Xylanase Supplement Enhances the Growth Performance of Broiler by Modulating Serum Metabolism, Intestinal Health, Short-Chain Fatty Acid Composition, and Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Design and Sample Collection
2.3. Sample Collection and Storage
2.4. Intestinal Index Determinations
2.5. Morphology Detection
2.6. Gene Expression Analysis
2.7. Short-Chain Fatty Acids (SCFAs)
2.8. Gut Microbiota Analysis
2.9. Metabolite Extraction and Analysis
2.10. Statistical Analysis
3. Results
3.1. Broiler Performance
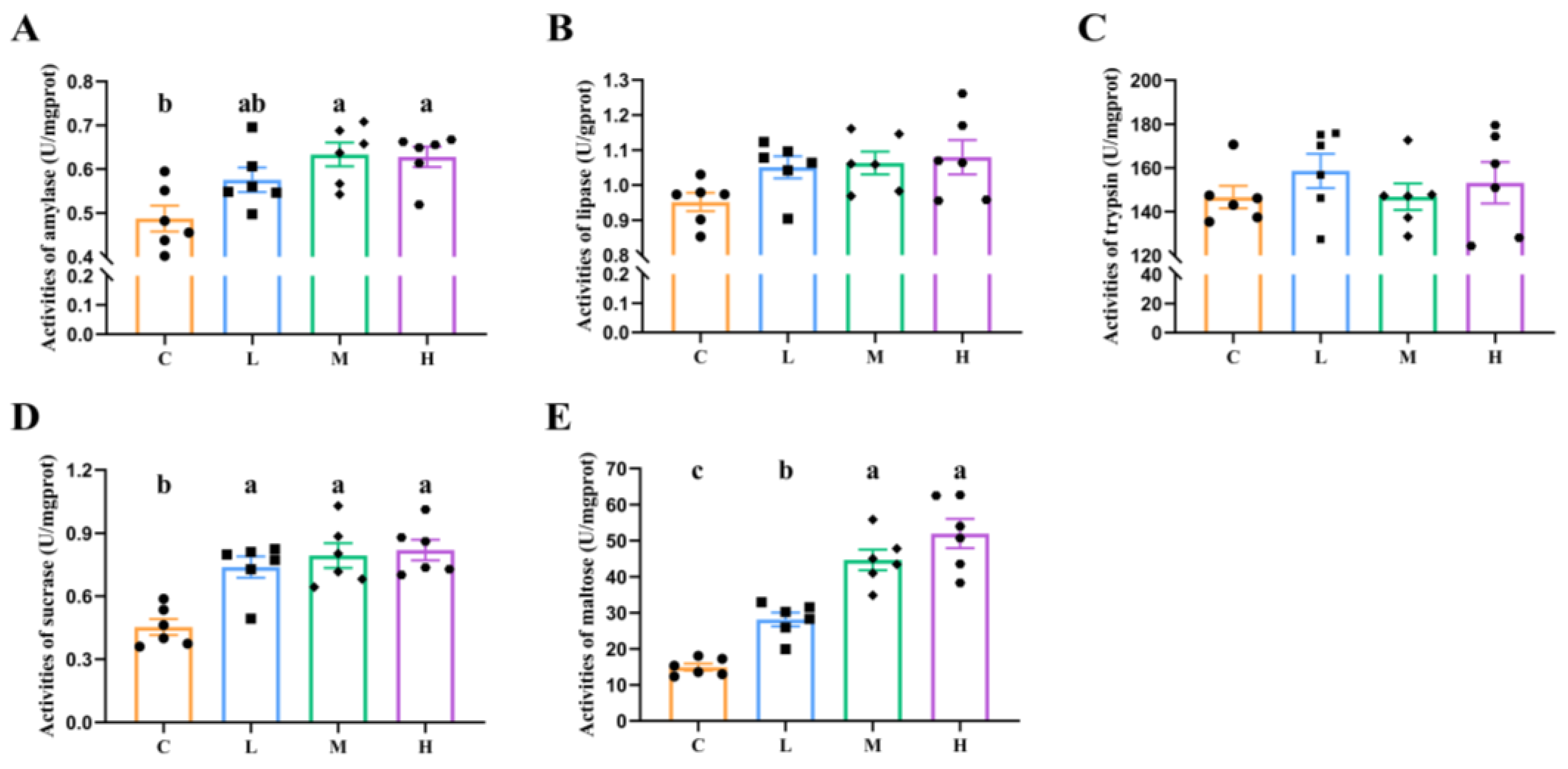
3.2. Major Digestive Enzyme Activities
3.3. Intestinal Permeability
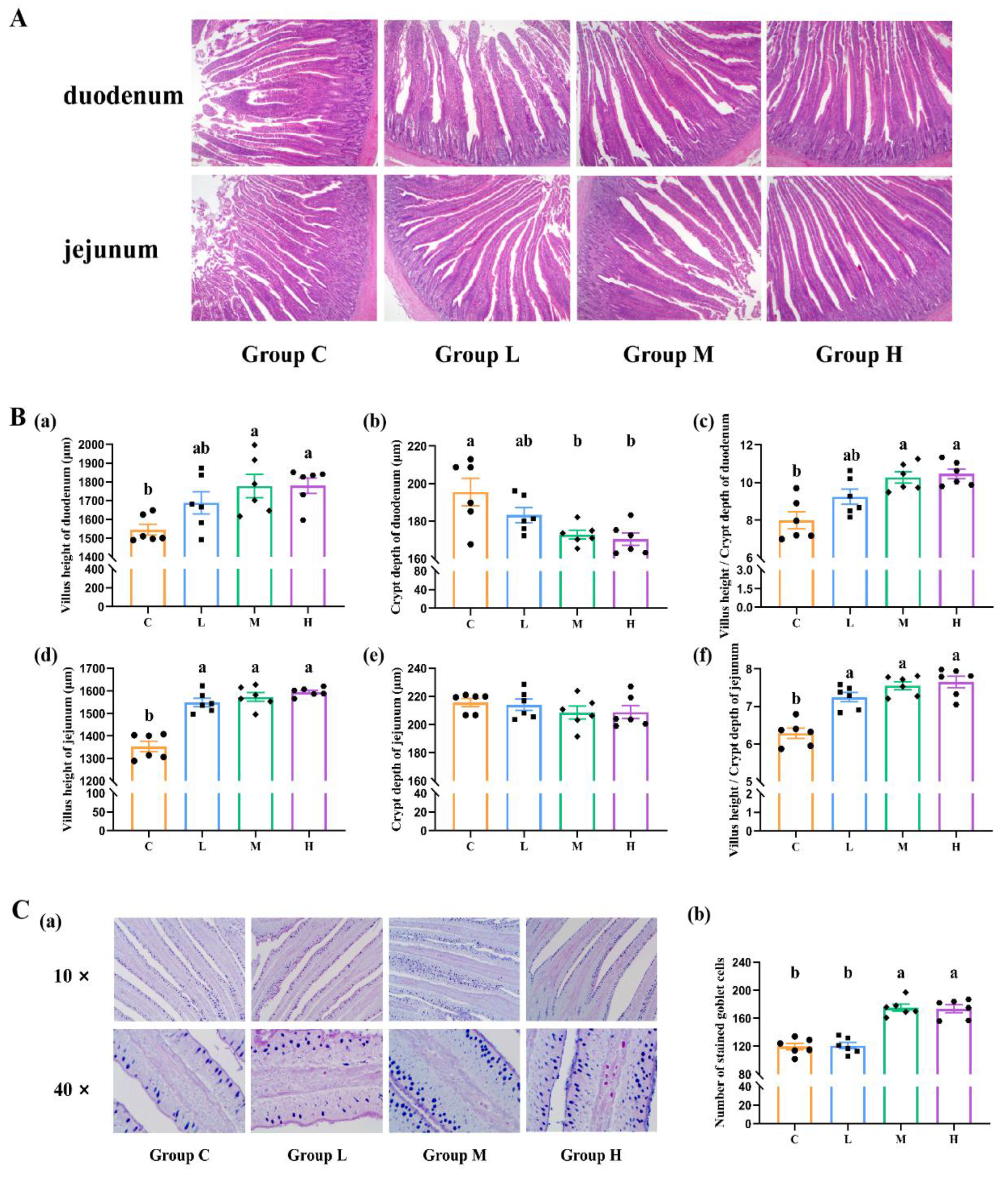
3.4. Intestinal Histomorphology
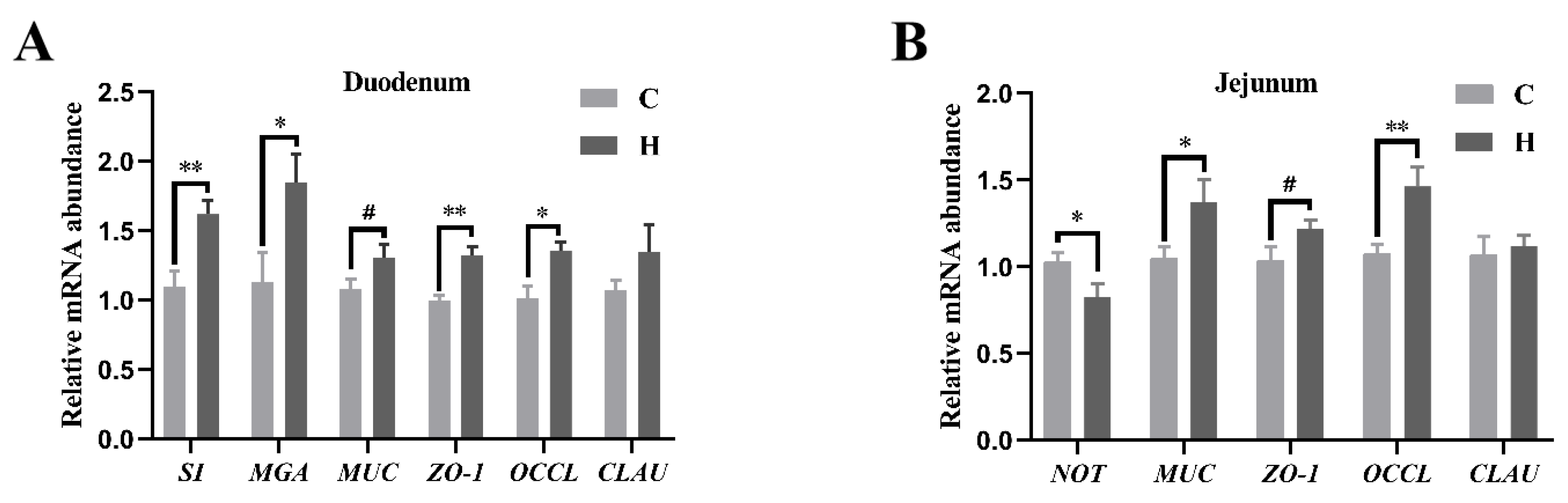
3.5. Intestinal Abundance of Relative mRNA
3.6. Short-Chain Fatty Acid
3.7. Cecal Microbiota Analysis
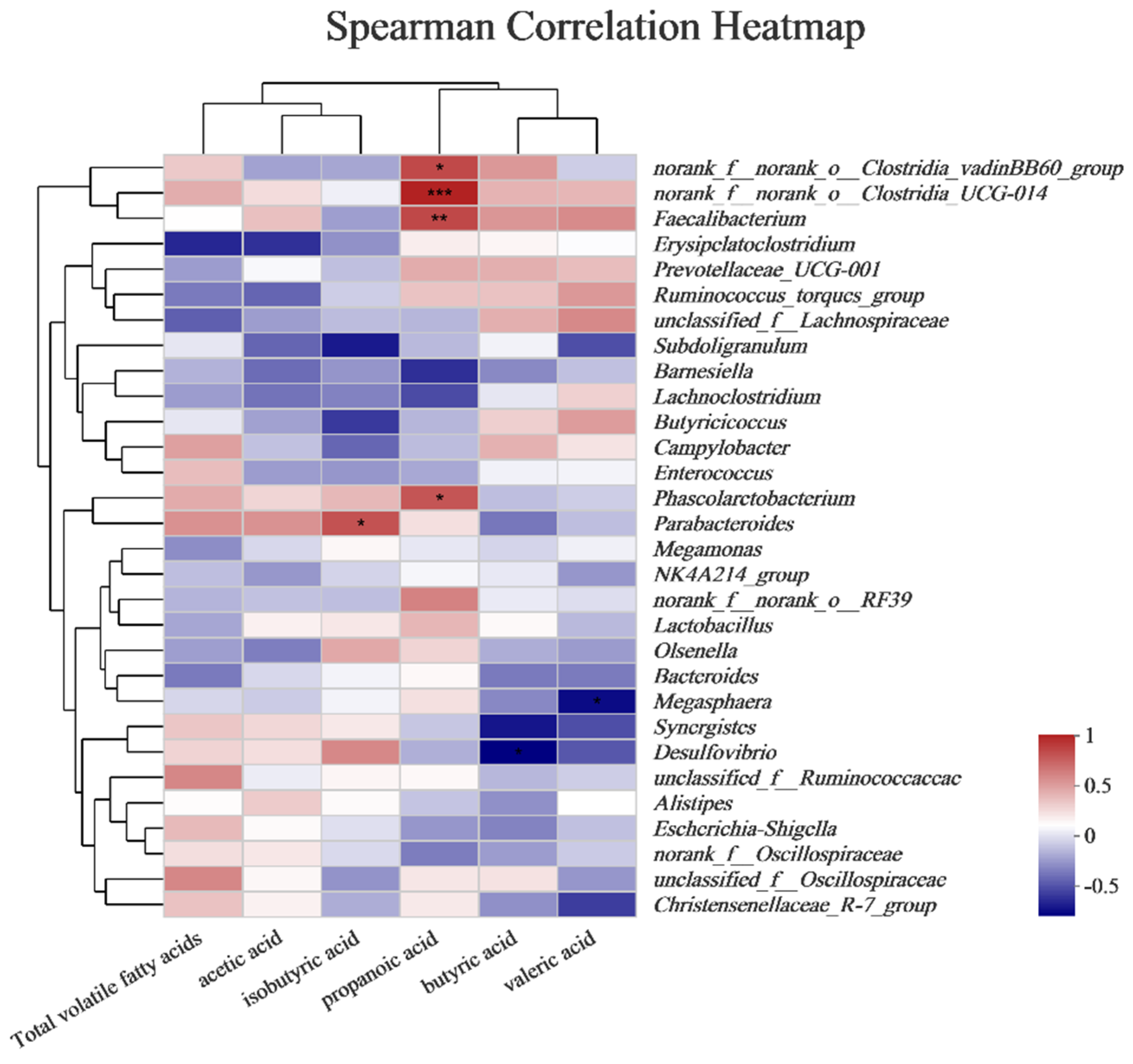
3.8. Correlation Analysis
3.9. Analysis of Metabolic and Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Zheng, M.; Zhao, G.; Wang, J.; Liu, J.; Wang, S.; Feng, F.; Liu, D.; Zhu, D.; Li, Q.; et al. Identification of QTL regions and candidate genes for growth and feed efficiency in broilers. Genet. Sel. Evol. 2021, 53, 13. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Adjemian, M.K. The 2019 government shutdown increased uncertainty in major agricultural commodity markets. Food Policy 2021, 102, 102064. [Google Scholar] [CrossRef]
- Goswami, A.; Adjemian, A.K.; Karali, B. The impact of futures contract storage rate policy on convergence expectations in domestic commodity markets. Food Policy 2022, 111, 102301. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, L.; Khan, A.; Zhao, R.; Wei, S.; Jing, X. Fermented wheat bran by xylanase-producing Bacillus cereus boosts the intestinal microflora of broiler chickens. Poult. Sci. 2020, 99, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Moss, A.F.; Morgan, N.K.; Gharib-Naseri, K.; Ader, P.; Choct, M. Non-starch polysaccharide-degrading enzymes may improve performance when included in wheat- but not maize-based diets fed to broiler chickens under subclinical necrotic enteritis challenge. Anim. Nutr. 2022, 10, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Guan, D.Y.; Fan, B.; Sun, Y.F.; Wang, F.Z. Untargeted mass spectrometry-based metabolomics approach unveils molecular changes in heat-damaged and normal soybean. LWT 2022, 171, 114136. [Google Scholar] [CrossRef]
- Cardoso, V.; Fernandes, E.A.; Santos, H.M.M.; Maçãs, B.; Lordelo, M.M.; Telo da Gama, L.; Ferreira, L.M.A.; Fontes, C.M.G.A.; Ribeiro, T. Variation in levels of non-starch polysaccharides and endogenous endo-1,4-β-xylanases affects the nutritive value of wheat for poultry. Br. Poult. Sci. 2018, 59, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Marcotuli, I.; Colasuonno, P.; Hsieh, Y.S.Y.; Fincher, G.B.; Gadaleta, A. Non-Starch Polysaccharides in Durum Wheat: A Review. Int. J. Mol. Sci. 2020, 21, 2933. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wang, X.; Wang, Y.; Lv, T.; Zhao, H.; Wang, Y.; Zhu, S.; Qiu, H.; Zeng, J.; Dai, Q.; et al. Effects of Dietary Bacillus and Non-starch Polysaccharase on the Intestinal Microbiota and the Associated Changes on the Growth Performance, Intestinal Morphology, and Serum Antioxidant Profiles in Ducks. Front. Microbiol. 2021, 12, 786121. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R.; Apajalahti, J.H. The role of feed enzymes in maintaining poultry intestinal health. J. Sci. Food Agric. 2022, 102, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, T.J.; Caviedes-Vidal, E.; Karasov, W.H. The integration of digestion and osmoregulation in the avian gut. Biol. Rev. Camb. Philos. Soc. 2009, 84, 533–565. [Google Scholar] [CrossRef] [PubMed]
- Mappley, L.J.; La Ragione, R.M.; Woodward, M.J. Brachyspira and its role in avian intestinal spirochaetosis. Vet. Microbiol. 2014, 168, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2019, 97, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, C.; Li, Y.; Pan, K.; Ouyang, K.; Song, X.; Xiong, X.; Qu, M.; Zhao, X. Characteristics of recombinant xylanase from camel rumen metagenome and its effects on wheat bran hydrolysis. Int. J. Biol. Macromol. 2022, 220, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Hu, L.L.; Hou, Y.Q.; Zhang, J.; Ding, B.Y. The effects of enzyme supplementation on performance and digestive parameters of broilers fed corn-soybean diets. Poult. Sci. 2014, 93, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D.; Khattak, F.; Hastie, P.; Bedford, M.R.; Olukosi, O.A. Xylanase and xylo- oligosaccharide prebiotic improve the growth performance and concentration of potentially prebiotic oligosaccharides in the ileum of broiler chickens. Br. Poult. Sci. 2020, 61, 70–78. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Wu, S.B.; Bedford, M.R.; Nguyen, X.H.; Morgan, N.K. Dietary soluble non-starch polysaccharide level and xylanase influence the gastrointestinal environment and nutrient utilisation in laying hens. Br. Poult. Sci. 2022, 63, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Moita, V.H.C.; Duarte, M.E.; Kim, S.W. Functional roles of xylanase enhancing intestinal health and growth performance of nursery pigs by reducing the digesta viscosity and modulating the mucosa-associated microbiota in the jejunum. J. Anim. Sci. 2022, 100, skac116. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Qiu, K.; Chang, X.Y.; Zhang, H.J.; Wang, J.; Qi, G.H.; Sun, T.H.; Su, Y.B.; Wu, S.G. Enhancing egg production and quality by the supplementation of probiotic strains (Clostridium and Brevibacillus) via improved amino acid digestibility, intestinal health, immune response, and antioxidant activity. Front. Microbiol. 2022, 13, 987241. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Kim, I.H. Effects of dietary xylanase supplementation on performance and functional digestive parameters in broilers fed wheat-based diets. Poult. Sci. 2017, 96, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, X.; Yin, D.; Wang, M.; Yin, X.; Lei, Z.; Guo, Y. Effect of different amylases on the utilization of cornstarch in broiler chickens. Poult. Sci. 2017, 96, 1139–1148. [Google Scholar] [CrossRef]
- Ornelas, A.; Dowdell, A.S.; Lee, J.S.; Colgan, S.P. Microbial Metabolite Regulation of Epithelial Cell-Cell Interactions and Barrier Function. Cells 2022, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 19–330. [Google Scholar] [CrossRef] [PubMed]
- Lilburn, M.S.; Loeffler, S. Early intestinal growth and development in poultry. Poult. Sci. 2015, 94, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Ma, J.; Piao, X. Changes in Growth Performance and Ileal Microbiota Composition by Xylanase Supplementation in Broilers Fed Wheat-Based Diets. Front. Microbiol. 2021, 12, 706396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Wang, J.; Zhang, H.J.; Wu, S.G.; Hui, Q.R.; Yang, C.B.; Fang, R.J.; Qi, G.H. Intestinal Morphologic and Microbiota Responses to Dietary Bacillus spp. in a Broiler Chicken Model. Front. Physiol. 2019, 9, 1968. [Google Scholar] [CrossRef]
- Cui, Y.; Han, C.; Li, S.; Geng, Y.; Wei, Y.; Shi, W.; Ba, Y. High-throughput sequencing-based analysis of the intestinal microbiota of broiler chickens fed with compound small peptides of Chinese medicine. Poult. Sci. 2021, 100, 100897. [Google Scholar] [CrossRef] [PubMed]
- Orellana, L.H.; Francis, T.B.; Ferraro, M.; Hehemann, J.H.; Fuchs, B.M.; Amann, R.I. Verrucomicrobiota are specialist consumers of sulfated methyl pentoses during diatom blooms. ISME J. 2022, 16, 630–641. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, J.; Jiang, S.; Jiao, N.; Huang, L.; Yuan, X.; Guan, Q.; Li, Y.; Yang, W. Effects of Dietary Isoleucine Supplementation on the Production Performance, Health Status and Cecal Microbiota of Arbor Acre Broiler Chickens. Microorganisms 2023, 11, 236. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, Y.; Zheng, L.; Rong, N.; Yang, Y.; Gong, P.; Yang, Y.; Siwu, X.; Zhang, C.; Zhu, L.; et al. Bifidobacterium and Lactobacillus improve inflammatory bowel disease in zebrafish of different ages by regulating the intestinal mucosal barrier and microbiota. Life Sci. 2023, 324, 121699. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, H.; Chen, Y.; Guo, Y.; Yuan, J. Debranching enzymes decomposed corn arabinoxylan into xylooligosaccharides and achieved prebiotic regulation of gut microbiota in broiler chickens. J. Anim. Sci. Biotechnol. 2023, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, K.W.; Bedford, M.R.; Kerr, B.J.; Dozier, W.A. Effects of age and supplemental xylanase in corn- and wheat-based diets on cecal volatile fatty acid concentrations of broilers. Poult. Sci. 2019, 98, 4787–4800. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.K.; Wallace, A.; Bedford, M.R.; González-Ortiz, G. Impact of fermentable fiber, xylo-oligosaccharides and xylanase on laying hen productive performance and nutrient utilization. Poult. Sci. 2022, 101, 102210. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Mishra, B.; Bedford, M.R.; Jha, R. Effects of supplemental xylanase and xylooligosaccharides on production performance and gut health variables of broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Isayama, K.; Rini, D.M.; Yamamoto, Y.; Suzuki, T. Propionate regulates tight junction barrier by increasing endothelial-cell selective adhesion molecule in human intestinal Caco-2 cells. Exp. Cell Res. 2023, 425, 113528. [Google Scholar] [CrossRef] [PubMed]
- Dale, T.; Hannay, I.; Bedford, M.R.; Tucker, G.A.; Brameld, J.M.; Parr, T. The effects of exogenous xylanase supplementation on the in vivo generation of xylooligosaccharides and monosaccharides in broilers fed a wheat-based diet. Br. Poult. Sci. 2020, 61, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Liu, C.; Sun, L.; Wang, T.; Dai, H.; Wang, K.; Bao, L.; Li, H.; Wang, W.; Liu, S.J.; et al. Gut Parabacteroides merdae protects against cardiovascular damage by enhancing branched-chain amino acid catabolism. Nat. Metab. 2022, 4, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bao, X.; Liu, S.; Ye, K.; Xiang, S.; Yu, L.; Xu, Q.; Zhang, Y.; Wang, X.; Zhu, X.; et al. Gut microbiota affect the formation of calcium oxalate renal calculi caused by high daily tea consumption. Appl. Microbiol. Biotechnol. 2021, 105, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Ammar, E.M.; Philippidis, G.P. Fermentative production of propionic acid: Prospects and limitations of microorganisms and substrates. Appl. Microbiol. Biotechnol. 2021, 105, 6199–6213. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Goodarzi, P.; Shili, C.N.; Sutton, J.; Wileman, C.M.; Kim, D.M.; Lin, D.; Pezeshki, A. A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile. Int. J. Mol. Sci. 2022, 23, 3300. [Google Scholar] [CrossRef] [PubMed]
- Diniz, A.L.; da Silva, D.I.R.; Lembke, C.G.; Costa, M.D.L.; Ten-Caten, F.; Li, F.; Vilela, R.D.; Menossi, M.; Ware, D.; Endres, L.; et al. Amino Acid and Carbohydrate Metabolism Are Coordinated to Maintain Energetic Balance during Drought in Sugarcane. Int. J. Mol. Sci. 2020, 21, 9124. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Furukawa, K.; Bailey, C.A.; Wu, G. Oxidation of amino acids, glucose, and fatty acids as metabolic fuels in enterocytes of post-hatching developing chickens. J. Anim. Sci. 2022, 100, skac053. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Fujii, J. Application of Glutathione as Anti-Oxidative and Anti-Aging Drugs. Curr. Drug Metab. 2015, 16, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; De Carolis, C.; Perricone, R. Glutathione: A key player in autoimmunity. Autoimmun. Rev. 2009, 8, 697–701. [Google Scholar] [CrossRef]




| Items | 1 to 21 Days | 22 to 56 Days |
|---|---|---|
| Ingredients (%) | ||
| Wheat | 40.00 | 40.00 |
| Corn | 23.55 | 32.80 |
| Soybean Meal (CP44%) | 24.50 | 14.20 |
| CGM (CP60%) | 5.00 | 7.00 |
| Soybean Oil | 2.55 | 2.20 |
| NaCl | 0.30 | 0.30 |
| CaHPO4 | 0.88 | 0.71 |
| Limestone | 1.74 | 1.50 |
| Zeolite | 0.48 | 0.29 |
| Premix 1 | 1.00 | 1.00 |
| Total | 100.0 | 100.0 |
| Nutrient levels 2 (%) | ||
| ME (MJ/kg) | 12.37 | 12.78 |
| CP | 21.00 | 18.52 |
| Ca | 0.90 | 0.76 |
| TP | 0.59 | 0.52 |
| Lys | 1.15 | 0.98 |
| Met | 0.49 | 0.42 |
| Met+Cys | 0.85 | 0.76 |
| Thr | 0.72 | 0.62 |
| Digestible Lys | 1.06 | 0.91 |
| Digestible Met | 0.47 | 0.40 |
| Digestible Met+Cys | 0.79 | 0.70 |
| Digestible Thr | 0.63 | 0.54 |
| Try | 0.22 | 0.18 |
| Digestible Try | 0.20 | 0.16 |
| Genes | Forward | Reverse |
|---|---|---|
| β-actin | 5′-GAGAAATTGTGCGTGACATCA-3′ | 5′-CCTGAACCTCTCATTGCCA-3′ |
| SI | 5′-GTGCTCCATGAGTTTGCACAAG-3′ | 5′-CAGCGGGCATTGGGTAAGTA-3′ |
| MGA | 5′-GGGACCAGATGACAAAATCCAT-3′ | 5′-CAGCGGGCATTGGGTAAGTA-3′ |
| ZO-1 | 5′-CTTCAGGTGTTTCTCTTCCTCCTC-3′ | 5′-CTGTGGTTTCATGGCTGGATC-3′ |
| MUC-2 | 5′-ATTGTGGTAACACCAACATTCATC-3′ | 5′-CTTTATAATGTCAGCACCAACTTCTC-3′ |
| Occludin | 5′-GAGCCCAGACTACCAAAGCAA-3′ | 5′-GCTTGATGTGGAAGAGCTTGTTG-3′ |
| Claudin-1 | 5′-TGGCCACGTCATGGTATGG-3′ | 5′-AACGGGTGTGAAAGGGTCATAG-3′ |
| Items | Treatments 2 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | L | M | H | |||
| Starter (1~21 days) | ||||||
| 1 day BW (g) | 33.10 | 33.40 | 33.28 | 33.38 | 0.052 | 0.135 |
| 21 days BW (g) | 424.8 | 433.0 | 448.2 | 426.8 | 3.455 | 0.078 |
| ADFI (g/d) | 30.98 | 30.98 | 31.59 | 30.68 | 0.153 | 0.233 |
| ADG (g/d) | 18.65 | 19.03 | 19.76 | 18.74 | 0.164 | 0.077 |
| F/G | 1.66 a | 1.63 ab | 1.60 b | 1.64 ab | 0.008 | 0.049 |
| Grower (22~56 days) | ||||||
| 56 days BW (g) | 1788 | 1788 | 1820 | 1802 | 12.38 | 0.818 |
| ADFI (g/d) | 94.36 | 93.19 | 94.02 | 92.97 | 0.746 | 0.911 |
| ADG (g/d) | 38.85 | 38.62 | 39.13 | 39.34 | 0.324 | 0.884 |
| F/G | 2.43 | 2.41 | 2.40 | 2.36 | 0.008 | 0.082 |
| Overall (1~56 days) | ||||||
| ADFI (g/d) | 70.68 | 69.90 | 70.60 | 69.51 | 0.462 | 0.798 |
| ADG (g/d) | 31.34 | 31.34 | 31.91 | 31.58 | 0.221 | 0.818 |
| F/G | 2.26 a | 2.23 ab | 2.21 ab | 2.20 b | 0.008 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, D.; Xu, Y.; Ding, X.; Liang, S.; Xie, L.; Wang, Y.; Zhan, X. Xylanase Supplement Enhances the Growth Performance of Broiler by Modulating Serum Metabolism, Intestinal Health, Short-Chain Fatty Acid Composition, and Microbiota. Animals 2024, 14, 1182. https://doi.org/10.3390/ani14081182
Wang X, Li D, Xu Y, Ding X, Liang S, Xie L, Wang Y, Zhan X. Xylanase Supplement Enhances the Growth Performance of Broiler by Modulating Serum Metabolism, Intestinal Health, Short-Chain Fatty Acid Composition, and Microbiota. Animals. 2024; 14(8):1182. https://doi.org/10.3390/ani14081182
Chicago/Turabian StyleWang, Xiaoli, Danlei Li, Yibin Xu, Xiaoqing Ding, Shuang Liang, Lingyu Xie, Yongxia Wang, and Xiuan Zhan. 2024. "Xylanase Supplement Enhances the Growth Performance of Broiler by Modulating Serum Metabolism, Intestinal Health, Short-Chain Fatty Acid Composition, and Microbiota" Animals 14, no. 8: 1182. https://doi.org/10.3390/ani14081182




