 736642. [Google Scholar] [CrossRef]
- Xie, C.X.; Xiong, C.X.; Zhou, J.; Wan, X.M.; Jin, H. Feeding intensity and dynamics of juvenile northern snakehead, Channa argus, under different illumination. Acta Hydrobiol. Sin. 1997, 21, 213–218. [Google Scholar]
- Qiao, Z.G.; Zhang, G.L.; Zhang, Y.Y.; Peng, X.L. Diel feeding rhythm of juvenile oriental sheatfish Silurus asotus in different photoperiods. Chin. J. Ecol. 2008, 27, 791–796. [Google Scholar]
- Savino, J.F.; Stein, R.A. Behavior of fish predators and their prey: Habitat choice between open water and dense vegetation. Environ. Biol. Fishes 1989, 24, 287–293. [Google Scholar] [CrossRef]
- Heck, K.L.; Crowder, L.B. Habitat structure and predator—Prey interactions in vegetated aquatic systems. In Habitat Structure; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Alexander, M.E.; Dick, J.T.A.; O’Connor, N.E. Born to kill: Predatory functional responses of the littoral amphipod Echinogammarus marinus Leach throughout its life history. J. Exp. Mar. Biol. Ecol. 2013, 439, 92–99. [Google Scholar] [CrossRef]
- Yu, J.X.; Xiong, M.T.; Ye, S.W.; Li, W.; Zhang, T.L. Effects of stocking density and artificial macrophyte shelter on survival, growth and molting of juvenile red swamp crayfish (Procambarus clarkii) under experimental conditions. Aquaculture 2020, 521, 735001. [Google Scholar] [CrossRef]
- Wu, L.M.; Han, G.M.; Tan, B.L.; Zhang, J.H.; Wang, S.H. Effects of different aquatic plants on growth performance, physiological indices and muscle nutrients of red swamp crayfish (Procambarus clarkii). J. Dalian Ocean Univ. 2023, 38, 779–786. [Google Scholar]
- Stoner, A.W. The influence of benthic macrophytes on the foraging behavior of pinfish, Lagodon rhomboides (Linnaeus). J. Exp. Mar. Biol. Ecol. 1982, 58, 271–284. [Google Scholar] [CrossRef]
- Howard, R.K.; Koehn, J.D. Population dynamics and feeding ecology of pipefish (Syngnathidae) associated with eelgrass beds of Western Port, Victoria. Mar. Freshw. Res. 1985, 36, 361–370. [Google Scholar] [CrossRef]
- Ostrand, K.G.; Braeutigam, B.J.; Wahl, D.H. Consequences of Vegetation Density and Prey Species on Spotted Gar Foraging. Trans. Am. Fish. Soc. 2004, 133, 794–800. [Google Scholar] [CrossRef]
- Gotceitas, V. Variation in plant stem density and its effects on foraging success of juvenile bluegill sunfish. Environ. Biol. Fishes 1990, 27, 63–70. [Google Scholar] [CrossRef]
- Figler, M.H.; Cheverton, H.M.; Blank, G.S. Shelter competition in juvenile red swamp crayfish (Procambarus clarkii): The influences of sex differences, relative size, and prior residence. Aquaculture 1999, 178, 63–75. [Google Scholar] [CrossRef]
- Everett, R.A.; Ruiz, G.M. Coarse woody debris as a refuge from predation in aquatic communities: An experimental test. Oecologia 1993, 93, 475–486. [Google Scholar] [CrossRef]
- Karnofsky, E.B.; Atema, J.; Elgin, R.H. Natural Dynamics of Population Structure and Habitat Use of the Lobster, Homarus americanus, in a Shallow Cove. Biol. Bull. 1989, 176, 247–256. [Google Scholar] [CrossRef]
- Vince, S.; Valiela, I.; Backus, N.; Teal, J.M. Predation by the salt marsh killifish Fundulus heteroclitus (L.) in relation to prey size and habitat structure: Consequences for prey distribution and abundance. J. Exp. Mar. Biol. Ecol. 1976, 23, 255–266. [Google Scholar] [CrossRef]
- Stein, R.A. Selective Predation, Optimal Foraging, and Resource Depression within the Predator-Prey Interaction between Fish and Crayfish; University of Wisconsin-Madison: Madison, WI, USA, 1975. [Google Scholar]
- Valley, R.D.; Bremigan, M.T. Effects of Macrophyte Bed Architecture on Largemouth Bass Foraging: Implications of Exotic Macrophyte Invasions. Trans. Am. Fish. Soc. 2002, 131, 234–244. [Google Scholar] [CrossRef]
- Eggleston, D.B.; Lipcius, R.N. Shelter selection by spiny lobster under variable predation risk, social conditions, and shelter size. Ecology 1992, 73, 992–1011. [Google Scholar] [CrossRef]
- Mintz, J.D.; Lipcius, R.N.; Eggleston, D.B.; Seebo, M.S. Survival of juvenile caribbean spiny lobster: Effects of shelter size, geographic location and conspecific abundance. Mar. Ecol. Prog. Ser. 1994, 112, 255. [Google Scholar] [CrossRef]
- Nilsson, P.A.; Bronmark, C. Prey Vulnerability to a Gape-Size Limited Predator: Behavioural and Morphological Impacts on Northern Pike Piscivory. Oikos 2008, 88, 539–546. [Google Scholar] [CrossRef]
- Webb, P.W. Effect of Body Form and Response Threshold on the Vulnerability of Four Species of Teleost Prey Attacked by Largemouth Bass (Micropterus salmoides). Can. J. Fish. Aquat. Sci. 2011, 43, 763–771. [Google Scholar] [CrossRef]
- Huang, W.; Hu, T.; Mao, J.; Montzka, C.; Bol, R.; Wan, S.; Li, J.; Yue, J.; Dai, H. Hydrological Drivers for the Spatial Distribution of Wetland Herbaceous Communities in Poyang Lake. Remote Sens. 2022, 14, 4870. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Xu, X.L.; Li, Y.L.; Jiang, J.H.; Zhang, Q. Wetland landscape pattern evolution of large yangtze-connected lakes in the middle reaches of yangtze river. Resour. Environ. Yangtze Basin 2017, 26, 1619–1624. [Google Scholar]
- Eversole, A.G.; Mazlum, Y. Comparative fecundity of three Procambarus species. J. Shellfish Res. 2002, 21, 255–258. [Google Scholar]
- Lei, X.Q.; Yan, B.H.; Yao, Y.; Jiang, Q.L.; Wang, Y.L. Study on biological characteristics and propagation techniques of wheat ear fish. Jiangxi Fish. Sci. Technol. 2013, 16–18. [Google Scholar]
- Xie, D.S.; Luo, L.Z. Diapause and its roles in the population dynamics of the beet webworm, Loxostege sticticalis. Plant Prot. 2011. [Google Scholar]
- Zhao, M.G.; Chen, J.H.; Feng, G.P.; Wang, H.H.; Zhang, Y.P. The Predation Selectivity of Ferocious Fish (Silurus asotus and Channa argus) to Procambarus clarkii Larvae in Poyang Lake. Acta Hydrobiol. Sin. 2024, 48, 799–807. [Google Scholar]
- Correia, A.M.; Ferreira, Ó. Burrowing behavior of the introduced red swamp crayfish Procambarus clarkii (Decapoda: Cambaridae) in Portugal. J. Crustac. Biol. 1995, 15, 248–257. [Google Scholar] [CrossRef]
- Groza, M.I.; Pop-Vancia, V.; Mireşan, V. Diel activity and use of multiple artificially constructed shelters in Astacus leptodactylus (Decapoda: Astacidae). Biologia 2016, 71, 1369–1379. [Google Scholar] [CrossRef]
- Li, R.R.; Kaufman, Y.J.; Gao, B.C.; Davis, C.O. Remote sensing of suspended sediments and shallow coastal waters. IEEE Trans. Geosci. Remote Sens. 2003, 41, 559–566. [Google Scholar]
- Levin, I.M.; Radomyslskay, T.M. Estimate of water inherent optical properties from secchi depth. Izv. Atmos. Ocean. Phys. 2012, 48, 214–221. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Li, M.Y.; Wu, X.K. Study on the growth and feeding rhythm of larval and juvenile of Lateolabrax Japonicus. J. Zhejiang Ocean Univ. 2002, 21, 210–215. [Google Scholar]
- Li, K.G.; Zhen, D.S.; Fu, S.J. Effect of feeding on excess post-exercise oxygen consumption in juvenile chinese catfish (Silurus asotus Linnaeus). Acta Hydrobiol. Sin. 2012, 36, 1035–1040. [Google Scholar] [CrossRef]
- Perry, W.L.; Lodge, D.M.; Lamberti, G.A. Impact of crayfish predation on exotic zebra mussels and native invertebrates in a lake-outlet stream. Can. J. Fish. Aquat. Sci. 1997, 54, 120–125. [Google Scholar] [CrossRef]
- Dorn, N.J.; Mittelbach, G.G. More than predator and prey: A review of interactions between fish and crayfish. Life Environ. 1999, 49, 229–237. [Google Scholar]
- Hoyle, J.A.; Allen, K. The effect of prey morphology and size on handling time in a piscivore, the largemouth bass (Micropterus salmoides). Can. J. Zool. 1987, 65, 1972–1977. [Google Scholar] [CrossRef]
- Gill, A.B. The dynamics of prey choice in fish: The importance of prey size and satiation. J. Fish Biol. 2010, 63, 105–116. [Google Scholar] [CrossRef]



| Species | Number | TL (mm) | BW (g) | MB (mm) | OFL (mm) | BH (mm) | BW (mm) |
|---|---|---|---|---|---|---|---|
| S. asotus | 42 | 311.65 ± 26.53 | 205.67 ± 30.84 | 35.03 ± 3.62 | 27.88 ± 4.11 | / | / |
| P. clarkii | 672 | 38.40 ± 1.75 | 1.94 ± 0.59 | / | / | 10.48 ± 0.25 | 9.35 ± 0.77 |
| Species | Number of S. asotus | Number of P. clarkii | Number | Shelter Type (Coverage Rate: 50%) | ||
|---|---|---|---|---|---|---|
| Water Grass | PVC Pipes | Stone | ||||
| S. asotus | 1 | 16 | I | √ | ||
| 1 | 16 | II | √ | |||
| 1 | 16 | III | √ | |||
| 1 | 16 | IV | √ | √ | ||
| 1 | 16 | V | √ | √ | ||
| 1 | 16 | VI | √ | √ | ||
| 1 | 16 | VII | √ | √ | √ | |
| Shelter Type | Time Period | ||||
|---|---|---|---|---|---|
| 8~20 | 20~8 | ||||
| RPR (%) | NOA | RPR (%) | NOA | ||
| Water grass (WG) | 25% | 2.08 ± 2.08 | 7.33 ± 0.66 | 22.92 ± 1.20 a | 20.67 ± 1.69 a |
| 50% | 0 | 7.33 ± 0.33 | 12.50 ± 0.00 a | 17.33 ± 1.32 a | |
| 75% | 4.17 ± 2.05 | 8.67 ± 0.47 | 18.75 ± 0.00 a | 19.33 ± 1.48 a | |
| PVC pipes (PP) | 25% | 0 | 5.67 ± 0.67 | 4.17 ± 2.05 | 14.67 ± 0.88 a |
| 50% | 0 | 5.67 ± 0.33 | 6.25 ± 0.00 a | 9.67 ± 0.67 a | |
| 75% | 0 | 2.33 ± 0.33 | 0 | 5.33 ± 0.88 | |
| Stone (ST) | 25% | 4.17 ± 2.05 | 9.33 ± 0.88 | 22.92 ± 1.20 a | 19.00 ± 1.16 a |
| 50% | 2.08 ± 2.08 | 7.00 ± 0.58 | 10.42 ± 1.94 a | 12.67 ± 0.33 a | |
| 75% | 0 | 4.00 ± 0.58 | 4.17 ± 2.05 | 9.67 ± 0.33 a | |
| Coverage rate: 50% | IV | 0 | 5.67 ± 0.67 | 10.42 ± 1.94 a | 12.67 ± 1.82 a |
| V | 4.17 ± 2.05 | 6.67 ± 0.33 | 12.50 ± 0.00 a | 15.33 ± 1.88 a | |
| VI | 2.08 ± 2.08 | 4.33 ± 0.33 | 6.25 ± 3.61 | 12.00 ± 2.00 a | |
| VII | 0 | 4.66 ± 0.33 | 8.33 ± 2.05 a | 16.67 ± 1.77 a | |
| Control | 6.25 ± 0.00 | 9.67 ± 0.87 | 29.17 ± 2.06 a | 22.33 ± 0.67 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Feng, G.; Wang, H.; Shen, C.; Fu, Y.; Zhang, Y.; Zhang, H.; Yao, Y.; Chen, J.; Xu, W. The Influence of Shelter Type and Coverage on Crayfish (Procambarus clarkii) Predation by Catfish (Silurus asotus): A Controlled Environment Study. Animals 2024, 14, 1147. https://doi.org/10.3390/ani14081147
Zhao M, Feng G, Wang H, Shen C, Fu Y, Zhang Y, Zhang H, Yao Y, Chen J, Xu W. The Influence of Shelter Type and Coverage on Crayfish (Procambarus clarkii) Predation by Catfish (Silurus asotus): A Controlled Environment Study. Animals. 2024; 14(8):1147. https://doi.org/10.3390/ani14081147
Chicago/Turabian StyleZhao, Mingguang, Guangpeng Feng, Haihua Wang, Chenchen Shen, Yilong Fu, Yanping Zhang, Haixin Zhang, Yuan Yao, Jianhua Chen, and Weikang Xu. 2024. "The Influence of Shelter Type and Coverage on Crayfish (Procambarus clarkii) Predation by Catfish (Silurus asotus): A Controlled Environment Study" Animals 14, no. 8: 1147. https://doi.org/10.3390/ani14081147





