Simple Summary
We designed a common garden experiment to collect data on female reproductive traits from three populations of Takydromus sexlineatus, testing the hypothesis that geographically separated populations should share a species-specific pattern of seasonal shifts in reproductive allocation. Six traits differed among populations, with four of the six also differing among successive clutches. Females grew longer during the breeding season and produced more eggs in the first clutch than in the subsequent clutches; egg size was unchanged throughout the breeding season. After removing the influence of female size or postpartum mass we found that (1) postpartum body mass, clutch mass, and relative clutch mass were greater in the Wuzhishan population than in the Shaoguan and Zhaoqing populations; (2) egg size was greatest in the Wuzhishan population and smallest in the Zhaoqing population; and (3) clutch size was greatest in the Wuzhishan population and smallest in the Shaoguan population. Our data validate the hypothesis tested.
Abstract
We designed a common garden design to collect data on female reproductive traits from three populations of the southern grass lizard Takydromus sexlineatus, testing the hypothesis that a species-specific pattern of seasonal shifts in reproductive allocation should be shared by geographically separated populations. Of the seven examined traits, six differed among populations, with four of the six also differing among successive clutches. Females grew longer during the breeding season and produced more eggs in the first clutch than in the subsequent clutches; egg size was unchanged throughout the breeding season. After removing the influence of female size or postpartum body mass we found the following. First, postpartum body mass, clutch mass, and relative clutch mass were greater in the Wuzhishan population than in the Shaoguan and Zhaoqing populations. Second, egg size was greatest in the Wuzhishan population and smallest in the Zhaoqing population. Third, clutch size was greatest in the Wuzhishan population and smallest in the Shaoguan population. Females did not trade-off egg size against number within each population × clutch combination. Our study validates the hypothesis tested, supports the conventional view that reproductive output is highly linked to maternal body size in lizards, and follows the classic prediction that females with different amounts of resources to invest in reproduction should give priority to adjusting the total number rather than size of their offspring.
1. Introduction
Reproductive traits include the timing and duration of the breeding season, the size range of reproductive individuals, total investment per reproductive episode or season, reproductive frequency (the number of clutches or litters produced per breeding season), and reproductive allocation to offspring (egg or neonate) size versus number. These variables can vary between geographically separated populations, especially those of widespread species adapting to different habitats [1,2,3]. Previous studies generally conclude that patterns of geographical variation in reproductive traits are jointly determined by two mutually non-exclusive mechanisms, phenotypic plasticity and local adaptation, of which the relative importance on individual traits in question can be disentangled by using a reciprocal transplant or common garden design [4,5,6,7]. Many studies have investigated geographical variation in reproductive traits in a diverse array of animal taxa, including lizards of the families Agamidae [8,9], Dactyloidae [10,11], Gekkonidae [12], Lacertidae [13,14,15,16,17,18,19,20], Phrynosomatidae [21,22,23,24,25,26,27,28,29], Polychrotidae [30], Scincidae [31,32,33,34], and Xenosauridae [35]. However, these studies have rarely covered the whole breeding season to compare reproductive traits among populations. For the species where females reproduce multiple times per breeding season, geographical variations in reproductive traits cannot be accurately, objectively, and comprehensively presented without taking seasonal shifts in reproductive allocation into account.
Many lizards of the family Lacertidae are multiple-clutched species [13,14,15,16,17,18,19,20]. Reproductive investment per episode and its allocation to offspring number versus size are not fixed in lacertid lizards but vary seasonally, even when the energy and maternal abdominal space available to hold the clutch have unlimited availability. For example, female common wall lizards (Podarcis muralis) produce more and larger eggs in the first clutch and fewer and smaller eggs in the subsequent clutches [36]; female white-striped grass lizards (Takydromus wolteri) produce more eggs in the first clutch and fewer eggs in the subsequent clutches but keep egg size unchanged between successive clutches in a breeding season [15]; and female northern grass lizards (Takydromus septentrionalis) produce more and smaller eggs in the first clutch and fewer and larger eggs in the subsequent clutches [37]. The Mongolian racerunner Eremias argus provides a case where females from a cold-climate population produce more and smaller eggs in the first clutch and fewer and larger eggs in the subsequent clutches [38], whereas their warm-climate conspecifics produce smaller eggs in the first clutch and larger eggs in the subsequent clutches but keep clutch size unchanged between successive clutches throughout the breeding season [39]. What can be inferred from the above examples is that patterns of seasonal shifts in reproductive allocation to offspring size versus number differ among species and even among climatically distinct populations of the same species. However, as studies comparing reproductive traits among populations are far sparser than those of within-population comparisons, a broader collection of data from multiple populations of more species is needed to elucidate not only the life-history consequences of reproductive allocation but also the adaptive significance of seasonal shifts in reproductive investment in individual species.
Here, we have used a common garden design to collect data on seven female reproductive traits [female body size, postpartum body mass, egg size (mass), within-clutch variability in egg mass, clutch size, clutch mass, and relative clutch mass] from three populations of the southern grass lizard Takydromus sexlineatus, paying particular attention to the traits varying among populations and between successive clutches in a breeding season. Data from multiple populations are useful in identifying whether the patterns of seasonal shifts in reproductive allocation observed in individual species are species-specific or population-specific patterns. We hypothesize that a species-specific pattern should be shared by geographically separated populations, whereas a population-specific should differ among geographically separated populations.
2. Materials and Methods
2.1. Study Species
Takydromus sexlineatus is a small oviparous lacertid lizard described by Daudin (1802) [40]. The lizard is basically a tropical species that can be found in the southeastern part (Guangxi, Guizhou, Fujian, Hainan, Hong Kong, Hunan, Guangdong, and Yunnan) of Chinese mainland; it also occurs in Burma, Cambodia, Laos, Thailand, Vietnam, the Malaysian Peninsula, and Indonesia (Borneo, Java, Sumatra and Natuna islands) [41]. Despite its wide distribution and the fact that it is phylogenetically and taxonomically well known, the biology and ecology of T. sexlineatus remain poorly studied. Earlier studies of T. sexlineatus conclude the following: (1) prolonged exposure to temperatures lower than 6 °C or higher than 42 °C is lethal to the lizard, and adults in thermal gradients prefer to maintain their body temperatures within the range from 28 °C to 34 °C, with a mean of 31.5 °C; (2) the body temperature that maximizes locomotor performance (sprint speed) is higher in T. sexlineatus (~33 °C) compared with its congeneric counterparts (~28 °C in T. wolteri and ~31 °C in T. septentrionalis) with more northerly distributions; (3) T. sexlineatus is more vulnerable to climate change than T. wolteri and T. septentrionalis due to the lack of the mechanisms adopted by the latter two species to enhance thermal acclimatization capacity; and (4) the mean incubation length at a given temperature is longest in T. sexlineatus and shortest in T. wolteri, with T. septentrionalis in between [42,43,44].
2.2. Animal Collection and Care
We collected adult males and females with various-sized yolking follicles in late March 2015 and 2016 from three localities (populations): one in Wuzhishan (18°50′ N, 109°31′ E), Hainan Island (Province), and the other two in Shaoguan (24°41′ N, 113°48′ E) and Zhaoqing (23°03′ N, 112°27′ E), Guangdong Province (Figure 1). The annual mean temperature is highest in Wuzhishan (22.4 °C) and lowest in Shaoguan (20.9 °C), with Zhaoqing (22.1 °C) in between. The lizards collected in each year were transported to our laboratory where they were sexed and marked via unique combinations of clipped toes for later identification. Following a previous study on P. muralis on mating purpose [36], we housed 9–12 lizards (with a female-to-male ratio of approximately 2 to 1) from the same population in each 900 × 650 × 350 mm (length × width × height) communal cage with a moist soil substrate (~50 mm depth), grasses, and pieces of clay tile that served as shelters. We placed the communal cages in a room where temperatures varied from 22 °C to 28 °C. Thermoregulatory opportunities were provided daily between 07:00–19:00 h by a 60 W full-spectrum lamp suspended at one end of each communal cage. Mealworms (Tenebrio molitor), house crickets (Achetus domestica), and water enriched with multivitamins and minerals were provided ad libitum. We palpated females at 3 d intervals for the presence of eggs in the oviduct. Females with shelled oviductal eggs were removed from the communal cages and housed individually in 200 × 200 × 200 mm egg-laying cages with moist soil (~40 mm depth). In no case did any female remain in an egg-laying cage for >48 h.
Figure 1.
Map of China (top right corner), showing the three localities where we collected adult T. sexlineatus.
We collected, weighed, and measured eggs within 6 h post-laying. After recording the egg-laying date, snout-vent length (SVL), and body mass, we moved postpartum females back to the communal cages, where they remained until they again carried shelled oviductal eggs and, at that time, they were once again transferred to the egg-laying cages. Females were allowed to be fertilized and produce as many clutches as they could in the laboratory. We incubated the eggs under multiple thermal conditions, and we will report data on the hatching success, incubation length, and temperature-induced variation in the hatchling phenotypes elsewhere. We released the lizards collected in each year at their point of capture in late August soon after the egg-laying season.
2.3. Data Analyses
Clutch size, clutch mass, clutch mean egg mass (hereafter, egg mass), relative clutch mass (RCM), and within-clutch variability in each egg mass were calculated for each clutch. Clutch size was the total number of eggs in a clutch, clutch mass was the total mass of eggs in a clutch, RCM was calculated by dividing clutch mass by postpartum female mass, and within-clutch variability in egg mass was calculated as the coefficient of variation [CV = 100 × (standard deviation/clutch mean egg mass)].
All statistical analyses were performed with Statistica 10.0 (Tulsa, OK, USA). Before parametric analyses, data were tested for normality using the Kolmogorov–Smirnov test and for homogeneity of variances using Bartlett’s test. We used linear regression analysis to examine the relationship between a selected pair of dependent and independent variables and ANCOVA to examine the slope homogeneity of regression lines. We used data on the first three clutches with large enough sample sizes (≥18) to examine geographic and seasonal variation in female reproductive traits. More specifically, we used a two-way ANOVA (female SVL, egg mass, and CV of egg mass, of which the latter two were found to be independent of female SVL) or ANCOVA (postpartum body mass, clutch size, and clutch mass using female SVL as the covariate, and relative clutch mass using postpartum body mass as the covariate), with the population origin and clutch order as the fixed factors, to examine whether these female reproductive traits differed among populations and among successive clutches. Tukey’s post hoc test was performed on a trait that differed among populations and/or clutches. We used partial correlation analysis to examine whether females traded egg size (mass) off against egg number while holding female size (SVL) constant. Throughout this paper, values are presented as mean ± standard error (SE) and range, and the significance level is set at p = 0.05.
3. Results
Body sizes ranged from 49.6 to 64.2 mm SVL in Wuzhishan females, 44.5 to 57.9 mm SVL in Shaoguan females, and 46.3 to 61.4 mm SVL in Zhaoqing females (Table 1). Females from the three populations laid eggs in the months from April to August. Wuzhishan and Shaoguan females laid up to five clutches and Zhaoqing females laid up to four clutches per egg-laying season, with no females in either population laying more than four eggs per clutch (Table 1).

Table 1.
Descriptive statistics, expressed as mean ± SE and range, for post-laying female size and mass, egg mass, within-clutch variability [CV = 100 × (standard deviation/mean)] in egg mass, clutch size, clutch mass, and relative clutch mass in T. sexlineatus from the Wuzhishan, Shaoguan, and Zhaoqing populations. N = the number of females laying eggs.
Data on the first three clutches show that the mean values for female SVL differed among the three populations and among the first three clutches, and that the interaction between population (origin) and clutch (order) was not a significant source of variation in female SVL (Table 2). More specifically, the mean SVL was greater in Wuzhishan females than in females from the other two populations, and it was greater in females laying the subsequent clutches than in females laying their first clutch.

Table 2.
Results of two-way ANOVA (female SVL, egg mass, and CV of egg mass) and ANCOVA (female postpartum body mass, clutch size, and clutch mass using female SVL as the covariate, and relative clutch mass using female postpartum body mass as the covariate) with population origin and clutch order as the fixed factors on the first three clutches. Populations and clutches with different superscripts differ significantly (Tukey’s post hoc test, α = 0.05, a > b > c). WZS, SG, and ZQ indicate the Wuzhishan, Shaoguan, and Zhaoqing populations, respectively. F, S, and T indicate the first, second, and third clutches, respectively.
From a series of linear regression analyses within each population × clutch combination, we knew the following. First, postpartum body mass, clutch size, and clutch mass were positively related to female SVL (all p < 0.05). Second, egg mass and within-clutch variability in egg mass (CV of egg mass) were independent of female SVL (all p > 0.05). Third, clutch mass was positively related to postpartum female mass (all p < 0.05). Postpartum body mass differed among the three populations but not among the first three clutches after accounting for female SVL, with the SVL-adjusted mean mass being greater in Wuzhishan females than in females from other two populations (Table 2). Clutch size and clutch mass differed among the three populations and among the first three clutches after accounting for female SVL, and so did relative clutch mass after accounting for postpartum mass (Table 2). The SVL-adjusted mean clutch size was greatest in Wuzhishan females and smallest in Shaoguan females, with Zhaoqing females in between; the SVL-adjusted mean clutch size was greater in the first clutch than in the subsequent two clutches (Table 2). The mean values for clutch mass and RCM were greater in Wuzhishan females than in females from the other two populations and were greater in the first clutch than in the subsequent two clutches after accounting for female SVL or postpartum body mass (Table 2). The mean egg mass was greatest in Wuzhishan females and smallest in Zhaoqing females, with Shaoguan females in between; the mean egg mass did not vary among the first three clutches (Table 2). The within-clutch variability in egg mass remained remarkably consistent across the three populations and the first three clutches (Table 2). Females did not trade-off egg size against egg number, as was revealed by the fact that the negative correlation between egg mass and clutch size was not significant within each population × clutch combination after controlling for female SVL (partial correlation analysis; all r > −0.34 and all p > 118). Female postpartum mass was a significant determinant of egg mass (linear regression analysis, all p < 0.05) but only explained a very small proportions (from 3.8% in the Zhaoqing population to 7.0% in the Wuzhishan population) of variation in the egg mass within each population (Figure 2). After accounting for female postpartum mass, we once again found that the mean egg mass was greatest in Wuzhishan females and smallest in Zhaoqing females, with Shaoguan females in between (Tukey’s post hoc test, all p < 0.01).
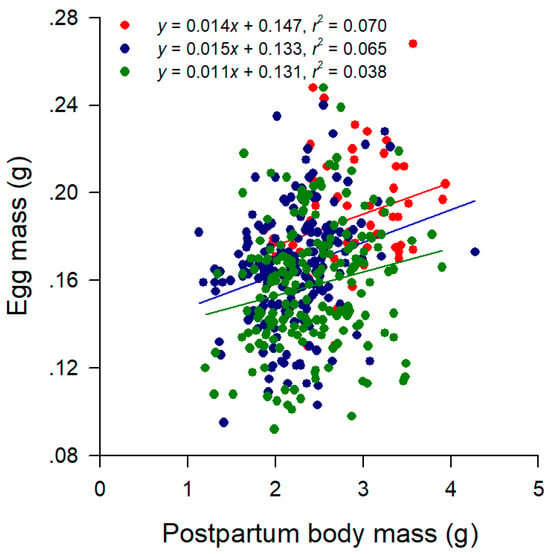
Figure 2.
Linear regressions of egg mass on postpartum body mass in Wuzhishan (red dots and line), Shaoguan (blue dots and line), and Zhaoqing (green dots and line) females. Regression equations and coefficients are given the figure.
4. Discussion
Of the seven female reproductive traits examined in this study, six differed among the three populations, and four of the six differed among the first three clutches (Table 2). The CV of egg mass was the only trait that did not differ among three populations, nor among the first three clutches. This finding suggests that female southern grass lizards from different populations and in different seasons tend to produce eggs of similar size in the same clutch. Such a strategy of reproductive investment per offspring is often adopted by species existing in fairly constant or predictable environments, following the prediction from the parent–offspring conflict theory for maternal investment in individual offspring [45,46]. The interaction between population origin and clutch order was not a significant source of variation in any examined female reproductive trait (Table 2). This finding is of particular interest because it suggests that the three populations of T. sexlineatus share the same patterns of seasonal shifts in all the examined female reproductive traits, thus validating the hypothesis tested. Our finding that reproductive female T. sexlineatus grew longer (SVL) but kept postpartum body mass unchanged between successive clutches provides evidence for the existence of two mechanisms of energy allocation during the breeding season. First, as in other animal taxa [47,48,49,50], somatic growth has a higher priority over reproduction in energy allocation in T. sexlineatus. Second, like other multiple-clutched lacertid females [15,36,37,38,39], female T. sexlineatus maximize annual fecundity by channeling surplus energy not used in the current reproductive episode into the next clutch. It is worth noting that the increases in SVL and optimal reproductive allocation along this study were achieved in females having ad libitum conditions of food and thermal requirements, which are very unlikely to be fulfilled in the field due to various limitations in resource acquisition.
Like female T. wolteri [15], female T. sexlineatus produced more eggs in the first clutch and fewer eggs in the subsequent clutches but kept egg mass unchanged between successive clutches (Table 2). This suggests that, although these two congeneric species are distributed in climatically different regions (T. wolteri in the temperate region, and T. sexlineatus in the tropics), they share the same patterns of seasonal shifts in clutch size and egg size, which differ from the patterns recorded in T. septentrionalis where females produce more and smaller eggs in the first clutch than in the subsequent clutches [37]. Of these three Takydromus species, only T. septentrionalis is endemic to China, with its distributional range overlapping south with T. sexlineatus and north with T. wolteri [41]. Therefore, the above comparisons provide an inference that seasonal shifts in clutch size and egg size do not necessarily vary among congeneric species in a geographically continuous way.
Body condition is crucial to the onset of vitellogenesis in squamate reptiles, with poorly conditioned females often reducing the number of offspring produced per episode or even skipping current reproduction [37,51,52]. Therefore, one plausible explanation for the larger SVL-adjusted mean clutch size in Wuzhishan females lies in their better body conditions, as was revealed by the fact that postpartum body mass was greater in Wuzhishan females than in females from the other two populations after accounting for SVL (Table 2). Alternatively, it might be possible that body conditions during the breeding season were always above the lower threshold required to initiate vitellogenesis of a clutch predicted by using female SVL in each population of T. sexlineatus.
We found that the SVL-adjusted mean clutch mass varied among the three populations of T. sexlineatus (Table 2). This variation resulted primarily from geographical variation in reproductive investment per episode and its allocation to egg number versus size, as was revealed by the fact that clutch mass, clutch size, and egg size all differed among the three populations (Table 2). RCM is determined by using postpartum female mass and clutch mass, both of which may change geographically and temporally in squamate reptiles [15,33,53]. With all else being equal, RCM should be greater in species where females produce heavier clutches but leave a smaller amount of energy reserves after laying, and geographical variation in RCM should be more apparent in widespread species living in contrasting environments [15].
It is common among reptiles that the total energy allocated to reproduction per episode is positively related to female size; however, reproductive investment per offspring varies both within and among species [52]. Larger female T. sexlineatus laid larger and heavier clutches, primarily by producing more eggs rather than larger eggs, as was revealed by the following two facts. First, egg mass was independent of female SVL within each population × clutch combination. Second, egg mass did not vary among successive clutches. Our study is the first to demonstrate that female postpartum body mass explains a significant proportion of variation in egg size in lizards (Figure 1). SVL-adjusted mean postpartum body mass was greater in Wuzhishan females (Table 2), suggesting that they accumulated a greater amount of energy throughout the breeding season and were therefore more likely to produce larger eggs. However, factors other than postpartum body mass also affect egg size in T. sexlineatus, as was revealed by the fact that the three populations differed from each other in mean egg mass after accounting for female postpartum mass (Figure 1). Presumably, the finding that the three populations of T. sexlineatus differ from each other in mean egg mass can be explained with respect to local adaptation of life history because organisms cannot better adapt to local environments without adjusting their life history traits plastically and/or genetically.
5. Conclusions
Larger females of T. sexlineatus laid more eggs and heavier clutches. This finding supports the conventional view that fecundity and reproductive output (clutch or litter mass) are highly linked to female body size in lizards. Egg size was less variable than clutch size in T. sexlineatus. Therefore, our study follows Smith and Fretwell’s (1974) [49] prediction that females with different amounts of resources to invest in a given reproductive episode are more likely to adjust the number rather than the size of their offspring. Female T. sexlineatus did not trade-off egg size against number, suggesting that the lizard is among species where egg size is independent of clutch size. Egg size was independent of female body size but was positively related to female postpartum body mass within each of the three populations of our study. These findings suggest that maternal body condition rather than body size is a determinant of egg size in T. sexlineatus. The three populations of T. sexlineatus differed from each other in mean egg mass after accounting for female postpartum body mass. This finding is of interest because it suggests that factors other than maternal body condition may also affect egg size in T. sexlineatus. Our data validate the hypothesis tested, which states that a species-specific pattern of seasonal shifts in reproductive allocation should be shared by all geographically separated populations.
Author Contributions
Conceptualization, C.-F.W. and X.J.; methodology, all authors; software, X.J.; validation, all authors; formal analysis, C.-F.W., Y.D. and X.J.; investigation, all authors; resources, Y.D. and X.J.; data curation, Y.D. and X.J.; writing—original draft preparation, C.-F.W. and X.J.; writing—review and editing, all authors; visualization, C.-F.W., Y.D. and X.J.; supervision, X.J.; project administration, X.J.; funding acquisition, X.J. and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (32370519 to X.J. and 32201268 to K.G.) and Wenzhou Ecological Park Research Project (KH2304012 to X.J.).
Institutional Review Board Statement
This work was carried out in compliance with laws on animal welfare and research in China and was approved by the Animal Research Ethical Committees of Wenzhou University (Approval number: WZU-0162023) and Hainan Tropical Ocean University (Approval number: HOPU 2015-03-006). The lizards in this study were collected under the permissions issued by the Guangdong and Hainan Provincial Forestry Departments.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Da-De Xu for assistance in the field, and Li Ma and Guang-Zheng Zhang for assistance in the laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Charnov, E.L. Life History Invariants; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Roff, D.A. Life History Evolution; Sinauer Associates Inc.: Sunderland, MA, USA, 2002. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Alice, B.W.; Sandercock, B.K.; Martin, K. Patterns and drivers of intraspecific variation in avian life history along elevational gradients: A meta-analysis. Biol. Rev. 2015, 91, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Hero, J.M. Geographic variation in life history characteristics of amphibians: A review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef]
- Trussell, G. Phenotypic clines, plasticity, and morphological trade-offs in an intertidal snail. Evolution 2000, 54, 151–166. [Google Scholar]
- Vila-Gispert, A.; Moreno-Amich, R. Life history patterns of 25 species from European freshwater fish communities. Environ. Biol. Fishes 2002, 65, 387–400. [Google Scholar] [CrossRef]
- Jin, Y.-T.; Li, J.-Q.; Liu, N.-F. Elevation-related variation in life history traits among Phrynocephalus lineages on the Tibetan Plateau: Do they follow typical squamate ecogeographic patterns? J. Zool. 2013, 290, 293–301. [Google Scholar] [CrossRef]
- Zeng, Z.-G.; Zhao, J.-M.; Sun, B.-J. Life history variation among geographically close populations of the toad-headed lizard (Phrynocephalus przewalskii): Exploring environmental and physiological associations. Acta Oecol. 2013, 51, 28–33. [Google Scholar] [CrossRef]
- Jaffe, A.L.; Campbell-Staton, S.C.; Losos, J.B. Geographical variation in morphology and its environmental correlates in a widespread North American lizard, Anolis carolinensis (Squamata: Dactyloidae). Biol. J. Linn. Soc. 2016, 117, 760–774. [Google Scholar] [CrossRef]
- Michaud, E.J.; Echternacht, A.C. Geographic variation in the life history of the lizard Anolis carolinensis and support for the pelvic constraint model. J. Herpetol. 1995, 29, 86–97. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Ping, J.; Hao, S.-L.; Zhou, H.-B. Temporal and spatial variation in life history traits of the Japanese gecko, Gekko japonicus. Herpetol. J. 2016, 26, 305–311. [Google Scholar]
- Díaz, J.A.; Iraeta, P.; Verdú-Ricoy, J.; Siliceo, I.; Salvador, A. Intraspecific variation of reproductive traits in a Mediterranean lizard: Clutch, population, and lineage effects. Evol. Biol. 2012, 39, 106–115. [Google Scholar] [CrossRef]
- Iraeta, P.; Salvador, A.; Díaz, J.A. Life-history traits of two Mediterranean lizard populations: A possible example of countergradient covariation. Oecologia 2013, 172, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, P.; Su, S.; Luo, L.-G.; Zhao, W.-G.; Ji, X. Life-history consequences of local adaptation in lizards: Takydromus wolteri (Lacertidae) as a model organism. Biol. J. Linn. Soc. 2019, 127, 88–99. [Google Scholar] [CrossRef]
- Monasterio, C.; Verdú-Ricoy, J.; Salvador, A.; Díaz, J.A. Living at the edge: Lower success of eggs and hatchlings at lower elevation may shape range limits in an alpine lizard. Biol. J. Linn. Soc. 2016, 118, 829–841. [Google Scholar] [CrossRef]
- Ortega, J.; López, P.; Martín, J. Altitudinally divergent adult phenotypes in Iberian wall lizards are not driven by egg differences or hatchling growth rates. Oecologia 2015, 177, 357–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortega, J.; López, P.; Martín, J. Environmental drivers of growth rates in Guadarrama wall lizards: A reciprocal transplant experiment. Biol. J. Linn. Soc. 2017, 122, 340–350. [Google Scholar] [CrossRef]
- Roitberg, E.S.; Eplanova, G.V.; Kotenko, T.I.; Amat, F.; Carretero, M.A.; Kuranova, V.N.; Bulakhova, N.A.; Zinenko, O.I.; Yakovlev, V.A. Geographic variation of life-history traits in the sand lizard, Lacerta agilis: Testing Darwin’s fecundity-advantage hypothesis. J. Evol. Biol. 2015, 28, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Roitberg, E.S.; Kuranova, V.N.; Bulakhova, N.A.; Orlova, V.F.; Eplanova, G.V.; Zinenko, O.I.; Shamgunova, R.R.; Hofmann, S.; Yakovlev, V.A. Variation of reproductive traits and female body size in the most widely-ranging terrestrial reptile: Testing the effects of reproductive mode, lineage, and climate. Evol. Biol. 2013, 40, 420–438. [Google Scholar] [CrossRef] [PubMed]
- Adolph, S.C.; Porter, W.P. Temperature, activity, and lizard life histories. Am. Nat. 1993, 142, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, R.E. Intraspecific variation in demography and life history of the lizard, Sceloporus jarrovi, along an altitudinal gradient in southeastern Arizona. Ecology 1979, 60, 901–909. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Ramíez-Bautista, A. Reproductive cycles and reproductive strategies among populations of the Rose-bellied Lizard Sceloporus variabilis (Squamata: Phrynosomatidae) from central Mexico. Ecol. Evol. 2016, 6, 1753–1768. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Ramírez-Bautista, A.; Stephenson, B.P.; Luja, V.H.; Hernández-Salinas, U. Variation in female reproduction between populations of the arboreal lizard Urosaurus bicarinatus (Squamata: Phrynosomatidae) from two different environments in Mexico. Salamandra 2017, 53, 359–367. [Google Scholar]
- Du, W.-G.; Warner, D.A.; Langkilde, T.; Robbins, T.R.; Shine, R. The roles of pre- and post-hatching growth rates in generating a latitudinal cline of body size in the eastern fence lizard (Sceloporus undulatus). Biol. J. Linn. Soc. 2012, 106, 202–209. [Google Scholar] [CrossRef]
- Dunham, A.E. Demographic and life-history variation among populations of the iguanid lizard Urosaurus ornatus: Implications for the study of life-history phenomena in lizards. Herpetologica 1982, 38, 208–221. [Google Scholar]
- Ramírez-Bautista, A.; Jiménez-Cruz, E.; Marshall, J.C. Comparative life history for populations of the Sceloporus grammicus (Squamata: Phrynosomatidae). West. N. Am. Nat. 2004, 64, 175–183. [Google Scholar]
- Ramírez-Bautista, A.; Lozano, A.; Herńandez-Salinas, U.; Cruz-Elizalde, R. Female reproductive characteristics among populations of the oviparous lizard Sceloporus aeneus (Squamata: Phrynosomatidae) from Central Mexico. Herpetologica 2016, 72, 196–201. [Google Scholar] [CrossRef]
- Ramírez-Bautista, A.; Stephenson, B.P.; Muñoz, C.S.; Cruz-Elizalde, R.; Herńandez-Salinas, U. Reproduction and sexual dimorphism in two populations of the polymorphic spiny lizard Sceloporus minor from Hidalgo, México. Acta Zool. 2014, 95, 397–408. [Google Scholar] [CrossRef]
- Garda, A.A.; Tavares-Bastos, L.; Costa, G.C.; França, F.G.R.; Giugliano, L.G.; Leite, G.S.; Mesquita, D.O.; Nogueira, C.; Vasconcellos, M.M.; Vieira, G.H.C.; et al. Reproduction, body size, and diet of Polychrus acutirostris (Squamata, Polychrotidae) in two contrasting environments in Brazil. J. Herpetol. 2012, 46, 2–8. [Google Scholar] [CrossRef]
- Forsman, A.; Shine, R. Parallel geographic variation in body shape and reproductive life history within the Australian scincid lizard Lampropholis delicata. Funct. Ecol. 1995, 9, 818–828. [Google Scholar] [CrossRef]
- Hasegawa, M. Inter-population radiation in life history of the lizard Eumeces okadae in the Ize islands, Japan. Copeia 1994, 1994, 732–747. [Google Scholar] [CrossRef]
- Lu, H.-L.; Lin, Z.-H.; Li, H.; Ji, X. Geographic variation in hatchling size in an oviparous skink: Effects of maternal investment and incubation thermal environment. Biol. J. Linn. Soc. 2014, 113, 283–296. [Google Scholar] [CrossRef]
- Wapstra, E.; Swain, R.; O’Reilly, J.M. Geographic variation in age and size at maturity in a small Australian viviparous skink. Copeia 2001, 2001, 646–655. [Google Scholar] [CrossRef]
- Rojas-González, R.I.; Zúñiga-Vega, J.J.; Lemos-Espinal, J.A. Reproductive variation of the lizard Xenosaurus platyceps: Comparing two populations of contrasting environments. J. Herpetol. 2008, 42, 332–336. [Google Scholar] [CrossRef]
- Ji, X.; Braña, F. Among clutch variation in reproductive output and egg size in the wall lizard (Podarcis muralis) from a lowland population of northern Spain. J. Herpetol. 2000, 34, 54–60. [Google Scholar] [CrossRef]
- Luo, L.-G.; Ding, G.-H.; Ji, X. Income breeding and temperature-induced plasticity in reproductive traits in lizards. J. Exp. Biol. 2010, 213, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, Y.; Ji, X. Clutch frequency affects the offspring size-number trade-off in lizards. PLoS ONE 2011, 6, e16585. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, K.; Su, S.; Lin, L.-H.; Xia, Y.; Ji, X. Age-related reproduction of female Mongolian racerunners (Eremias argus; Lacertidae): Evidence of reproductive senescence. J. Exp. Zool. A 2019, 331, 290–298. [Google Scholar]
- Daudin, F.M. Histoire Naturelle, Générale et Particulière des Reptiles, Ouvrage Faisant Suite, a l’Histoiure Naturelle, Générale et Particulière Composée par Leclerc de Buffon, et Redigée par C. S. Sonnini; F. Dufart: Paris, France, 1802; Volume 13. [Google Scholar]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 1 August 2023).
- Arnold, E.N. Interrelationships and evolution of the east Asian grass lizards, Takydromus (Squamata: Lacertidae). Zool. J. Linn. Soc. 1997, 119, 267–296. [Google Scholar] [CrossRef]
- Grismer, L.L.; Quah, E.S.H. An updated and annotated checklist of the lizards of Peninsular Malaysia, Singapore, and their adjacent archipelagos. Zootaxa 2019, 4545, 230–248. [Google Scholar] [CrossRef]
- Lin, S.-M.; Chen, C.A.; Lue, K.-Y. Molecular phylogeny and biogeography of the grass lizards genus Takydromus (Reptilia: Lacertidae) of East Asia. Mol. Phylogenet. Evol. 2002, 22, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Crespi, B.; Semeniuk, C. Parent-offspring conflict in the evolution of vertebrate reproductive mode. Am. Nat. 2004, 163, 635–653. [Google Scholar] [CrossRef]
- Smith, C.C.; Fretwell, S.D. The optimal balance between size and number of offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Congdon, J.D.; Dunham, A.E.; Tinkle, D.W. Biology of Reptilia; Academic Press: New York, NY, USA, 1982; Volume 13, pp. 233–271. [Google Scholar]
- Lucas, A. Bioenergetics of Aquatic Animals; Taylor and Francis Ltd.: London, UK, 1996. [Google Scholar]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Cornell University Press: New York, NY, USA, 2002. [Google Scholar]
- Sibly, R.M.; Grimm, V.; Martin, B.T.; Johnston, A.S.A.; Kułakowska, K.; Topping, C.J.; Calow, P.; Nabe-Nielsen, J.; Thorbek, P.; DeAngelis, D.L. Representing the acquisition and use of energy by individuals in agent-based models of animal populations. Methods Ecol. Evol. 2013, 4, 151–161. [Google Scholar] [CrossRef]
- Madsen, T.; Shine, R. The adjustment of reproductive threshold to prey abundance in a capital breeder. J. Anim. Ecol. 1999, 68, 571–580. [Google Scholar] [CrossRef]
- Shine, R. Life-history evolution in reptiles. Annu. Rev. Ecol. Syst. 2005, 36, 23–46. [Google Scholar] [CrossRef]
- Shine, R. Relative clutch mass and body shape in lizards and snakes: Is reproductive investment constrained or optimized? Evolution 1992, 46, 828–833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).