Antimicrobial Resistance and Biofilm Formation of Bordetella bronchiseptica in Central China, with Evidence of a Rare Heteroresistance Strain to Gentamicin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Identification
2.2. Multi-Locus Sequence Typing
2.3. Virulence Genes Detection
2.4. Antimicrobial Susceptibility Assay
2.5. Antimicrobial MIC Assay
2.6. Antimicrobial Heteroresistance Assay
2.7. Biofilm-Formation Ability Assay
2.8. Statistical Analysis
3. Results
3.1. B. bronchiseptica Isolation and Identification
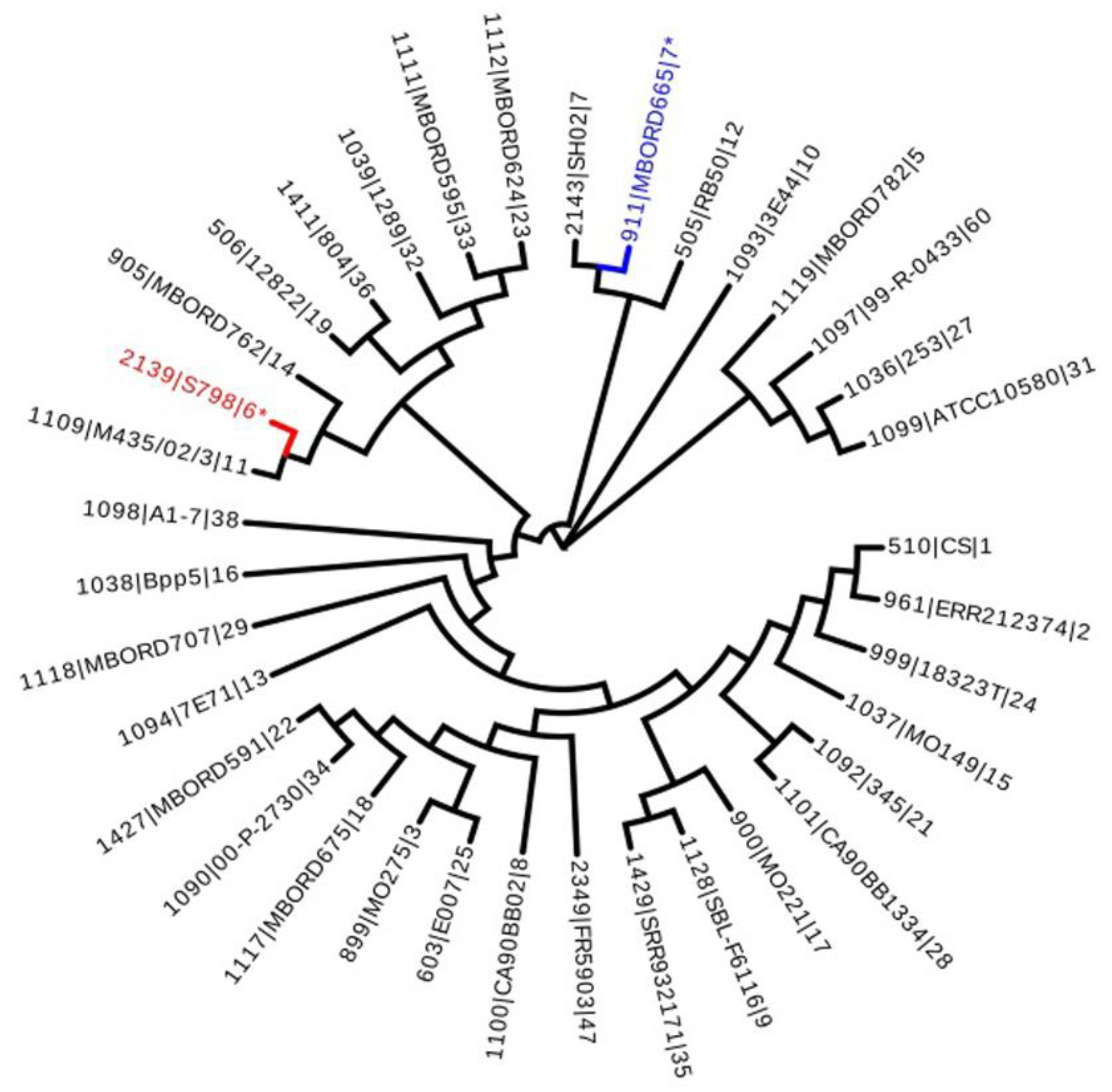
3.2. Multi-Locus Sequence Typing
3.3. Virulence Factors Encoding Genes Detection
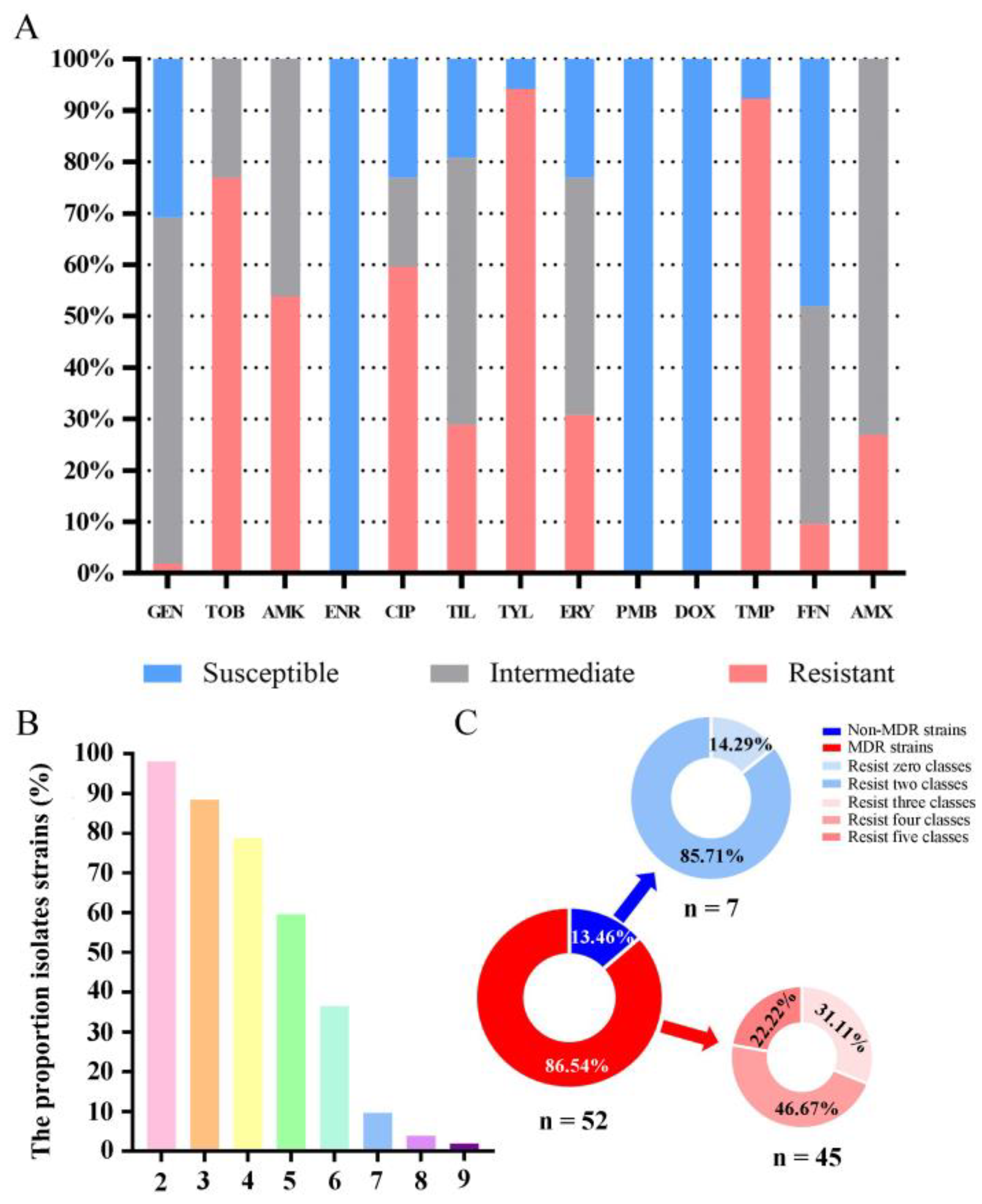
3.4. Antimicrobial Susceptibility Testing
3.5. Antimicrobial MIC Testing
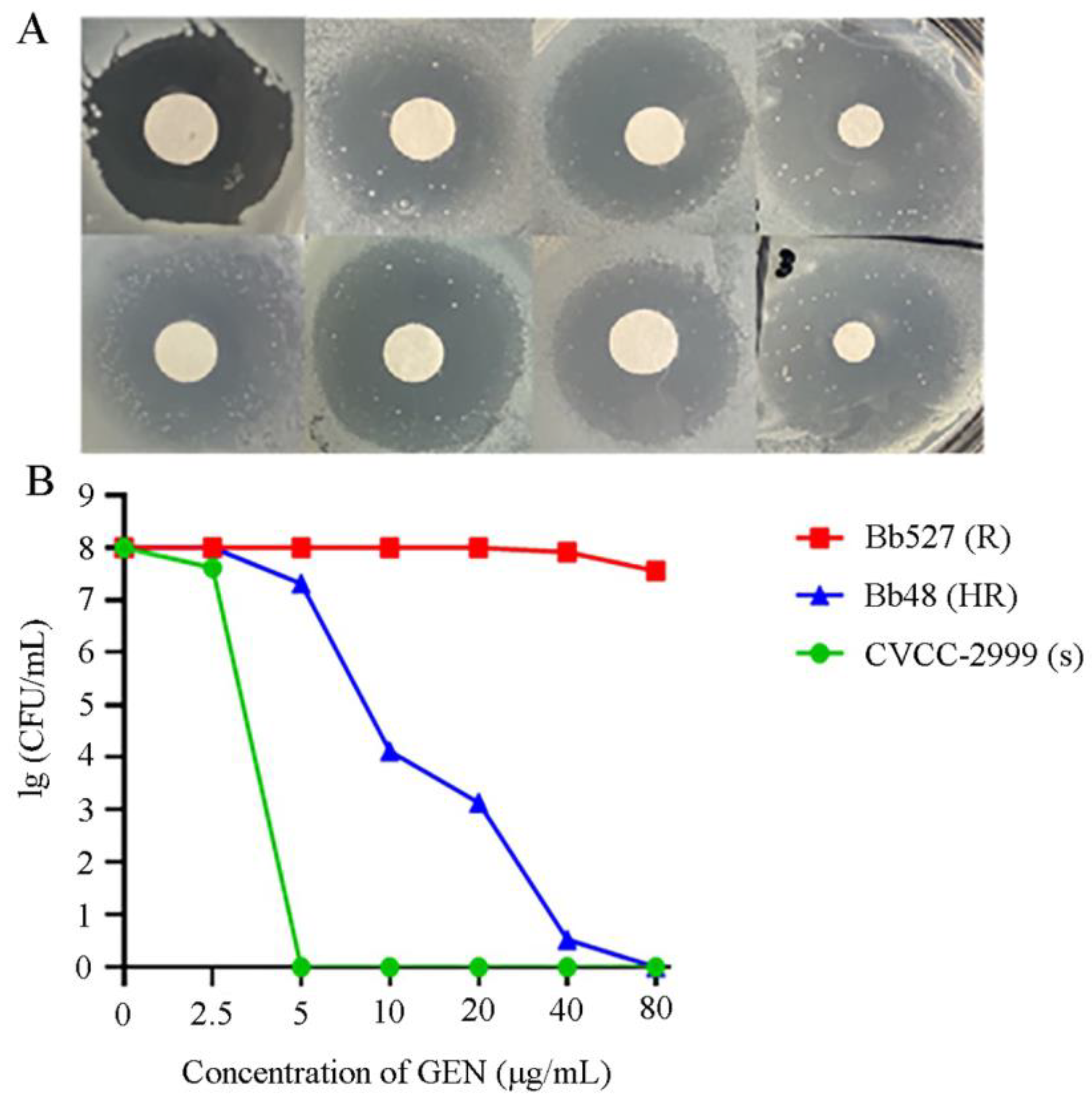
3.6. Heteroresistance Test
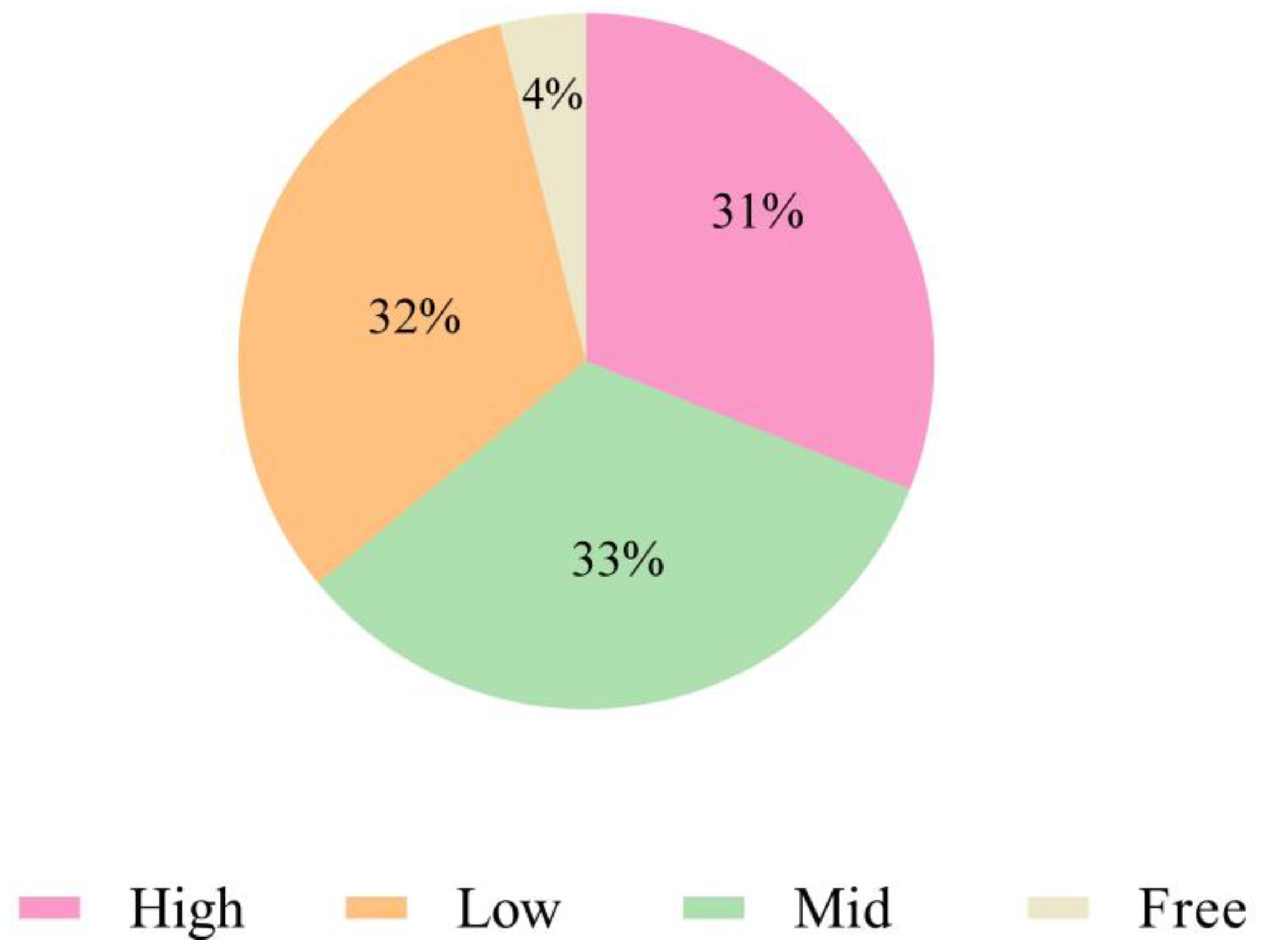
3.7. Biofilm-Formation Ability Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elizabeth, A.T.; Tracy, L.N.; Tod, J.M. Bordetella pertussis Transmission. Pathog. Dis. 2015, 73, ftv068. [Google Scholar]
- Chambers, J.K.; Matsumoto, I.; Shibahara, T.; Haritani, M.; Nakayama, H.; Uchida, K. An Outbreak of Fatal Bordetella bronchiseptica Bronchopneumonia in Puppies. J. Comp. Pathol. 2019, 167, 41–45. [Google Scholar] [CrossRef]
- Gujju, V.R.; Akram, B.; Shibib, D.R.; McGhee, M.A.; Drevets, D.A. Bordetella bronchiseptica Infections in Patients with HIV/AIDS: A Case Report and Review of the Literature. Medicine 2021, 100, e28244. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, L.; Wei, J.; Liu, K.; Shao, D.; Li, B.; Liu, L.; Widén, F.; Ma, Z.; et al. Comparative Genomic Analysis of Bordetella bronchiseptica Isolates from the Lungs of Pigs with Porcine Respiratory Disease Complex (PRDC)-ScienceDirect. Infect. Genet. Evol. 2020, 81, 104258. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, Y. Swine Atrophic Rhinitis Caused by Pasteurella multocida Toxin and Bordetella Dermonecrotic Toxin. Curr. Top. Microbiol. Immunol. 2012, 361, 113–129. [Google Scholar]
- Vötsch, D.; Willenborg, M.; Baumgärtner, W.; Rohde, M.; Valentin-Weigand, P. Bordetella bronchiseptica Promotes Adherence, Colonization, and Cytotoxicity of Streptococcus suis in A Porcine Precision-cut Lung Slice Model. Virulence 2021, 12, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Pomorska-Mól, M.; Kwit, K.; Pejsak, Z.; Rachubik, J.; Markowska-Daniel, I. Cytokine and Chemokine mRNA Expression Profiles in BALF Cells Isolated from Pigs Single Infected or Co-infected with Swine Influenza Virus and Bordetella bronchiseptica. Vet. Microbiol. 2014, 170, 206–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Guo, L.; Zhao, M.; Wang, F.; Song, W.; Hua, L.; Wang, L.; Liang, W.; Tang, X.; et al. Isolation, Antimicrobial Resistance Phenotypes, and Virulence Genes of Bordetella bronchiseptica from Pigs in China, 2018–2020. Front. Vet. Sci. 2021, 8, 638–648. [Google Scholar] [CrossRef]
- Chang, H.H.; Cohen, T.; Grad, Y.H.; Hanage, W.P.; O’Brien, T.F.; Lipsitch, M. Origin and Proliferation of Multiple-Drug Resistance in Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2015, 79, 101–116. [Google Scholar] [CrossRef]
- Digafie, Z.; Melaku, Y.; Belay, Z.; Eswaramoorthy, R. Synthesis, Antibacterial, Antioxidant, and Molecular Modeling Studies of Novel [2,3′-Biquinoline]-4-Carboxylic Acid and Quinoline-3-Carbaldehyde Analogs. Hindawi 2021, 2021, 9939506. [Google Scholar] [CrossRef]
- Zhao, H.; Zhong, L.; Yang, C.; Tang, N.; He, Y.; He, W.; Zhao, Z.; Wu, C.; Yuan, P.; Yang, Y.Y. Antibiotic-polymer Self-assembled Nanocomplex to Reverse Phenotypic Resistance of Bacteria Toward Last-resort Antibiotic Colistin. ACS Nano 2023, 17, 15411–15423. [Google Scholar] [CrossRef]
- Moreira, I.M.B.; Cortez, A.C.A.; de Souza, É.S.; Pinheiro, S.B.; de Souza Oliveira, J.G.; Sadahiro, A.; Cruz, K.S.; Matsuura, A.B.J.; Melhem, M.S.C.; Frickmann, H.; et al. Investigation of Fluconazole Heteroresistance in Clinical and Environmental Isolates of Cryptococcus neoformans Complex and Cryptococcus gattii Complex in the State of Amazonas, Brazil. Med. Mycol. 2022, 60, 3. [Google Scholar] [CrossRef]
- Band, V.; Weiss, D. Heteroresistance: A cause of Unexplained Antibiotic Treatment Failure? PLoS Pathog. 2019, 15, e1007726. [Google Scholar] [CrossRef]
- Woolfrey, B.F.; Moody, J.A. Human Infections Associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 1991, 4, 243–255. [Google Scholar] [CrossRef]
- Qi, J.; Wang, S.R.; Li, L.B.; Luo, Y.B.; Hu, M. Isolation and Identification of Duck Salmonella and Screening of Sensitive Antimicrobials in Shandong Province. Chin. J. Anim. Husb. Vet. Med. 2014, 41, 216–220. [Google Scholar]
- Wang, J.; Sun, S.; Chen, Y.; Chen, D.; Xie, X. Characterization of Bordetella bronchiseptica Isolated from Rabbits in Fujian, China. Epidemiol. Infect. 2020, 21, e237. [Google Scholar] [CrossRef]
- Stepniewska, K.; Urbaniak, K.; Markowska-Daniel, I. Phenotypic and Genotypic Characterization of Bordetella bronchiseptica Strains Isolated from Pigs in Poland. Pol. J. Vet. Sci. 2014, 17, 71–77. [Google Scholar] [CrossRef]
- Palmeira, J.D.; Ferreira, H. CLSI-EUCAST: Comparison of Antibiotic-susceptibility Profile of Enterobacteriaceae of Animal Origin According to The Standards. Acta Microbiol. Immunol. Hung. 2019, 66, 413–422. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B. Olsson-Liljequist, Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- El-Halfawy, O.M.; Valvano, M.A. Antimicrobial Heteroresistance: An Emerging Field in Need of Clarity. Microbiol. Rev. 2015, 28, 191–207. [Google Scholar] [CrossRef]
- Jo, J.; Ko, K. Tigecycline Heteroresistance and Resistance Mechanism in Clinical Isolates of Acinetobacter baumannii. Microbiol. Spect. 2021, 9, e0101021. [Google Scholar] [CrossRef]
- Yi, L.; Wang, Y.; Ma, Z.; Zhang, H.; Lu, C.P. Biofilm Formation of Streptococcus equi ssp. zooepidemicus and Comparative Proteomic Analysis of Biofilm and Planktonic Cells. Curr. Microbiol. 2014, 69, 227–233. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, L.; Wang, Y.; Wang, Y.; Cai, Y.; Zhao, W.; Ding, C. Isolation, Phylogenetic Group, Drug Resistance, Biofilm Formation, and Adherence Genes of Escherichia coli from Poultry in Central China. Poult. Sci. 2016, 95, 2895. [Google Scholar] [CrossRef]
- Kadlec, K.; Kehrenberg, C.; Wallmann, J.; Schwarz, S. Antimicrobial Susceptibility of Bordetella bronchiseptica Isolates from Porcine Respiratory Tract Infections. Antimicrob. Agents Chemother. 2004, 48, 4903–4906. [Google Scholar] [CrossRef]
- Lappin, M.R.; Blondeau, J.; Boothe, D.; Breitschwerdt, E.B.; Guardabassi, L.; Lloyd, D.H.; Papich, M.G.; Rankin, S.C.; Sykes, J.E.; Turnidge, J. Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J. Vet. Intern. Med. 2017, 31, 279–294. [Google Scholar] [CrossRef]
- Matajira, C.; Moreno, L.; Poor, A.; Gomes, V.; Dalmutt, A.; Parra, B.; Oliveira, C.; Barbosa, M.; Sato, M.; Calderaro, F.; et al. Streptococcus suis in Brazil: Genotypic, Virulence, and Resistance Profiling of Strains Isolated from Pigs between 2001 and 2016. Pathogens 2019, 9, 31. [Google Scholar] [CrossRef]
- Petrocchi-Rilo, M.; Martínez-Martínez, S.; Aguarón-Turrientes, Á.; Roca-Martínez, E.; García-Iglesias, M.; Pérez-Fernández, E.; González-Fernández, A.; Herencia-Lagunar, E.; Gutiérrez-Martín, C. Streptococcus suis Anatomical Site, Typing, Virulence Gene Profiling, Antimicrobial Susceptibility and Resistance Genes of Isolates Recovered from Pigs in Spain. Antibiotics 2021, 10, 707. [Google Scholar] [CrossRef]
- Russo, T.P.; Pace, A.; Varriale, L.; Borrelli, L.; Dipineto, L. Prevalence and Antimicrobial Resistance of Enteropathogenic Bacteria in Yellow-Legged Gulls (Larus michahellis) in Southern Italy. Animals 2021, 11, 275. [Google Scholar] [CrossRef]
- Shin, S.J.; Kang, S.G.; Nabin, R.; Kang, M.L.; Yoo, H.S. Evaluation of the Antimicrobial Activity of Florfenicol Against Bacteria Isolated from Bovine and Porcine Respiratory Disease. Vet. Microbiol. 2005, 106, 73–77. [Google Scholar] [CrossRef]
- Kristina, K.; Stefan, S. Antimicrobial Resistance in Bordetella bronchiseptica. Microbiol. Spect. 2018, 6. [Google Scholar]
- Costerton, J.W.; Greenberg, P.S.S.P. Greenberg, Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A. Respiration-induced Biofilm Formation as A Driver for Bacterial Niche Colonization. Trends Microbiol. 2023, 31, 120–134. [Google Scholar] [CrossRef]
- Oana, C.; Claus, M.; Strup, J.P.; Niels, H. Tolerance and Resistance of Microbial Biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar]
- Ramezani, M.; Monroe, M.B.B. Bacterial Protease-responsive Shape Memory Polymers for Infection Surveillance and Biofilm Inhibition in Chronic Wounds. J. Biomed. Mater. Res. A 2023, 111, 921–937. [Google Scholar] [CrossRef]
- Chakraborty, S.; Roychoudhury, P.; Samanta, I.; Subudhi, P.K.; Das, M.; De, A.; Bandyopadhayay, S.; Joardar, S.N.; Mandal, M.; Qureshi, A.; et al. Molecular Detection of Biofilm, Virulence and Antimicrobial Resistance Associated Genes of Salmonella Serovars Isolated from Pig and Chicken of Mizoram, India. Indian J. Anim. Res. 2020, 54, 608–613. [Google Scholar] [CrossRef]
- Pandey, P.K.; Laxmi, M.S.; Kumar, S. In Vitro Evaluation of Natural and Synthetic Substrate for Biofilm Formation and Their Effect on Water Qualities. Indian J. Anim. Res. 2014, 48, 585–592. [Google Scholar] [CrossRef]
- Yang, W.; Yuxin, W.; Liyun, S.; Daniel, G.; Li, Y. Streptococcus suis Biofilm: Regulation, Drug-resistance Mechanisms, and Disinfection Strategies. Appl. Microbiol. Biotechnol. 2019, 102, 9121–9129. [Google Scholar]

| Primers | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| fhaB P1 | GCGCAGAACATCACCAATG | 475 |
| fhaB P2 | TGAAATACTCCATGGCGGAC | |
| prn P1 | GACCTCGCTCAGTCGATC | 555 |
| prn P2 | GAAGACATTCATGCGGAACAG | |
| cyaA P1 | CTACGAGCAGTTCGAGTTTC | 377 |
| cyaA P2 | TATTCATGTCGCCGTCGTA | |
| dnt P1 | TGATCCTGCAGTGGTTGATC | 491 |
| dnt P2 | ATCGGCATACGCCAGATC | |
| bteA P1 | TGTTGAGCAACAACGTCAATC | 474 |
| bteA P2 | TATGCAGGTCTTCGAGGTTC | |
| fla P1 | AGGCTCCCAAGAGAGAAAGGCT | 237 |
| fla P2 | TGGCGCCTGCCCTATC | |
| bfrZ P1 | GCAATGACCTGAACCTGTATTT | 368 |
| bfrZ P2 | CATGGGCATGTTCTTCTTGT |
| Antibiotics | MIC Frequency Distribution (% of Isolates) | Antibiotics | MIC Frequency Distribution (% of Isolates) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gentamicin | ≤4 | 8 | ≥16 | Erythromycin | ≤8 | 16 | ≥32 | ||||
| 30.8% | 67.3% | 1.9% | 23.1% | 48.1% | 28.8% | ||||||
| Tobramycin | ≤4 | 8 | ≥16 | Polymyxin B | ≤0.5 | 1 | 2 | ≥4 | |||
| 0 | 23.1% | 76.9% | 100% | 0 | |||||||
| Amikacin | ≤16 | 32 | ≥64 | Doxycycline | ≤2 | 4 | 8 | ≥16 | |||
| 0 | 46.2% | 53.8% | 100% | 0 | 0 | ||||||
| Enrofloxacin | ≤0.25 | 0.5 | ≥1 | Trimethoprim | ≤8 | 16 | ≥32 | ||||
| 100% | 0 | 0 | 7.7% | 92.3% | |||||||
| Ciprofloxacin | ≤0.125 | 0.25 | 0.5 | 1 | ≥2 | Florfenicol | ≤2 | 4 | ≥8 | ||
| 23.1% | 17.3% | 59.6% | 48.1% | 42.3% | 9.6% | ||||||
| Tilmicosin | ≤5 | 10 | 20 | ≥40 | Amoxicillin | ≤8 | 16 | ≥32 | |||
| 19.2% | 51.9% | 28.9% | 0 | 73.1% | 26.9% | ||||||
| Tylosin | ≤24 | 48 | ≥96 | Vertical red lines indicate the CLSI-approved breakpoint for susceptible, intermediate, and resistant in B. bronchiseptica; numbers in the lowest concentration of the tested antibacterial drug range represent the percentage of isolates that had MICs less than or equal to the lowest drug concentration tested per year, while numbers above the highest antibacterial drug concentration represent the percentage of isolates that had MICs greater than the highest drug concentration tested that year. | |||||||
| 5.8% | 94.2% | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, L.; Fan, H.; Yuan, S.; Li, R.; Wang, H.; Quan, Y.; Zhang, H.; Wang, Y.; Wang, Y. Antimicrobial Resistance and Biofilm Formation of Bordetella bronchiseptica in Central China, with Evidence of a Rare Heteroresistance Strain to Gentamicin. Animals 2024, 14, 1301. https://doi.org/10.3390/ani14091301
Yi L, Fan H, Yuan S, Li R, Wang H, Quan Y, Zhang H, Wang Y, Wang Y. Antimicrobial Resistance and Biofilm Formation of Bordetella bronchiseptica in Central China, with Evidence of a Rare Heteroresistance Strain to Gentamicin. Animals. 2024; 14(9):1301. https://doi.org/10.3390/ani14091301
Chicago/Turabian StyleYi, Li, Haoran Fan, Shuo Yuan, Rishun Li, Haikun Wang, Yingying Quan, Hui Zhang, Yuxin Wang, and Yang Wang. 2024. "Antimicrobial Resistance and Biofilm Formation of Bordetella bronchiseptica in Central China, with Evidence of a Rare Heteroresistance Strain to Gentamicin" Animals 14, no. 9: 1301. https://doi.org/10.3390/ani14091301
APA StyleYi, L., Fan, H., Yuan, S., Li, R., Wang, H., Quan, Y., Zhang, H., Wang, Y., & Wang, Y. (2024). Antimicrobial Resistance and Biofilm Formation of Bordetella bronchiseptica in Central China, with Evidence of a Rare Heteroresistance Strain to Gentamicin. Animals, 14(9), 1301. https://doi.org/10.3390/ani14091301






