Simple Summary
Clarifying the relationship between signal variation and social interactions is important for understanding the evolution of signal complexity. While sound and color signals have been well studied, less is known about how social communication shapes the movement-based visual signals. In this study, we examined the relationship between the variation in tail displays and social interactions in the Asian agamid lizard Phrynocephalus vlangalii. We found that males significantly reduced the duration of tail coiling during the mating season, while females increased both the duration and variation in tail movements to meet the demands of parental care. Additionally, females that invested more in reproduction displayed their tails for longer periods. These findings demonstrate that social interaction influences movement-based visual signals, providing new evidence for the evolution of signal complexity from a new signal pattern.
Abstract
The social complexity hypothesis suggests that complex social interactions drive the evolution of sophisticated communicative signals. While the relationship between social communication and the complexity of sound and color signals has been extensively studied, the correlation between social communication and movement-based visual signal complexity remains underexplored. In this study, we selected the Asian agamid lizard, Phrynocephalus vlangalii, as our model system. Through a combination of controlled experiments, behavioral observations, and signal quantification, we examined the relationship between social communications and variation in movement-based visual signals and tested our social complexity hypothesis. Our experiments revealed that males significantly decreased the tail coil duration during the mating season to deal with high social interaction. Conversely, females significantly increased the tail display duration and variation in mean tail coil amplitude in line with the intensity of parental care, and tail display duration showed a significant and positive correlation with female reproductive investment. These findings suggest that social communication plays an important role in shaping the changes in movement-based visual signals, providing new evidence for the social complexity hypothesis.
1. Introduction
Animal social communication depends on a variety of signals. The signaler influences behavioral response of the recipient through signal change, and its behavioral response may impact the signal structure accordingly, thereby forming a complete communication loop [1]. Nevertheless, the signal structure is not fixed. Signalers likely adjust their signals according to specific situations, such as climate change [2], season variation [3], and environment noise [4,5,6], so as to ensure the signal efficacy under background noise and physiological constraint [7]. For example, in the context of climate change, the male spadefoots (Spea multiplicata) reduced their call speed in response to the colder environment [8]. The neotropical lizard Liolaemus pacha (Iguania: Liolaemidae) exhibits more frequent tongue flicking and visual displays during the breeding season [9]. The tropical Anolis lizards have evolved diverse visual signaling strategies to resist interference from environment noise, such as visual motion and wind-blown vegetation [10].
Recently, the social complexity hypothesis focusing on the relationship between signal complexity and social complexity has garnered a significant attention due to its intimate connection with human language [11,12,13,14]. The hypothesis posits that the complexity of social communication drives the evolution of signal complexity, and that maintaining complex social structures necessitates complex signals [15,16]. This hypothesis has been evidenced across numerous animal groups [17,18,19,20,21]. For example, in macaque taxa, species experiencing complex social interactions exhibit greater vocal diversity and flexibility [22]. In birds, species that depend on cooperative reproduction display a larger sound repertoire [23]. Living in groups promotes the differentiation of sound signals in striped dolphins between different ages and sexes [24]. Movement-based visual signals (e.g., claw wave in fiddler crab, head bod in Anolis lizard) have been widely used in many species [20,25,26] and play important roles in social communication. Nevertheless, whether the relationship between the variation in movement-based display signals and complexity of social interactions is in line with social complexity hypothesis remains largely unclear.
The Asian agamid lizard, Phrynocephalus vlangalii, serves as an excellent model system to study the relationship between the variation in movement-based visual signals and social communication complexity. This species primarily communicates through movement-based visual signals (tail coiling, tail lashing) during male–male competition, male courtship, and female territorial defense [27]. More importantly, a lot of research has shown that P. vlangalii can adjust the tail coiling and tail lashing according to social context. For example, the female resident would display toward the intruder female more quickly when a neighboring male is present, indicating the mate defense role of female movement-based visual signals [28]. The resident male would adjust their response according to the tail coil and tail lash speed of intruder [29]. In addition, the movement-based visual signals in P. vlangalii encode information on individual physical condition and burrow quality [30]. More interestingly, when the offspring are born, they live in the female burrow for a while and female aggression increases accordingly [31], suggesting the burrow share functions in parental care, and the duration of burrow sharing provides a nice indicator of parental care investment. These studies provide important perspectives on the links between movement variation and social communication.
Here, we used P. vlangalii as a model system to examine the links between the variation in movement-based visual signals and the complexity of social communication. If the complexity of social interactions indeed influences the variation in movement-based visual signals, we predict that the variation in these signals is higher during the mating season compared to the non-mating season for males, due to the frequent male–male competition and male courtship. For females, we predict that the variation in movement-based visual signals is associated with investment in reproduction, such as energy and parental care: individuals with high reproductive investment are likely to exhibit more variable signals.
2. Materials and Methods
2.1. Study Site and Species
Our study was conducted near the Xiaman field station of Zoige Wetland Nature Reserve, in southwestern China (33°43′25.0″ N, 102°29′04.0″ E, with an elevation of 3475 m a.s.l.). The climate in this area is characterized by drought, low temperature, and strong sunshine, with average monthly precipitation of 54.88 ± 47.99 mm, average monthly minimum temperature of −5.2 ± 8.7 °C, and average monthly sunshine duration of 12.2 ± 1.6 h (data from Weather Atlas). The population density of P. vlangalii in this area is approximately 0.1128 ± 0.0172 lizards per square meter [32], mainly distributed around sand dunes. The active season of the lizards lasts from late April to early September, with the mating season in May and June, and young emerging in late August and early September [33].
2.2. Male Display Collection
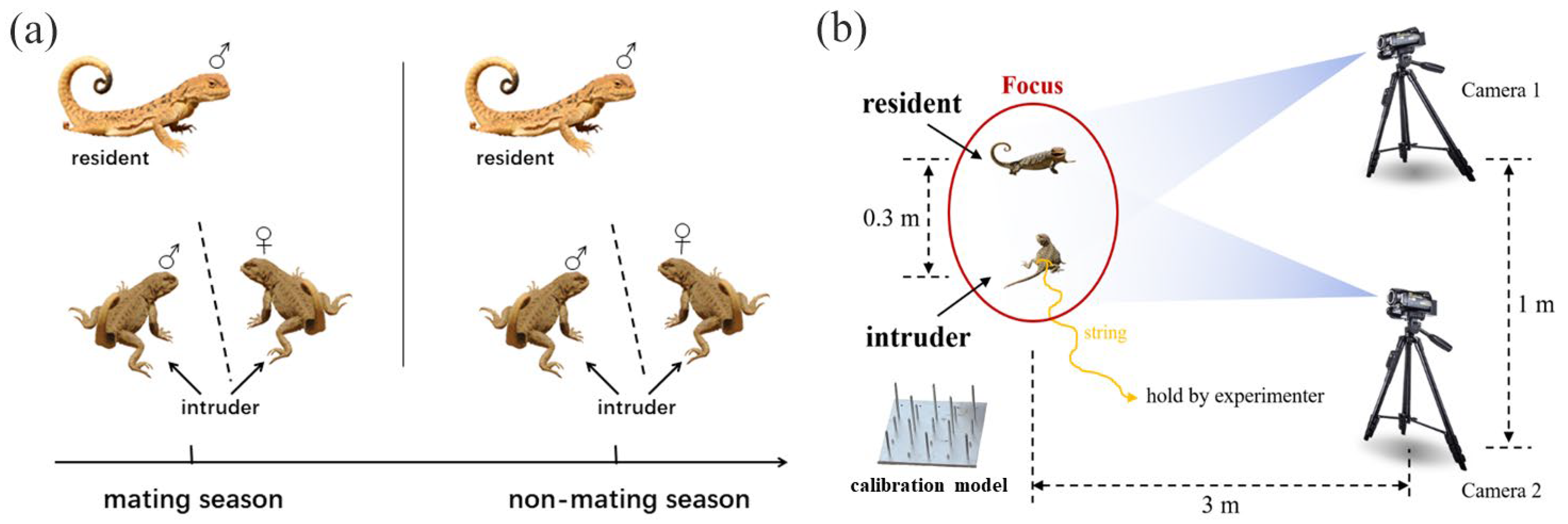
To test the relationship between the variation in movement-based visual signals and the complexity of male social interaction, we employed the most recent 3D method and collected the movement-based visual signals of males both in mating and non-mating seasons from 2016 to 2018 [30]. To examine whether the relationship is variable with social contexts, each resident male experienced two social contexts, including male–male competition and male courtship. For the male–male competition context, we presented focal resident males with an intruder male, while for male courtship context, we presented focal resident males with an intruder female. We waited at least 30 min before presenting the next intruder, based on the natural display rates of one every 20 min (Figure 1a). To facilitate size matching, we first captured the resident males with a lasso, measured their snout–vent length (SVL) and tail length (TL) separately to the nearest 0.01 mm using a vernier caliper (PD-151, Mitutoyo Corporation, Kawasaki, Japan), and weighed their mass to the nearest 0.01 g using an electronic balance (ES-08B, Want Balance Instrument Co., Ltd., Guangzhou, China). To facilitate individual identification, we marked them using a nontoxic paint pen and returned them to the original burrow with a mark flag planted near the burrow entrance. To avoid the potential impact of previous social interactions, individuals used as intruders were collected at least three kilometers away from the habitat of residents, and their burrows were also marked to facilitate subsequent releasing. The snout–vent length (SVL) and tail length (TL) of the intruders were measured using the similar method.
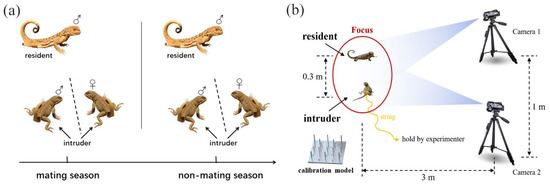
Figure 1.
Male experimental scheme design. (a) Collection of movement-based visual signals in males. ♂ represents male individuals, while ♀ represents female individuals. (b) Schematic of collecting method on movement-based visual signals.
After at least 24 h, we went back to the marked residents and collected the display signals. As shown in Figure 1b, we first confirmed the presence of the resident using binoculars; when the resident was locked down, we immediately set up two cameras (50 frames per second, Sony HDR PJ670, Sony Corporation, Tokyo, Japan) at 3 m away from the resident. The distance between the two cameras was kept at least 1 m apart. We waited for at least 10 min for the resident to acclimatize before commencing filming. At the same time, the other experimenter introduced the intruder from 3 m away using a fishing rod. The intruder was tethered to the rod using 30 cm of dental floss tied around the waist. We terminated a trial after a resident completed its display or before the interaction between resident and intruder escalating into fight. We also terminated a trial if a resident did not exhibit a display after 10 min of presenting an intruder. Before we turned off the camera, a calibration object was placed in the site where the signal from the tail of the resident was displayed for subsequent spatial digitization. We minimized the size difference between the intruder and resident by size-matching. To avoid the effects of physiological stress, each intruder was used a maximum of three times and then released back into their original burrow.
2.3. Female Display Collection and Reproductive Investment Measurement
To test the relationship between the variation in movement-based visual signals and female reproductive investment, we collected movement-based visual signals from postpartum females in August 2021. To confirm pregnancy, we constructed a plot (50 m × 50 m) in the field from late July and the pregnancy status of the females was checked daily. When the female showed obvious signs of pregnancy, we captured 30 pregnant females using a lasso, measured their snout–vent length (SVL, to the nearest of 0.01 mm) and mass (to the nearest of 0.01 g), marked them using a nontoxic paint pen, and returned them to the original burrow with a color flag planted nearby the burrow entrance.
We then followed the 30 pregnant females and observed their reproductive status using binoculars. Each lizard was checked twice a day from 9:00 am to 4:00 pm to accurately determine the delivery time. To estimate the energetic investment, we measured the mass of the females after giving birth, calculated the female mass variation before and after postpartum, and used the ratio between female mass variation and mass after postpartum as an estimate of female energetic investment [34,35]. Three days after the female gave birth, we collected the female movement-based visual signals by presenting an intruder female to postpartum females using the same method as the males. We size-matched the resident and intruders and returned the intruders after three trials.
After the display collection on postpartum females, we continued the field observation twice a day to determine the duration that females and babies shared the burrow. When the females and offspring were no longer in the same burrow, we recorded this day as the separation time. Three days of extra observation were conducted to ensure the accuracy of the separation time. We then used the number of days the females and offspring shared the burrow as an estimate of female parental care.
2.4. Display Digitization and Variation Estimation
The process of display digitization was consistent with the protocol proposed by Peters et al. [27]. Briefly, we used the video editing software Adobe Premiere Pro CC 2018 (version 12.0) to extract high-quality display clips from the original video and obtained a screenshot containing the calibration objects. The video was then calibrated using Direct Linear Transformation (DLT) in MATLAB 2016b (MathWorks Inc., Natick, MA, USA) with the calibration object screenshot. The 3D coordinates of the two points of the tail base and tail tip were digitized frame by frame for tail display reconstruction. Movements of the tail coil and tail lash were reconstructed for males, while movements of the tail coil were reconstructed for females. Using the reconstructed positional data, we extracted the average tail coil speed, tail coil duration, average tail coil amplitude, average tail lash speed, tail lash duration, and volume of space swept by the tail lash display (VT) for males. The average tail coil (or lash) speed was defined as the average distance moved by the tail tip per second in each display bout; tail coil (or lash) duration was defined as the total time of tail coil (or lash) in each display bout; and average tail coil amplitude was defined as the average distance between the tail tip and tail base. The smaller the average tail coil amplitude, the more tightly coiled the tail. VT was defined as the volume of space swept by the tail tip during lashing in each display bout [30]. Occasionally, there was a time interval between two successive display segments, and we regarded those two display segments as one display segment if the time interval was less than 2 s; otherwise, we classified them as two display segments. When digitizing the female signal, we only extracted the average tail coil speed, tail coil duration, and average tail coil amplitude because tail lash frequency was very low. Very short tail lash was found in five videos of the females; we included them in the overall display duration. Therefore, the female tail display duration used in subsequent analysis was the overall display duration, and the average duration was the ratio of the overall display duration to the number of signal segments. The variation in movement-based displays was estimated using the coefficient of variation (CV), calculated as the standard deviation divided by the mean [36,37], as well as the tail coil (lash) duration.
2.5. Statistical Analysis
All statistical analyses were conducted using the R version 4.3.2. The linear mixed models, constructed using the lmer function in lme4 package (version 1.1.35.1), were used to analyze the relationship between the variation in male movement-based visual signals and seasons [38]. Variables, including the tail coil duration after logarithmic transformation, CV of tail coil duration, CV of mean tail coil amplitude, CV of maximum tail coil speed, CV of mean tail coil speed, tail lash duration after logarithmic transformation, CV of tail lash duration, CV of tail lash volume, CV of maximum tail lash speed, and CV of mean tail lash speed, were considered dependent variables, while the season and social communication context were included as independents, and a Gaussian error distribution was assumed. In addition, we considered the resident’s SVL, body temperature, and mass as covariates to account for potential effects of size and physiological condition.
The linear model constructed using the lm function in the stats package was also used to test the relationship between the variation in movement-based visual signals and reproductive investment in postpartum females [39,40]. Variables, including tail display duration after logarithmic transformation, CV of display duration, CV of mean tail coil amplitude, CV of maximum tail coil speed, and CV of mean tail coil speed were considered as dependent variables, female energetic investment and parental care investment were used as independent variables, and a Gaussian error distribution was assumed. In addition, the resident’s SVL and body temperature were included as covariates to explain the potential impacts of size and physiological condition.
To visualize the relationship between the variation in male movement-based visual signals and seasons, and the relationship between the variation in movement-based visual signals and female parental care investment and energetic investment, we predicted the relationship based on the full model using the ggplot function in the ggplot2 package (version 3.5.1) [41].
3. Results
A total of 48 males were collected for movement-based visual signal analysis, including 29 individuals during the mating season and 19 during the non-mating season. Quantification of these signals revealed 380 movement-based events recorded during the mating season, averaging approximately 13 events per individual. In the non-mating season, 210 movement-based events were recorded, with an average of 11 events per individual. See the male movement-based visual signals in Video S1. Parental care behavior was followed and quantified in 30 pregnant females, with 14 giving birth and their parental care being measured. Of the 14 females with recorded parental care data, movement-based visual signals were collected from 12 individuals.
3.1. Differences in Variation in Male Movement-Based Visual Signals Between Mating and Non-Mating Seasons
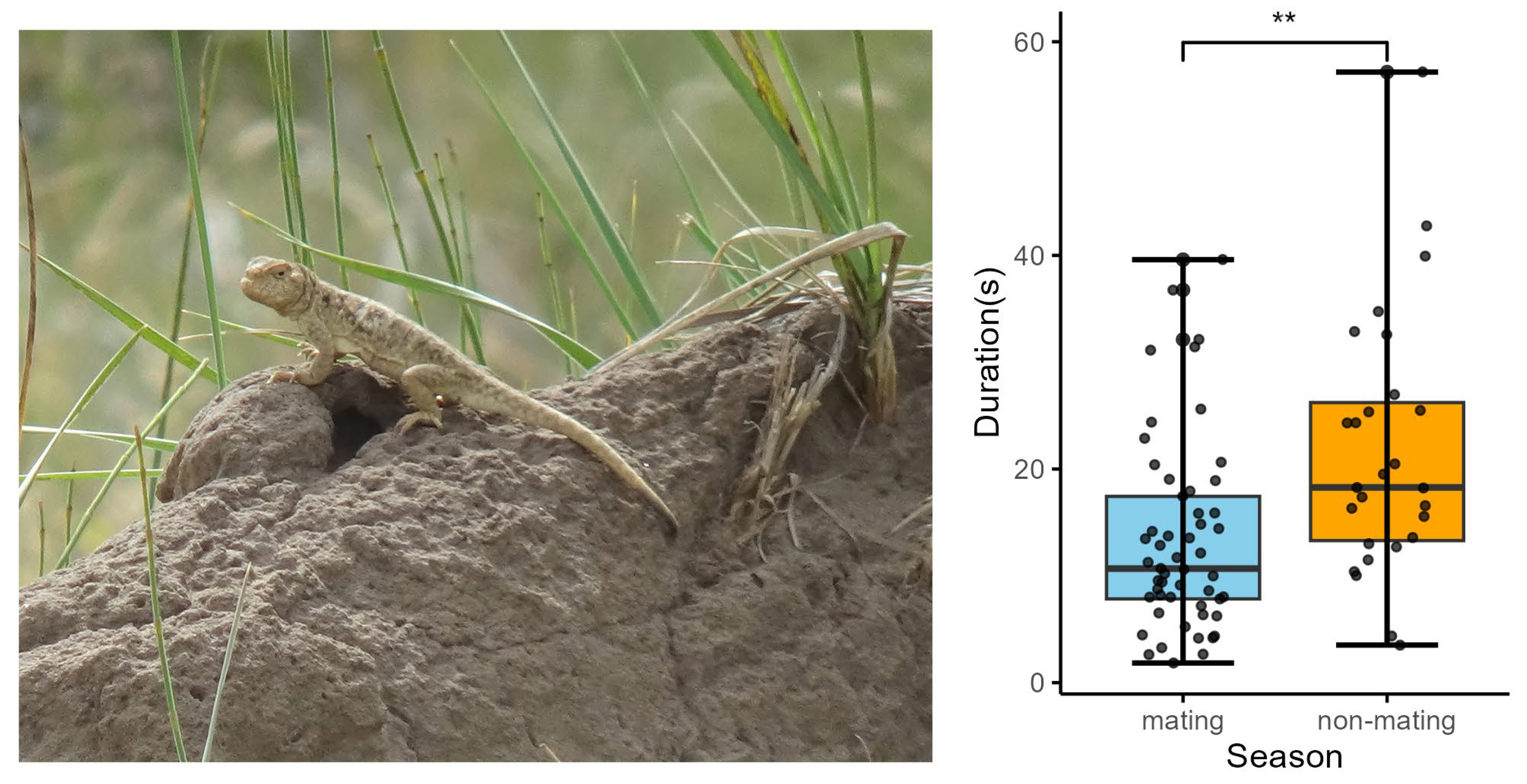
We found a significant change in tail coil duration between the mating and non-mating seasons. The tail coil duration was significantly shorter in the mating season compared with that in the non-mating season (p = 0.006; Table S1; Figure 2). No significant change was found in the CV of tail coil (or lash) duration, CV of mean tail coil amplitude, CV of maximum tail coil (or lash) speed, CV of mean tail coil (or lash) speed, CV of VT, or CV of lash duration between the two seasons (Table S1).
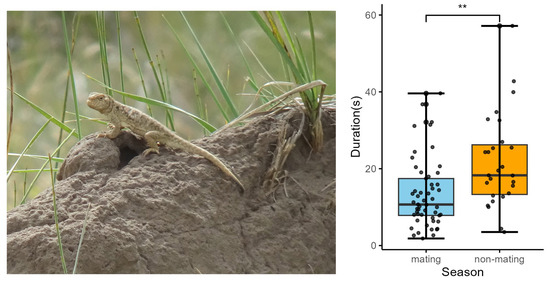
Figure 2.
Male image of P. vlangalii and the variation in tail coil duration in the male’s movement-based visual signals across seasons. Blue represents the breeding season, and orange represents the non-breeding season. Statistical analysis showed significant differences, with p values indicated by p < 0.01 (**).
3.2. Relationship Between Variation in Female Movement-Based Visual Signals and Parental Care
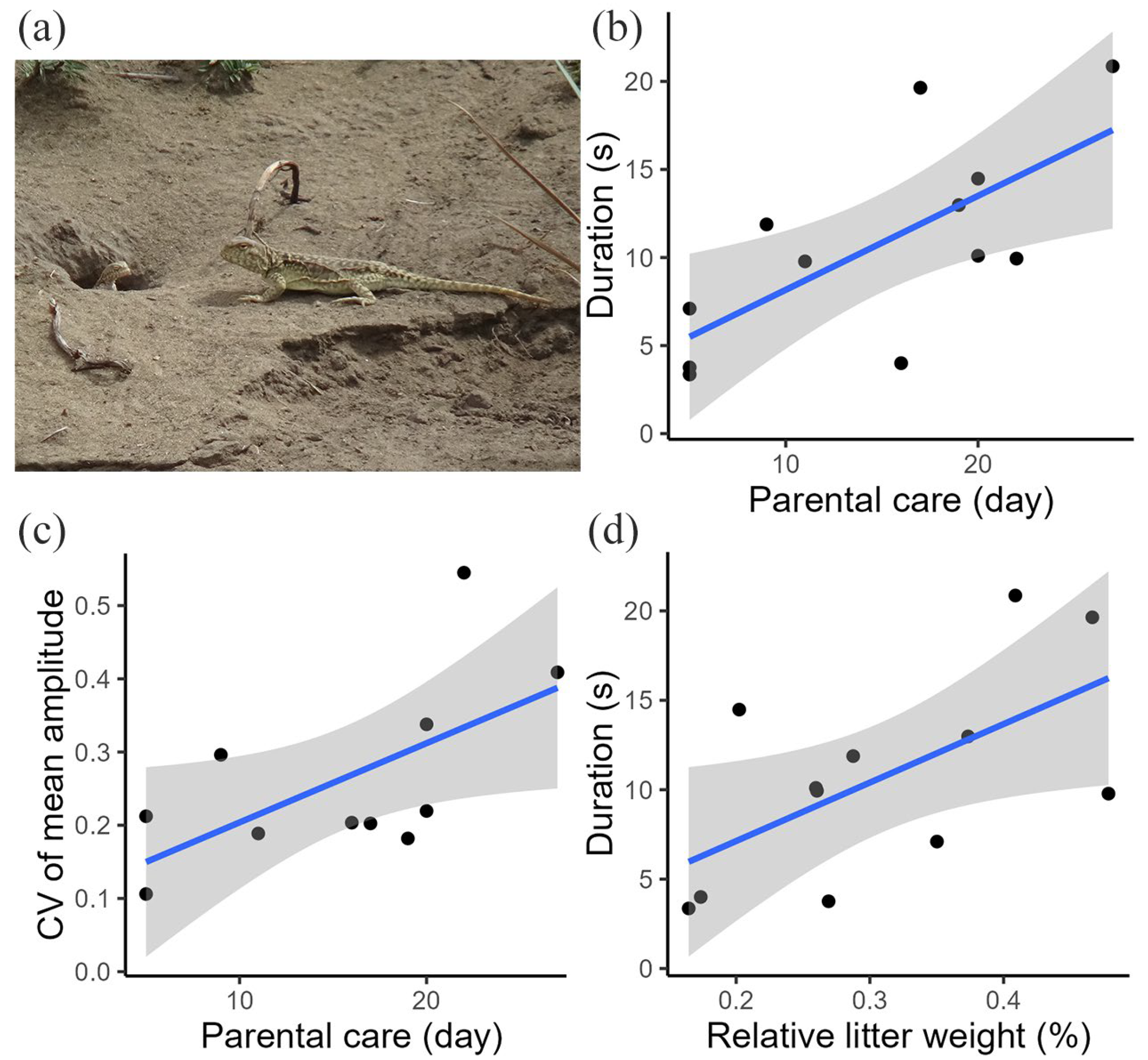
We found a link between the variation in female movement-based visual signals and parental care for postpartum females. The general tail display duration (p = 0.025; Table S2; Figure 3b) and CV of mean tail coil amplitude (p = 0.047; Table S2; Figure 3c) all increased with parental care time. No significant relationships were found between the CV of the maximum tail coil speed and CV of the mean tail coil speed and parental care (Table S2).
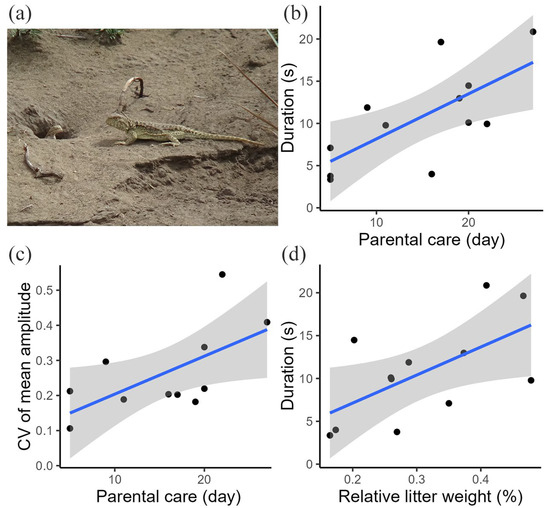
Figure 3.
(a) Image of a postpartum female and her offspring. (b) Relationship between the tail display duration and parental care. (c) Relationship between the CV of the tail coil amplitude and parental care. (d) Relationship between the tail display duration and reproductive investment. Blue lines represent the fitted regression lines, gray color indicates the confidence intervals, and black dots represent individual data points.
3.3. Relationship Between Variation in Female Movement-Based Visual Signals and Energetic Investment
We found a link between the variation in female movement-based visual signals and energetic investment for postpartum females. The general tail display duration increased significantly with energetic investment (p = 0.045; Table S3; Figure 3d), suggesting the role of reproductive investment in the variation in tail displays. We found no significant relationships between the CV of tail display duration, CV of mean tail coil amplitude, CV of maximum tail coil speed, CV of mean tail coil speed, and reproductive investment (Table S3).
4. Discussion
In this study, we examined the relationship between the variation in movement-based visual signals and social communication and tested the social complexity hypothesis from movement-based signals. Our data revealed that males significantly decreased tail coil duration during the mating compared with the non-mating season. Conversely, females significantly increased tail display duration and the CV of mean tail coil amplitude in line with the intensity of parental care, and tail display duration showed a significant positive correlation with female energetic investment, suggesting it is social communication that drives the variation in movement-based visual signals, providing new evidence for the social complexity hypothesis.
Males decreased tail coil duration during mating season, which is surprising because signal duration is generally considered an important indicator of signal complexity [22,37,42,43]. Typically, higher social complexity correlates with longer signal durations. For example, in some anurans like Hyla cinerea, males increase the duration and complexity of their calls in more competitive environments [44]. There is multiple evidence on the links between sexual selection pressure and signal complexity [45,46,47,48]. For example, in a study of male Physalaemus pustulosus (túngara frogs), females showed a preference for complex acoustic calls over simple ones [49]. However, we found that the tail coil duration in P. vlangalii during the mating season was significantly shorter than in non-mating seasons, which contradicts our expectations considering the influence of social complexity on signal complexity [16,21,50,51,52]. There are two potential explanations. First, males likely reduce tail coil duration to achieve high tail coil frequency to respond to frequent social interaction, such as male–male competition and male courtship [53,54]. This is evidenced by a lot of existing research, for example, higher frequency display instances enhance the chances of successful mate acquisition [55,56,57,58,59]. Alternatively, the shorter tail coil displays may reflect a trade-off between signal complexity and social interactions [60,61]. Males likely choose to decrease the tail coil duration to ensure the energy requirement for male–male competition and male courtship. Multiple intermittent displays allow males to navigate various social scenarios during the mating season [54,60]. There is also multiple evidence on this; for example, studies indicate that the evolution of calling songs in Gomphocerinae (Orthoptera, Acrididae), which involves changes in echeme duration and increased syllable complexity, facilitates the development of signals toward greater complexity [62].
More interestingly, we found obvious links between the variation in movement-based signals and female parental care. Individuals with prolonged parental care and high energetic investment exhibit more complex movement-based signals. This provides a novel insight into the social complexity hypothesis. Parental care is very rare in lizards, and, more importantly, movement-based signals are mainly observed in males of other species. For example, male lizards often exhibit courtship-related behaviors such as head bobbing and push-ups [63,64,65]. The relationship between the variation in movement-based signals (e.g., duration and amplitude) and parental care suggests the applicability of the social complexity hypothesis to females. Postpartum females have to navigate various complex social scenarios, such as territory defense and offspring protection, while also recovering their physiological condition as quickly as possible. In this context, variable visual signals likely facilitate mutual evaluations during social interactions. A similar pattern has been found in Liolaemus pacha lizards, where visual displays are crucial for assessing social dynamics [66]. Additionally, we also found a link between the variation in movement-based signals and female energetic investment. This may further strengthen the relationship between female reproductive investment and the variation in movement-based signals, because female energetic investment and parental care are important indicators of female reproductive investment, which in turn can influence offspring survival and fitness [67,68]. This may also promote the iterative inheritance of movement-based signals, as demonstrated in studies of behavioral adaptations and signal evolution in response to environmental and social pressures [69,70].
5. Conclusions
In summary, our study provides new insights into the universality of the social complexity hypothesis from movement-based visual signals, although the relationship between the variation in movement-based signals and social complexity is different between the sexes. Males likely achieve the signal complexity by reducing the signal duration, but females tend to adjust the amplitude and increase the signal duration so as to respond to different social scenarios. This fully shows that variation in movement-based display depends on specific social interaction and the life-history stage, thereby enhance our understanding of the factors driving signal complexity. Much more specific research should be carried out to elucidate the efficacy of different signal variations under different social contexts, and adaptation of male shorter displays in mating season should be figured out.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani15010038/s1. Table S1: The results of the general linear model summarized the relationship between the variation in male movement-based display and the season in male P. vlangalii lizards; Table S2: The results of the general linear model summarized the relationship between the postpartum female’s variation in movement-based display and parental care time in P. vlangalii lizards; Table S3: The results of the general linear model summarized the relationship between the postpartum female’s variation in movement-based display and reproductive investment in P. vlangalii lizards; Video S1: A segment of male movement-based visual signals.
Author Contributions
Conceived the idea and designed the study, Y.Q.; conducted the field collection, measurements, and behavioral trials, J.L. and Q.H.; analyzed data and wrote draft, J.L. and Q.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation grant (No. 32270527 and 32470506).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Chengdu Institute of Biology, Chinese Academy of Sciences (protocol code: CIBDWLL2022004).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data that support the findings of this study have been deposited in figshare at https://doi.org/10.6084/m9.figshare.27641712.v1.
Acknowledgments
We thank K.C., Suolangduoerji, and W.Z. from the Zoige Wetland Nature Reserve for assistance and support in the field work. We also thank X.Q. and L.C. for their valuable suggestions on manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Laidre, M.E.; Johnstone, R.A. Animal signals. Curr. Biol. 2013, 23, R829–R833. [Google Scholar] [CrossRef] [PubMed]
- Penar, W.; Magiera, A.; Klocek, C. Applications of bioacoustics in animal ecology. Ecol. Complex. 2020, 43, 100847. [Google Scholar] [CrossRef]
- Martins, E.P. Structural complexity in a lizard communication system: The Sceloporus graciosus push-up display. Copeia 1994, 1994, 944–955. [Google Scholar] [CrossRef]
- Ramos, J.A.; Peters, R.A. Habitat-dependent variation in motion signal structure between allopatric populations of lizards. Anim. Behav. 2017, 126, 69–78. [Google Scholar] [CrossRef]
- Sharma, N.; Prakash, S.V.; Kohshima, S.; Sukumar, R. Asian elephants modulate their vocalizations when disturbed. Anim. Behav. 2020, 160, 99–111. [Google Scholar] [CrossRef]
- Brumm, H.; Slabbekoorn, H. Acoustic communication in noise. Adv. Study Behav. 2005, 35, 151–209. [Google Scholar]
- Wiley, R.H. Noise Matters: The Evolution of Communication; Harvard University Press: Cambridge, MA, USA, 2015; p. xi + 502. ISBN 9780674744127. [Google Scholar]
- Calabrese, G.M.; Pfennig, K.S. Climate change alters sexual signaling in a desert-adapted frog. Am. Nat. 2023, 201, 91–105. [Google Scholar] [CrossRef]
- Vicente, N.S.; Halloy, M. Chemical recognition of conspecifics in a neotropical lizard, Liolaemus pacha (Iguania: Liolaemidae): Relation to visual displays, season and sex. J. Ethol. 2016, 34, 329–335. [Google Scholar] [CrossRef]
- Ord, T.J.; Charles, G.K.; Hofer, R.K. The evolution of alternative adaptive strategies for effective communication in noisy environments. Am. Nat. 2011, 177, 54–64. [Google Scholar] [CrossRef]
- Prieur, J.; Barbu, S.; Blois-Heulin, C.; Lemasson, A. The origins of gestures and language: History, current advances and proposed theories. Biol. Rev. 2020, 95, 531–554. [Google Scholar] [CrossRef]
- Freeberg, T.M.; Ord, T.J.; Dunbar, R.I.M. The social network and communicative complexity: Preface to theme issue. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1782–1784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nettle, D. Social scale and structural complexity in human languages. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, R.I.M. Bridging the bonding gap: The transition from primates to humans. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.A.; Blumstein, D.T. Evolving communicative complexity: Insights from rodents and beyond. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1869–1878. [Google Scholar] [CrossRef]
- Freeberg, T.M.; Dunbar, R.I.M.; Ord, T.J. Social complexity as a proximate and ultimate factor in communicative complexity. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1785–1801. [Google Scholar] [CrossRef]
- Fichtel, C.; Kappeler, P.M. Coevolution of social and communicative complexity in lemurs. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210297. [Google Scholar] [CrossRef]
- Krams, I.; Krama, T.; Freeberg, T.M.; Kullberg, C.; Lucas, J.R. Linking social complexity and vocal complexity: A parid perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1879–1891. [Google Scholar] [CrossRef]
- McComb, K.; Semple, S. Coevolution of vocal communication and sociality in primates. Biol. Lett. 2005, 1, 381–385. [Google Scholar] [CrossRef]
- Ord, T.J.; Martins, E.P. Tracing the origins of signal diversity in anole lizards: Phylogenetic approaches to inferring the evolution of complex behaviour. Anim. Behav. 2006, 71, 1411–1429. [Google Scholar] [CrossRef]
- Ord, T.J.; Garcia-Porta, J. Is sociality required for the evolution of communicative complexity? Evidence weighed against alternative hypotheses in diverse taxonomic groups. Philos. Trans. R. Soc. B-Biol. Sci. 2012, 367, 1811–1828. [Google Scholar] [CrossRef]
- Rebout, N.; De Marco, A.; Lone, J.-C.; Sanna, A.; Cozzolino, R.; Micheletta, J.; Sterck, E.H.M.; Langermans, J.A.M.; Lemasson, A.; Thierry, B. Tolerant and intolerant macaques show different levels of structural complexity in their vocal communication. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200439. [Google Scholar] [CrossRef] [PubMed]
- Leighton, G.M. Cooperative breeding influences the number and type of vocalizations in avian lineages. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171508. [Google Scholar] [CrossRef] [PubMed]
- Papale, E.; Fanizza, C.; Buscaino, G.; Ceraulo, M.; Cipriano, G.; Crugliano, R.; Grammauta, R.; Gregorietti, M.; Renò, V.; Ricci, P.; et al. The social role of vocal complexity in striped dolphins. Front. Mar. Sci. 2020, 7, 584301. [Google Scholar] [CrossRef]
- How, M.J.; Zeil, J.; Hemmi, J.M. Differences in context and function of two distinct waving displays in the fiddler crab, Uca perplexa (Decapoda: Ocypodidae). Behav. Ecol. Sociobiol. 2007, 62, 137–148. [Google Scholar] [CrossRef]
- How, M.J.; Zeil, J.; Hemmi, J.M. Variability of a dynamic visual signal: The fiddler crab claw-waving display. J. Comp. Physiol. A-Neuroethol. Sens. Neural Behav. Physiol. 2009, 195, 55–67. [Google Scholar] [CrossRef]
- Peters, R.A.; Ramos, J.A.; Hernandez, J.; Wu, Y.; Qi, Y. Social context affects tail displays by Phrynocephalus vlangalii lizards from China. Sci. Rep. 2016, 6, 31573. [Google Scholar] [CrossRef]
- Wu, Y.; Ramos, J.A.; Qiu, X.; Peters, R.A.; Qi, Y. Female–female aggression functions in mate defence in an Asian agamid lizard. Anim. Behav. 2018, 135, 215–222. [Google Scholar] [CrossRef]
- Qiu, X.; Fu, J.; Qi, Y. Tail waving speed affects territorial response in the toad-headed agama Phrynocephalus vlangalii. Asian Herpetol. Res. 2018, 9, 182–187. [Google Scholar]
- Qiu, X.; Hu, Q.; Peters, R.; Yue, B.; Fu, J.; Qi, Y. Unraveling the content of tail displays in an Asian agamid lizard. Behav. Ecol. Sociobiol. 2021, 75, 117. [Google Scholar] [CrossRef]
- Wu, Y.; Whiting, M.J.; Fu, J.; Qi, Y. The driving forces behind female-female aggression and its fitness consequence in an Asian agamid lizard. Behav. Ecol. Sociobiol. 2019, 73, 73. [Google Scholar] [CrossRef]
- Liu, J.; Qi, Y. The Driving Role of Social Complexity in the Dynamic Visual Signals of Phrynocephalus vlangalii; Chengdu Institute of Biology, Chinese Academy of Sciences: Chengdu, China, 2024; manuscript in preparation. [Google Scholar]
- Wu, Y.; Fu, J.; Yue, B.; Qi, Y. An atypical reproductive cycle in a common viviparous Asia Agamid Phrynocephalus vlangalii. Ecol. Evol. 2015, 5, 5138–5147. [Google Scholar] [CrossRef] [PubMed]
- Castro-Franco, R.; Bustos-Zagal, M.G.; Mendez-De la Cruz, F.R. Variation in parental investment and relative clutch mass of the spiny-tail iguana, Ctenosaura pectinata (Squamata: Iguanidae) in central Mexico. Rev. Mex. Biodivers. 2011, 82, 199–204. [Google Scholar] [CrossRef]
- Cuellar, O. Reproduction in a parthenogenetic lizard: With a discussion of optimal clutch size and a critique of the clutch weight/body weight ratio. Am. Midl. Nat. 1984, 111, 242–258. [Google Scholar] [CrossRef]
- Eberhard, M.J.; Treschnak, D. Variation of vibrational communication signals in animals depends on trait duration. Ethology 2018, 124, 855–861. [Google Scholar] [CrossRef]
- Lin, Y.S.; Qiu, X.; Fu, J.Z.; Peters, R.; Qi, Y. Testing the factors on the evolution of movement-based visual signal complexity in an Asian agamid lizard. Behav. Ecol. Sociobiol. 2023, 77, 135. [Google Scholar] [CrossRef]
- Bates, D.; Kliegl, R.; Vasishth, S.; Baayen, H. Parsimonious mixed models. arXiv 2015, arXiv:1506.04967. [Google Scholar]
- Wilkinson, G.N.; Rogers, C.E. Symbolic description of factorial models for analysis of variance. R. Stat. Soc. Ser. C-Appl. Stat. 1973, 22, 392–399. [Google Scholar] [CrossRef]
- Chambers, J.; Hastie, T. Linear models. In Statistical Models in S; Wadsworth Brooks/Cole; Routledge: London, UK, 1992; Chapter 4. [Google Scholar]
- Wickham, H. A layered grammar of graphics. J. Comput. Graph. Stat. 2010, 19, 3–28. [Google Scholar] [CrossRef]
- Roberts, A.I.; Chakrabarti, A.; Roberts, S.G.B. Gestural repertoire size is associated with social proximity measures in wild chimpanzees. Am. J. Primatol. 2019, 81, e22954. [Google Scholar] [CrossRef]
- Terhune, J.M. The underwater vocal complexity of seals (Phocidae) is not related to their phylogeny. Can. J. Zool. 2019, 97, 232–240. [Google Scholar] [CrossRef]
- Neelon, D.P.; Höbel, G. Staying ahead of the game-plasticity in chorusing behavior allows males to remain attractive in different social environments. Behav. Ecol. Sociobiol. 2019, 73, 124. [Google Scholar] [CrossRef]
- D’Ammando, G.; Bro-Jorgensen, J. Sexual selection and species recognition promote complex male courtship displays in ungulates. Behav. Ecol. 2024, 35, arae027. [Google Scholar] [CrossRef]
- Choi, N.; Adams, M.; Fowler-Finn, K.; Knowlton, E.; Rosenthal, M.; Rundus, A.; Santer, R.D.; Wilgers, D.; Hebets, E.A. Increased signal complexity is associated with increased mating success. Biol. Lett. 2022, 18, 20220052. [Google Scholar] [CrossRef] [PubMed]
- Ord, T.J.; Blumstein, D.T.; Evans, C.S. Intrasexual selection predicts the evolution of signal complexity in lizards. Proc. R. Soc. B-Biol. Sci. 2001, 268, 737–744. [Google Scholar] [CrossRef]
- Miles, M.C.; Fuxjager, M.J. Synergistic selection regimens drive the evolution of display complexity in birds of paradise. J. Anim. Ecol. 2018, 87, 1149–1159. [Google Scholar] [CrossRef]
- Stange, N.; Page, R.A.; Ryan, M.J.; Taylor, R.C. Interactions between complex multisensory signal components result in unexpected mate choice responses. Anim. Behav. 2017, 134, 239–247. [Google Scholar] [CrossRef]
- Grampp, M.; Samuni, L.; Girard-Buttoz, C.; León, J.; Zuberbühler, K.; Tkaczynski, P.; Wittig, R.M.; Crockford, C. Social uncertainty promotes signal complexity during approaches in wild chimpanzees (Pan troglodytes verus) and mangabeys (Cercocebus atys aty). R. Soc. Open Sci. 2023, 10, 231073. [Google Scholar] [CrossRef]
- Peckre, L.; Kappeler, P.M.; Fichtel, C. Clarifying and expanding the social complexity hypothesis for communicative complexity. Behav. Ecol. Sociobiol. 2019, 73, 11. [Google Scholar] [CrossRef]
- Zhu, B.C.; Zhao, X.M.; Zhang, H.D.; Wang, J.C.; Cui, J.G. The functions and evolution of graded complex calls in a treefrog. Bioacoust.-Int. J. Anim. Sound Its Rec. 2023, 32, 642–659. [Google Scholar] [CrossRef]
- López, B.D. Context-dependent and seasonal fluctuation in bottlenose dolphin (Tursiops truncatu) vocalizations. Anim. Cogn. 2022, 25, 1381–1392. [Google Scholar] [CrossRef]
- Weir, L.K.; Grant, J.W.A.; Hutchings, J.A. The influence of operational sex ratio on the intensity of competition for mates. Am. Nat. 2011, 177, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, P. Correlates of hoot rate and structure in male Tawny Owls Strix aluco: Implications for male rivalry and female mate choice. J. Avian Biol. 1998, 29, 25–32. [Google Scholar] [CrossRef]
- Koch, R.E.; Krakauer, A.H.; Patricelli, G.L. Investigating female mate choice for mechanical sounds in the male Greater Sage-Grouse. Auk 2015, 132, 349–358. [Google Scholar] [CrossRef]
- Pellitteri-Rosa, D.; Sacchi, R.; Galeotti, P.; Marchesi, M.; Fasola, M. Courtship displays are condition-dependent signals that reliably reflect male quality in Greek tortoises, Testudo graeca. Chelonian Conserv. Biol. 2011, 10, 10–17. [Google Scholar] [CrossRef]
- Singer, F.; Riechert, S.E.; Xu, H.F.; Morris, A.W.; Becker, E.; Hale, J.A.; Noureddine, M.A. Analysis of courtship success in the funnel-web spider Agelenopsis aperta. Behaviour 2000, 137, 93–117. [Google Scholar] [CrossRef]
- Smith, C.L.; Van Dyk, D.A.; Taylor, P.W.; Evans, C.S. On the function of an enigmatic ornament: Wattles increase the conspicuousness of visual displays in male fowl. Anim. Behav. 2009, 78, 1433–1440. [Google Scholar] [CrossRef]
- Milner, R.N.C.; Jennions, M.D.; Backwell, P.R.Y. Keeping up appearances: Male fiddler crabs wave faster in a crowd. Biol. Lett. 2011, 8, 176–178. [Google Scholar] [CrossRef]
- Perry, A.C. Behavioral tactics on the lek: Manipulating and modeling the social environment of courtship in greater sage-grouse (Centrocercus urophasianus). Ph.D. Thesis, University of California, Davis, CA, USA, 2017. [Google Scholar]
- Sevastianov, N.; Neretina, T.; Vedenina, V. Evolution of calling songs in the grasshopper subfamily Gomphocerinae (Orthoptera, Acrididae). Zool. Scr. 2023, 52, 154–175. [Google Scholar] [CrossRef]
- O’Bryant, E.L.; Wade, J. Sexual dimorphism in neuromuscular junction size on a muscle used in courtship by green anole lizards. J. Neurobiol. 2002, 50, 24–30. [Google Scholar] [CrossRef]
- Batabyal, A.; Thaker, M. Lizards assess complex social signals by lateralizing colour but not motion detection. J. Exp. Biol. 2018, 221, jeb173252. [Google Scholar] [CrossRef]
- Jun, W.U.; XiaoGuo, J.; Jian, C.; Yu, P.; FengXiang, L.I.U. Courtship and mating behaviors of the wolf spider Pardosa astrigera. Chin. J. Zool. 2008, 43, 9–12. [Google Scholar]
- Vicente, N.S. Headbob displays signal sex, social context and species identity in a Liolaemus lizard. Amphib.-Reptil. 2018, 39, 203–218. [Google Scholar] [CrossRef]
- Gross, M.R. The evolution of parental care. Q. Rev. Biol. 2005, 80, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Trivers, R.L. Parental investment and sexual selection. In Sexual Selection and the Descent of Man; Routledge: London, UK, 2017; pp. 136–179. [Google Scholar]
- Otte, D. Effects and functions in the evolution of signaling systems. Annu. Rev. Ecol. Syst. 1974, 5, 385–417. [Google Scholar] [CrossRef]
- Buchanan, K.L.; Buchanan, K.L. Stress and the evolution of condition-dependent signals. Trends Ecol. Evol. 2000, 15, 156–160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).