Surveillance of Sarcoptic Mange in Iberian Ibexes (Capra pyrenaica) and Domestic Goats (Capra hircus) in Southern Spain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
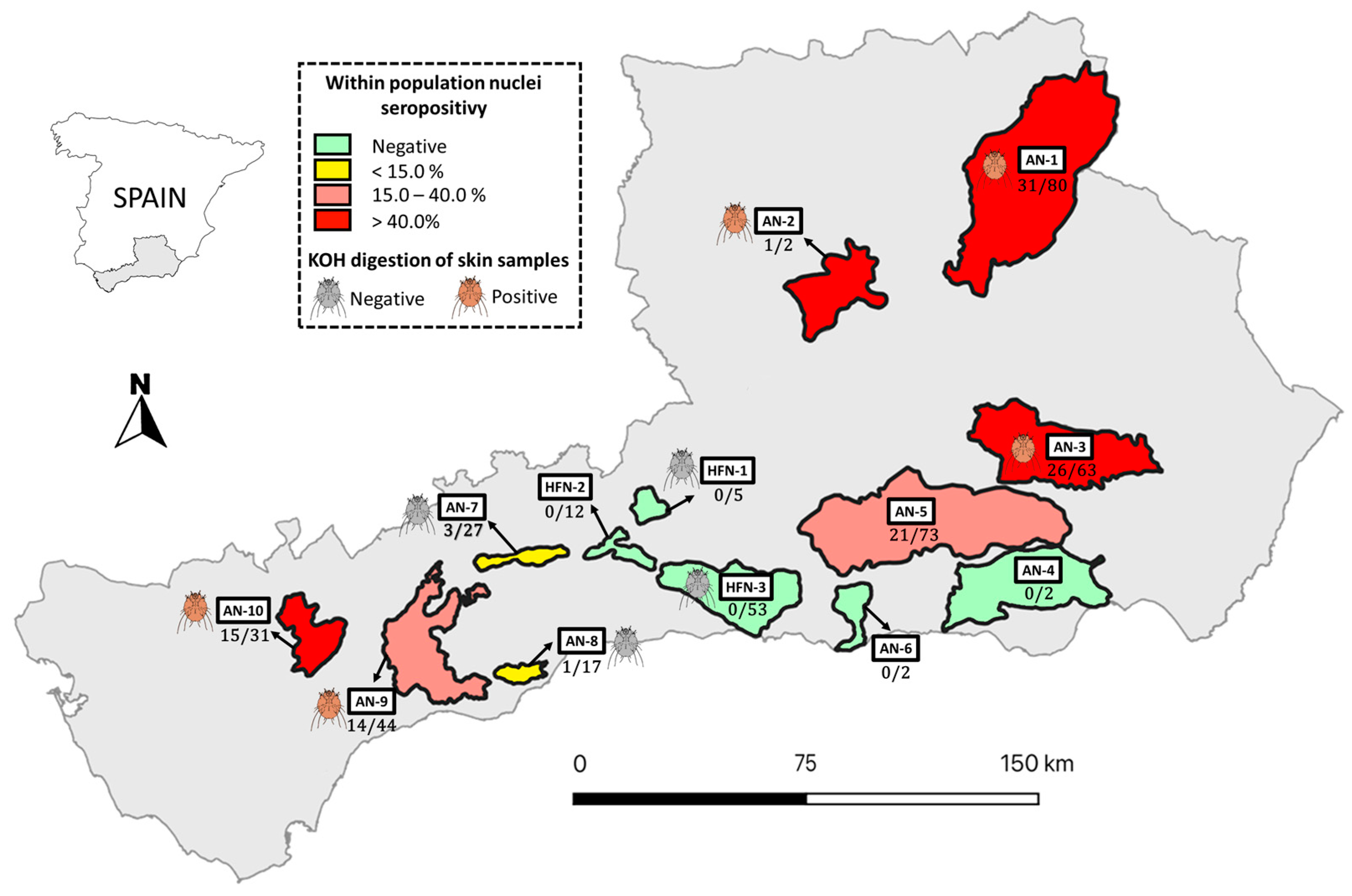
2.1. Study Area and Data Collection
2.2. Serological Analyses
2.3. Potassium Hydroxide (KOH) Digestion Procedure
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colebrook, E.; Wall, R. Ectoparasites of livestock in Europe and the Mediterranean region. Vet. Parasitol. 2004, 120, 251–274. [Google Scholar] [CrossRef] [PubMed]
- Arlian, L.G.; Morgan, M.S. A review of Sarcoptes scabiei: Past, present and future. Parasites Vectors 2017, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Carver, S.; Cross, P.C.; Rossi, L.; Almberg, E.S.; Yabsley, M.J.; Niedringhaus, K.D.; Van Wick, P.; Dominguez-Villegas, E.; Gakuya, F.; et al. Sarcoptic mange: An emerging panzootic in wildlife. Transbound. Emerg. Dis. 2022, 69, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Moroni, B.; Angelone, S.; Pérez, J.M.; Molinar Min, A.R.; Pasquetti, M.; Tizzani, P.; López-Olvera, J.R.; Valldeperes, M.; Granados, J.E.; Lavín, S.; et al. Sarcoptic mange in wild ruminants in Spain: Solving the epidemiological enigma using microsatellite markers. Parasit. Vectors 2021, 14, 171. [Google Scholar] [CrossRef]
- Walton, S.F.; Currie, B.J. Problems in diagnosing scabies, a global disease in human and animal populations. Clin. Microbiol. Rev. 2007, 20, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Granados, J.E.; Espinosa, J.; Ráez-Bravo, A.; López-Olvera, J.R.; Rossi, L.; Meneguz, P.G.; Angelone, S.; Fandos, P.; Soriguer, R.C. Biology and management of sarcoptic mange in wild Caprinae populations. Mamm. Rev. 2021, 51, 82–94. [Google Scholar] [CrossRef]
- Rossi, L.; Tizzani, P.; Rambozzi, L.; Moroni, B.; Meneguz, P.G. Sanitary emergencies at the wild/domestic Caprines interface in Europe. Animals 2019, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Turchetto, S.; Obber, F.; Rossi, L.; D’Amelio, S.; Cavallero, S.; Poli, A.; Parisi, F.; Lanfranchi, P.; Ferrari, N.; Dellamaria, D.; et al. Sarcoptic Mange in Wild Caprinae of the Alps: Could Pathology Help in Filling the Gaps in Knowledge? Front. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Cabrera, A. The Subspecies of the Spanish Ibex. Proc. Zool. Soc. Lond. 1911, 81, 963–977. [Google Scholar] [CrossRef]
- Herrero, J.; Acevedo, P.; Arnal, M.C.; Fernández de Luco, D.; Fonseca, C.; García-González, R.; Pérez, J.M.; Sourp, E. Capra pyrenaica. The IUCN Red List of Threatened Species 2021: e.T3798A195855497. 2021. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-1.RLTS.T3798A195855497.en (accessed on 2 April 2024).
- Fandos, P. La cabra montés (Capra pyrenaica) en el Parque Natural de las Sierras de Cazorla, Segura y las Villas; Serie Técnica ICONA: Madrid, Spain, 1991; ISBN 9788485496907. [Google Scholar]
- León-Vizcaíno, L.; Ruíz de Ybáñez, M.R.; Cubero, M.J.; Ortíz, J.M.; Espinosa, J.; Pérez, L.; Simón, M.A.; Alonso, F. Sarcoptic mange in Spanish ibex from Spain. J. Wildl. Dis. 1999, 35, 647–659. [Google Scholar] [CrossRef]
- Granados, J.E.; Ros-Candeira, A.; Pérez-Luque, A.J.; Moreno-Llorca, R.; Cano-Manuel, F.J.; Fandos, P.; Soriguer, R.C.; Cerrato, J.E.; Jiménez, J.M.P.; Ramos, B.; et al. Long-Term Monitoring of the Iberian Ibex Population in the Sierra Nevada of the Southeast Iberian Peninsula. Sci. Data 2020, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Muñoz, M.J.; Castillo-Contreras, R.; Pérez, J.M.; Granados, J.E.; Márquez, F.J.; López-Montoya, A.J. Co-infection patterns in the ectoparasitic community affecting the Iberian ibex Capra pyrenaica. Parasit. Vectors 2023, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Valldeperes, M.; Yerro, P.P.; López-Olvera, J.R.; Fandos, P.; Lavín, S.; Escofet, R.C.S.; Mentaberre, G.; León, F.J.C.-M.; Espinosa, J.; Ráez-Bravo, A.; et al. Diseases of Iberian ibex (Capra pyrenaica). Eur. J. Wildl. Res. 2023, 69, 63. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.; Mörner, T.; Samuel, W.M. Sarcoptes scabiei and Sarcoptic Mange. In Parasitic Diseases of Wild Mammals; Iowa State University Press: Ames, IA, USA, 2001; pp. 107–119. [Google Scholar]
- Pence, D.B.; Ueckermann, E. Sarcoptic manage in wildlife. Rev. Sci. Tech. (Int. Off. Epizoot.) 2002, 21, 385–398. [Google Scholar] [CrossRef]
- Pérez, J.M.; Molina, L.; Ureña-Gutiérrez, B.; Espinosa, J.; López-Montoya, A.J.; Boos, M.; Granados, J.E.; Cano-Manuel, F.J.; Azorit, C. Individual stress responses to Sarcoptes scabiei infestation in Iberian ibex, Capra pyrenaica. Gen. Comp. Endocrinol. 2019, 281, 1–6. [Google Scholar] [CrossRef] [PubMed]
- CSMAEA. Consejería de Sostenibilidad, Medio Ambiente y Economía Azul. Junta de Andalucía. Available online: https://www.juntadeandalucia.es/medioambiente/portal/documents/20151/1487460/pacam_web_2013.pdf/131a3a48-0885-b3a2-ff5e-a58d0a1d2c19 (accessed on 2 February 2024).
- Pérez, J.M.; Granados, J.E.; Sarasa, M.; Serrano, E. Usefulness of estimated surface area of damaged skin as a proxy of mite load in the monitoring of sarcoptic mange in free-ranging populations of Iberian wild goat, Capra pyrenaica. Vet. Parasitol. 2011, 176, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ráez-Bravo, A.; Granados, J.E.; Cerón, J.J.; Cano-Manuel, F.J.; Fandos, P.; Pérez, J.M.; Espinosa, J.; Soriguer, R.C.; López-Olvera, J.R. Acute phase proteins increase with sarcoptic mange status and severity in Iberian ibex (Capra pyrenaica, Schinz 1838). Parasitol. Res. 2015, 114, 4005–4010. [Google Scholar] [CrossRef]
- MAPA. Ministerio de Agricultura Pesca y Alimentación. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/resultados_definitivos_nov17_ovino-caprino_webmapama_tcm30-450864.pdf (accessed on 6 March 2024).
- Rambozzi, L.; Menzano, A.; Lavin, S.; Rossi, L. Biotin-avidin amplified ELISA for detection of antibodies to Sarcoptes scabiei in chamois (Rupicapra spp.). Vet. Res. 2004, 35, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ráez-Bravo, A.; Granados, J.E.; Serrano, E.; Dellamaria, D.; Casais, R.; Rossi, L.; Puigdemont, A.; Cano-Manuel, F.J.; Fandos, P.; Pérez, J.M.; et al. Evaluation of three enzyme-linked immunosorbent assays for sarcoptic mange diagnosis and assessment in the Iberian ibex, Capra pyrenaica. Parasit. Vectors 2016, 9, 558. [Google Scholar] [CrossRef]
- Wall, R.; Shearer, D. Veterinary Entomology: Arthropod Ectoparasites of Veterinary Importance; Springer Science & Business Media: Dordrecht, The Netherland, 1997; ISBN 9780412615108. [Google Scholar]
- Thrusfield, M.; Christley, R. Veterinary Epidemiology; Wiley: Hoboken, NJ, USA, 2018; ISBN 9781118280287. [Google Scholar]
- Arenas, A.J.; Gómez, F.; Salas, R.; Carrasco, P.; Borge, C.; Maldonado, A.; O’Brien, D.J.; Martínez Moreno, F.J. An evaluation of the application of infrared thermal imaging to the tele-diagnosis of sarcoptic mange in the Spanish ibex (Capra pyrenaica). Vet. Parasitol. 2002, 109, 111–117. [Google Scholar] [CrossRef]
- Alasaad, S.; Permunian, R.; Gakuya, F.; Mutinda, M.; Soriguer, R.C.; Rossi, L. Sarcoptic-mange detector dogs used to identify infected animals during outbreaks in wildlife. BMC Vet. Res. 2012, 8, 110. [Google Scholar] [CrossRef]
- Angelone-Alasaad, S.; Molinar Min, A.; Pasquetti, M.; Alagaili, A.N.; D’Amelio, S.; Berrilli, F.; Obanda, V.; Gebely, M.A.; Soriguer, R.C.; Rossi, L. Universal conventional and real-time PCR diagnosis tools for Sarcoptes scabiei. Parasit. Vectors 2015, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Rampton, M.; Walton, S.F.; Holt, D.C.; Pasay, C.; Kelly, A.; Currie, B.J.; McCarthy, J.S.; Mounsey, K.E. Antibody responses to Sarcoptes scabiei apolipoprotein in a porcine model: Relevance to immunodiagnosis of recent infection. PLoS ONE 2013, 8, e65354. [Google Scholar] [CrossRef] [PubMed]
- Valldeperes, M.; Granados, J.E.; Pérez, J.M.; Castro, I.; Ráez-Bravo, A.; Fandos, P.; López-Olvera, J.R.; Serrano, E.; Mentaberre, G. How sensitive and specific is the visual diagnosis of sarcoptic mange in free-ranging Iberian ibexes? Parasit. Vectors 2019, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Sarasa, M.; Rambozzi, L.; Rossi, L.; Meneguz, P.G.; Serrano, E.; Granados, J.-E.; González, F.J.; Fandos, P.; Soriguer, R.C.; Gonzalez, G.; et al. Sarcoptes scabiei: Specific immune response to sarcoptic mange in the Iberian ibex Capra pyrenaica depends on previous exposure and sex. Exp. Parasitol. 2010, 124, 265–271. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, H.M.J.F.; Rambags, P.G.M.; Elbers, A.R.W.; van Maanen, C.; Hunneman, W.A. Validation of ELISAs for the detection of antibodies to Sarcoptes scabiei in pig. Vet. Parasitol. 2000, 89, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.; Zakrisson, G.; Thebo, P. Klinisk bild och antikroppssvar vid experimentell infektion av Sarcoptes scabiei var, vulpes hos rödräv (Vulpes vulpes). Acta Vet. Scand. 1995, 36, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Tarigan, S. Antibody response in naïve and sensitised goats infested by Sarcoptes Scabiei. J. Ilmu Ternak Dan Vet. 2014, 9, 258–265. [Google Scholar]
- Casais, R.; Dalton, K.P.; Millán, J.; Balseiro, A.; Oleaga, Á.; Solano, P.; Goyache, F.; Prieto, J.M.; Parra, F. Primary and secondary experimental infestation of rabbits (Oryctolagus cuniculus) with Sarcoptes scabiei from a wild rabbit: Factors determining resistance to reinfestation. Vet. Parasitol. 2014, 203, 173–183. [Google Scholar] [CrossRef]
- Castro, I.; de la Fuente, A.; Fandos, P.; Cano-Manuel, F.; Granados, J.-E.; Soriguer, R.C.; Alasaad, S.; Pérez, J.M. On the population biology of Sarcoptes scabiei infesting Iberian ibex (Capra pyrenaica). Int. J. Acarol. 2016, 42, 7–11. [Google Scholar] [CrossRef]
- Niedringhaus, K.D.; Brown, J.D.; Ternent, M.A.; Peltier, S.K.; Van Wick, P.; Yabsley, M. Serology as a tool to investigate sarcoptic mange in American black bears (Ursus americanus). J. Wildl. Dis. 2019, 56, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Valldeperes, M.; Granados, J.E.; Pérez, V.; López-Olvera, J.R.; Ráez-Bravo, A.; Fandos, P.; Pérez, J.M.; Mentaberre, G.; Tampach, S.; Soriguer, R.C.; et al. The local skin cellular immune response determines the clinical outcome of sarcoptic mange in Iberian ibex (Capra pyrenaica). Front. Vet. Sci. 2023, 10, 1183304. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Ruiz-Martínez, I.; Granados, J.E.; Soriguer, R.C.; Fandos, P. The dynamics of sarcoptic mange in the ibex population of Sierra Nevada in Spain—Influence of climatic factors. J.Wild. Res. 1997, 2, 86–89. [Google Scholar]
- Rossi, L.; Fraquelli, C.; Vesco, U.; Permunian, R.; Sommavilla, G.M.; Carmignola, G.; Da Pozzo, R.; Meneguz, P.G. Descriptive epidemiology of a scabies epidemic in chamois in the Dolomite Alps, Italy. Eur. J. Wildl. Res. 2007, 53, 131–141. [Google Scholar] [CrossRef]
- Fernández-Morán, J.; Gómez, S.; Ballesteros, F.; Quirós, P.; Benito, J.; Feliu, C.; Nieto, J. Epizootiology of sarcoptic mange in a population of cantabrian chamois (Rupicapra pyrenaica parava) in Northwestern Spain. Vet. Parasitol. 1997, 73, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Menzano, A.; Rambozzi, L.; Rossi, L. A severe episode of wildlife-derived scabies in domestic goats in Italy. Small Rumin. Res. 2007, 70, 154–158. [Google Scholar] [CrossRef]
- Falconi, C.; Oleaga, Á.; López-Olvera, J.R.; Casais, R.; Prieto, M.; Gortázar, C. Prevalence of antibodies against selected agents shared between Cantabrian chamois (Rupicapra pyrenaica parva) and domestic goats. Eur. J. Wildl. Res. 2010, 56, 319–325. [Google Scholar] [CrossRef]
| Variable | Categories | Iberian Ibexes with Skin Lesions Compatible with Sarcoptic Mange | Clinically Healthy Iberian Ibexes | ||||
|---|---|---|---|---|---|---|---|
| % ELISA Positive | Seropositives/ Overall a | p-Value | % ELISA Positive | Seropositives/ Overall a | p-Value | ||
| Location b | AN HFN | 68.4 0.0 | 104/152 0/5 | 0.004 | 4.2 0.0 | 8/189 0/65 | 0.09 |
| Age | Juvenile Sub-adult Adult | 100.0 67.1 63.9 | 7/7 49/73 46/72 | 0.152 | 10.0 4.5 2.1 | 1/10 4/88 3/140 | 0.305 |
| Sex | Male Female | 68.1 58.5 | 77/113 24/41 | 0.179 | 3.7 1.6 | 7/187 1/62 | 0.366 |
| Sampling year | 2015 2016 2017 2018 2019 2020 2021 | - 0.0 60.6 73.3 66.1 80.0 68.8 | - 0/3 20/33 11/15 39/59 12/15 22/32 | 0.153 | 20.0 2.8 1.7 6.8 1.5 3.4 0.0 | 1/5 1/36 1/58 3/44 1/66 1/29 0/16 | 0.231 |
| Percentage of skin surface area affected | Grade I (≤25%) Grade II (25–50%) Grade III (50–75%) Grade IV (≥75%) | 51.1 76.3 83.3 88.2 | 23/45 29/38 10/12 30/34 | 0.002 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Guillamón, F.; Jiménez-Martín, D.; Dellamaria, D.; Arenas, A.; Rossi, L.; Citterio, C.V.; Camacho-Sillero, L.; Moroni, B.; Cano-Terriza, D.; García-Bocanegra, I. Surveillance of Sarcoptic Mange in Iberian Ibexes (Capra pyrenaica) and Domestic Goats (Capra hircus) in Southern Spain. Animals 2024, 14, 1194. https://doi.org/10.3390/ani14081194
Gómez-Guillamón F, Jiménez-Martín D, Dellamaria D, Arenas A, Rossi L, Citterio CV, Camacho-Sillero L, Moroni B, Cano-Terriza D, García-Bocanegra I. Surveillance of Sarcoptic Mange in Iberian Ibexes (Capra pyrenaica) and Domestic Goats (Capra hircus) in Southern Spain. Animals. 2024; 14(8):1194. https://doi.org/10.3390/ani14081194
Chicago/Turabian StyleGómez-Guillamón, Félix, Débora Jiménez-Martín, Debora Dellamaria, Antonio Arenas, Luca Rossi, Carlo V. Citterio, Leonor Camacho-Sillero, Barbara Moroni, David Cano-Terriza, and Ignacio García-Bocanegra. 2024. "Surveillance of Sarcoptic Mange in Iberian Ibexes (Capra pyrenaica) and Domestic Goats (Capra hircus) in Southern Spain" Animals 14, no. 8: 1194. https://doi.org/10.3390/ani14081194
APA StyleGómez-Guillamón, F., Jiménez-Martín, D., Dellamaria, D., Arenas, A., Rossi, L., Citterio, C. V., Camacho-Sillero, L., Moroni, B., Cano-Terriza, D., & García-Bocanegra, I. (2024). Surveillance of Sarcoptic Mange in Iberian Ibexes (Capra pyrenaica) and Domestic Goats (Capra hircus) in Southern Spain. Animals, 14(8), 1194. https://doi.org/10.3390/ani14081194







