Defecation Site Preferences and Spatial Ecological Segregation of Forest Musk Deer and Siberian Roe Deer in North China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
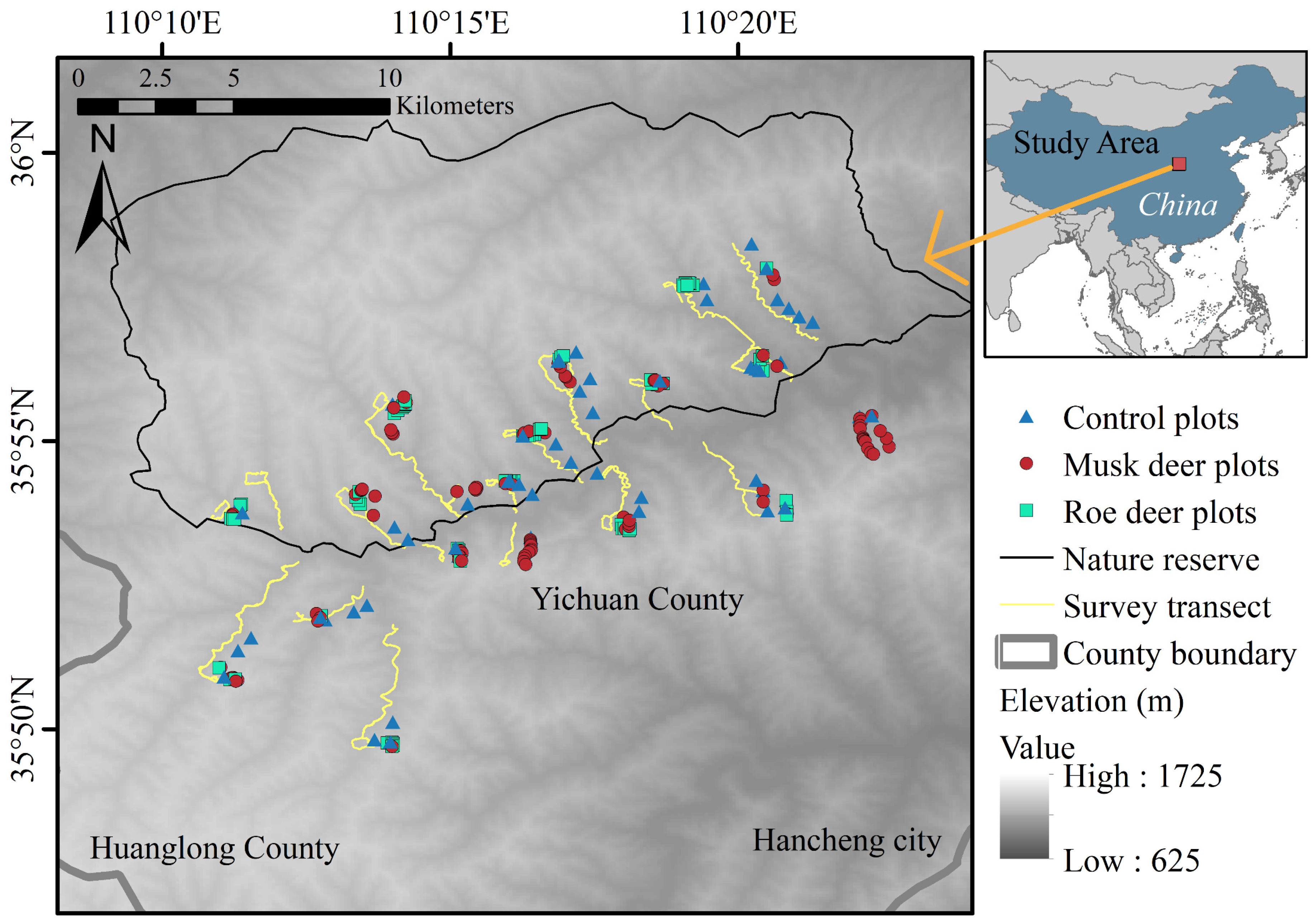
2.1. Study Area
2.2. Field Survey and Data Collection
2.3. Species Identification
2.4. Data Analysis
3. Results
3.1. Species Identification and Number of Valid Sample Plots
3.2. Model Analysis of Defecation Sites for Forest Musk Deer and Roe Deer
3.3. Spatial Utilization Differences Between Forest Musk Deer and Siberian Roe Deer
4. Discussion
4.1. The Impact of Abiotic Factors on the Defecation Site Preferences of Forest Musk Deer and Siberian Roe Deer
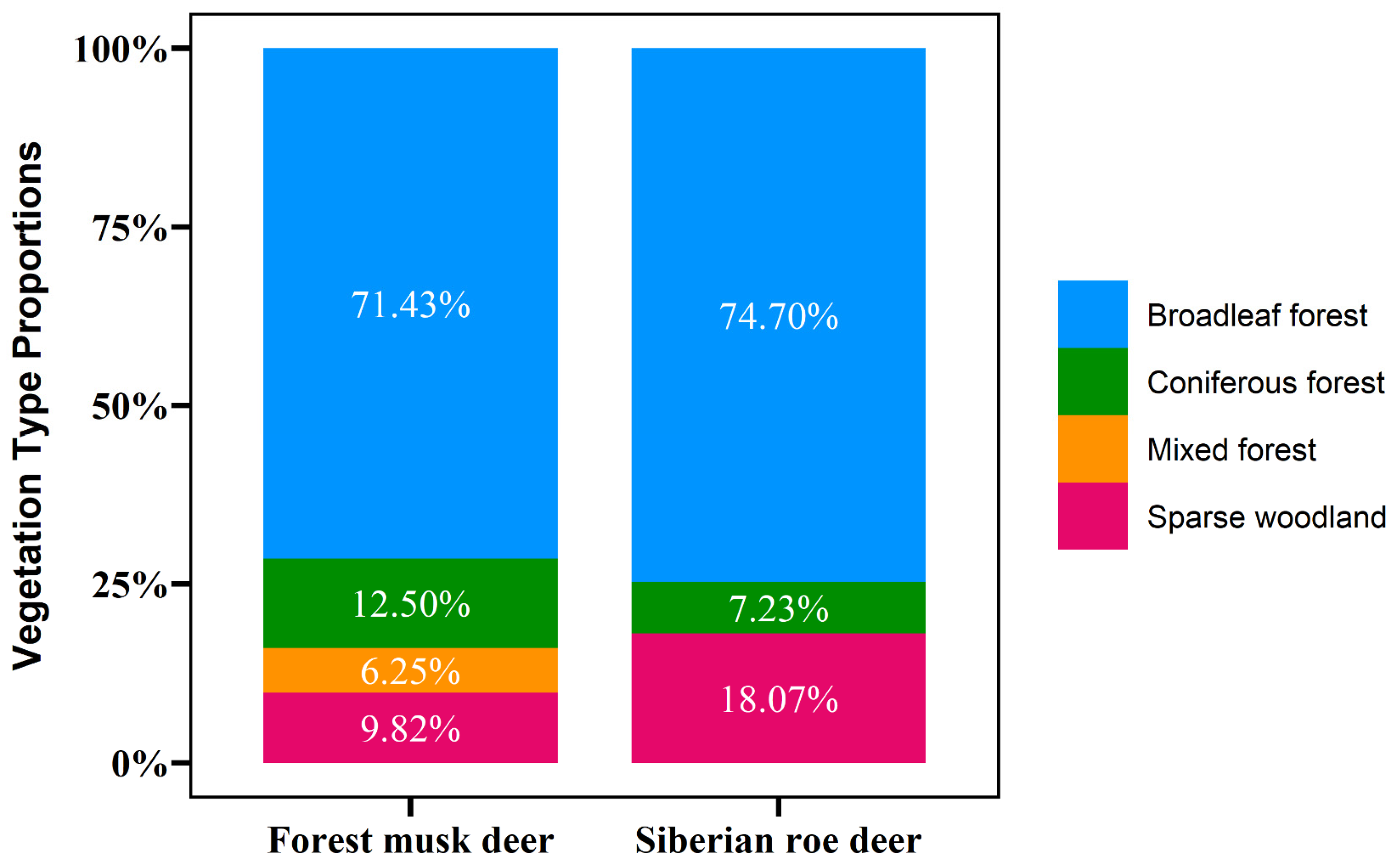
4.2. Influence of Biotic Factors on Forest Musk Deer and Siberian Roe Deer Defecation Site Preferences
4.3. Spatial Ecological Segregation Between Forest Musk Deer and Siberian Roe Deer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, R. Principles of Animal Ecology; Beijing Normal University Press: Beijing, China, 1987. [Google Scholar]
- Davies, J.T.; Meiri, S.; Barraclough, T.G.; Gittleman, J.L. Species Co-Existence and Character Divergence across Carn12ivores. Ecol. Lett. 2007, 10, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.A.; Matsuda, H. Positive Indirect Effects Between Prey Species That Share Predators. Ecology 1996, 77, 610–616. [Google Scholar] [CrossRef]
- Latham, J. Interspecific Interactions of Ungulates in European Forests: An Overview. For. Ecol. Manag. 1999, 120, 13–21. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Qu, Y.; He, J.; Liu, K.; Xue, H.; Cui, P.; Ruan, X.; Wu, H. Camera Trap Reveals the Co-Occurrence Patterns of Two Sympatric Muntjac Species in Southern Anhui Province, China: No Spatial Segregation. Ecol. Evol. 2021, 11, 17801–17809. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Hu, L.; Zhao, S.; Dong, X.; Feng, W.; Zhang, D.; Zhang, J.; Zhou, C.; Bai, W. Spatiotemporal Ecological Niche Characteristics of the Tibetan Antelope (Pantholops hodgsonii) and the Sichuan Goral (Naemorhedus griseus) in Their Sympatric Distribution. Acta Ecol. Sin. 2022, 42, 5275–5284. (In Chinese) [Google Scholar]
- Liu, W.; Li, X.; Li, Z.; Li, Y.; You, Z.; Jiang, Y.; Ruan, G.; Lu, B.; Yang, N. Spatio-temporal Distribution and Overlap of the Sichuan Goral and the Tibetan Antelope on Mount Gongga, Sichuan, China. J. Appl. Ecol. 2023, 34, 1630–1638. (In Chinese) [Google Scholar]
- Wang, Y.-X. IUCN Red List of Threatened Species: Moschus berezovskii. IUCN Red List of Threatened Species 2015. Available online: https://www.iucnredlist.org/species/13894/103431781 (accessed on 17 December 2024).
- Wu, J.Y.; Wang, W. Chinese Musk Deer; China Forestry Publishing House: Beijing, China, 2006. [Google Scholar]
- Feng, H.; Wang, L.; Cao, F.; Ma, J.; Tang, J.; Feng, C.; Su, Z. Forest Musk Deer (Moschus berezovskii) in China: Research and Protection. J. Vertebr. Biol. 2023, 72, 1–13. [Google Scholar] [CrossRef]
- Lovari, S.; Masseti, M.; Lorenzini. IUCN Red List of Threatened Species: Capreolus pygargus. IUCN Red List of Threatened Species 2015. Available online: https://www.iucnredlist.org/species/42396/22161884 (accessed on 17 December 2024).
- Cao, K.Q. Chinese Deer Species. East China Normal University Press: Shanghai, Chinia, 1992. [Google Scholar]
- Jiang, Z.G. Diversity and Geographical Distribution of Chinese Mammals; Science Press: Beijing, China, 2015. [Google Scholar]
- Ohtaishi, N.; Gao, Y. A Review of the Distribution of All Species of Deer (Tragulidae, Moschidae and Cervidae) in China. Mammal Rev. 1990, 20, 125–144. [Google Scholar] [CrossRef]
- Sheng, H.L. Current status of musk deer resources in China and rescue measures. Wildlife 1996, 3, 10–12. (In Chinese) [Google Scholar] [CrossRef]
- Green, M.J.B. The Distribution, Status and Conservation of the Himalayan Musk Deer Moschus chrysogaster. Biol. Conserv. 1986, 35, 347–375. [Google Scholar] [CrossRef]
- Brashares, J.S.; Arcese, P. Scent Marking in a Territorial African Antelope: II. Econ. Marking Faeces. Anim. Behav. 1999, 57, 11–17. [Google Scholar] [CrossRef]
- Singh, P.B.; Saud, P.; Cram, D.; Mainali, K.; Thapa, A.; Chhetri, N.B.; Poudyal, L.P.; Baral, H.S.; Jiang, Z. Ecological Correlates of Himalayan Musk Deer Moschus leucogaster. Ecol. Evol. 2019, 9, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Gosling; Wright, K.H.M. Scent Marking and Resource Defence by Male Coypus (Myocastor coypus). J. Zool. 1994, 234, 423–436. [Google Scholar] [CrossRef]
- Boero, D.L. Scent-Deposition Behaviour in Alpine Marmots (Marmota marmota L.): Its Role in Territorial Defence and Social Communication. Ethology 1995, 100, 26–38. [Google Scholar] [CrossRef]
- Gosling; Atkinson, N.W.; Dunn, S.; Collins, S.A. The Response of Subordinate Male Mice to Scent Marks Varies in Relation to Their Own Competitive Ability. Anim. Behav. 1996, 52, 1185–1191. [Google Scholar] [CrossRef]
- Putman, R.J. Facts from Faeces. Mammal Rev. 1984, 14, 79–97. [Google Scholar] [CrossRef]
- Ralls, K. Mammalian Scent Marking. Science 1971, 171, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Dhami, B.; Bhusal, A.; Neupane, B.; Kc, N.; Lamichhane, S.; Bhattarai, D.; Shrestha, B. Fine-Scale Habitat Characteristics Influence the Winter Habitat Use of Swamp Deer (Rucervus duvaucelii) in Shuklaphanta National Park, Nepal. Glob. Ecol. Conserv. 2023, 47, e02648. [Google Scholar] [CrossRef]
- Zou, S.J.; Song, Y.C.; Yang, D.D.; Li, P.F. Microhabitat selection of winter bedding sites for the Père David’s deer in Shishou Père David’s Deer National Nature Reserve, Hubei, China. Ecol. J. 2013, 32, 899–904. (In Chinese) [Google Scholar] [CrossRef]
- Singh, P.B.; Shrestha, B.B.; Thapa, A.; Saud, P.; Jiang, Z. Selection of Latrine Sites by Himalayan Musk Deer (Moschus leucogaster) in Neshyang Valley, Annapurna Conservation Area, Nepal. J. Appl. Anim. Res. 2018, 46, 920–926. [Google Scholar] [CrossRef]
- Zha, M.H.; Chen, L.M.; Yang, S.; Xu, S.H.; Guo, X.B.; Zhang, B.F.; Hu, D.F. Preference of defecation sites by forest musk deer in Tangjiahe National Nature Reserve. Chin. J. Zool. 2019, 54, 484–492. (In Chinese) [Google Scholar] [CrossRef]
- Hemami, M.-R.; Watkinson, A.R.; Dolman, P.M. Population Densities and Habitat Associations of Introduced Muntjac Muntiacus Reevesi and Native Roe Deer Capreolus capreolus in a Lowland Pine Forest. For. Ecol. Manag. 2005, 215, 224–238. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary Steps of Ecophysiological Adaptation and Diversification of Ruminants: A Comparative View of Their Digestive System. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, J.; Gao, H.; Cai, Z.; Zhou, X.; Li, S.; Zhang, T. Musk Deer (Moschus spp.) Face Redistribution to Higher Elevations and Latitudes under Climate Change in China. Sci. Total Environ. 2020, 704, 135335. [Google Scholar] [CrossRef] [PubMed]
- Argunov, A.V. Formation of the Range of the Siberian Roe Deer (Capreolus pygargus, Cervidae) and Its Present Distribution in Yakutia. Biol. Bull. 2013, 40, 692–697. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, Y.; Bian, K.; Tang, J.; Wang, W.F.; Guo, L.W.; Wang, B.; Fang, G.; Zhao, L.; Qi, X.G. Home Range Use and Individual Migration of Reintroduced Sika Deer. Acta Theriol. Sin. 2020, 40, 109–119. (In Chinese) [Google Scholar] [CrossRef]
- Zaitsev, V.; Maksimova, D.; Smirnov, Y.; Belotelov, N. Home Range Use by a Male Siberian Musk Deer (Moschus Moschiferus L.) Cent. Sikhote-Alin. Biol. Bull. 2021, 100, 462–480. [Google Scholar]
- Teng, Y.; Zhang, S.; Saihan; Han, Z.Q.; Bao, W.D. Home Range Dynamics of Siberian Roe Deer in Saihanwula Nature Reserve. J. Beijing For. Univ. 2021, 43, 73–82. (In Chinese) [Google Scholar] [CrossRef]
- Luo, C.; Xu, W.H.; Zhou, Z.X.; Ouyang, Z.Y.; Zhang, L. Habitat Prediction of Sika Deer in the Qinling Mountains Based on Ecological Niche Models. Acta Ecol. Sin. 2011, 31, 1221–1229. (In Chinese) [Google Scholar]
- Wang, A.; Wen, D.X.; Zhou, Y.Y.; Yu, G.Q.; Chen, J.; Peng, D.Y.; Huang, T.F.; Tian, S.R.; Xiao, F. Habitat Suitability Evaluation for Sika Deer in Hunan Province Based on the MaxEnt Model and ArcGIS Software. Hunan For. Sci. Technol. 2024, 51, 43–49. (In Chinese) [Google Scholar] [CrossRef]
- Tang, J.; Xia, J.; Zhang, B.; Yang, C.; Suo, L.J.; Bian, K.; Li, F.R.; Wang, Y. Development of a Management System for Sika Deer Based on the NET Framework. J. Chin. Herbiv. Anim. Sci. 2021, 41, 56–61+81. (In Chinese) [Google Scholar] [CrossRef]
- Eom, T.-K.; Lee, J.-K.; Lee, D.-H.; Ko, H.; Rhim, S.-J. Adaptive Response of Siberian Roe Deer Capreolus pygargus to Climate and Altitude in the Temperate Forests of South Korea. Wildlife Biol. 2023, 2023, e01138. [Google Scholar] [CrossRef]
- Feng, H.; Li, Y.; Li, Y.; Li, N.; Li, Y.; Hu, Y.; Yu, J.; Luo, H. Identifying and Evaluating the Ecological Network of Siberian Roe Deer (Capreolus pygargus) in Tieli Forestry Bureau, Northeast China. Glob. Ecol. Conserv. 2021, 26, e01477. [Google Scholar] [CrossRef]
- Mori, E.; Cicero, M.; Lovari, S.; Zaccaroni, M.; Salomoni, S.; Vendramin, A.; Augugliaro, C. Occupancy and Activity Rhythms of the Siberian Roe Deer. Biologia 2021, 76, 3001. [Google Scholar] [CrossRef]
- Stepanova, V.V.; Argunov, A.V. Spatiotemporal Dynamics of Geographical Ranges of Red Deer (Cervus elaphus, Cervidae) and Siberian Roe Deer (Capreolus pygargus, Cervidae) in Yakutia. Russ. J. Ecol. 2016, 47, 62–67. [Google Scholar] [CrossRef]
- Huang, X.; Mo, Z.; Li, M.; Zhang, L.; Wan, D.; Jiang, Y. Evaluation of habitat suitability for Capreolus pygargus in the eastern Yanshan Mountains based on MaxEnt model. Acta Theriol. Sin. 2024, 44, 478–488. (In Chinese) [Google Scholar] [CrossRef]
- Xiang, R.; Da, Z.; Wu, J.; Bu, X.; Wang, J.; Lu, Q.; Hao, Y.; Sheng, Y.; Meng, X. Summer Habitat Preferences of Roe Deer (Capreolus pygargus) in the Mountainous Areas Surrounding Beijing. J. Ecol. 2021, 40, 3252–3258. (In Chinese) [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Zhen, D.; Bu, X.; Xiang, R.; Shrestha, T.; Sheng, Y.; Meng, X. Summer Habitat Use and Coexistence of Chinese Goral (Naemorhedus griseus) and Siberian Roe Deer (Capreolus pygargus) in Taihang Mountain, China. North-West. J. Zool. 2022, 18, 77–86. (In Chinese) [Google Scholar]
- Eom, T.-K.; Lee, J.-K.; Lee, D.-H.; Ko, H.; Kim, J.-H.; Rhim, S.-J. Assessing Scale-Dependent Effects of Resource Availability on the Habitat Selection of Siberian Roe Deer (Capreolus pygargus) Using a Mixture Model for the Fecal Pellet Count. Hystrix-Ital. J. Mammal. 2023, 34, 98–104. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Tan, D.D.; Li, W.Z.; Wang, Q.; Wang, G.R. Spatial Distribution Pattern and Association of Main Tree Species in Mingled Forest of Pinus tabuliformis + Quercus liaotungensis in Huanglong Mountain. J. Northwest AF Univ. Nat. Sci. 2015, 43, 113–120. (In Chinese) [Google Scholar] [CrossRef]
- Xie, C.J.; Xue, X.F.; Wen, Y.L.; Lei, F.; Pang, Y.H.; Fan, S.Q. Current Status and Conservation Strategies of Wild Flora and Fauna Resources in the Huanglong Mountain Nature Reserve, Hancheng, Shaanxi. Shaanxi For. Sci. Technol. 2014, 76, 52–54. (In Chinese) [Google Scholar]
- Roberts, D.W.; Cooper, S.V. Concepts and techniques of vegetation mapping. In Land Classifications Based on Vegetation: Applications for Resource Management; US Department of Agriculture: Washington, DC, USA, 1989; pp. 90–96. [Google Scholar]
- Piggott, M.; Taylor, A. Extensive Evaluation of Faecal Preservation and DNA Extraction Methods in Australian Native and Introduced Species. Aust. J. Zool. 2003, 51, 341–355. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.J.; Wang, B.; Suo, L.J.; Li, F.R.; Liu, W.H.; Wang, Y.; Yan, X.R. Two Methods for DNA Extraction from Musk Deer Faeces. J. Northwest AF Univ. Agric. Sci. Technol. 2018, 27, 326–330. (In Chinese) [Google Scholar] [CrossRef]
- Feng, H.; Huang, Y.; Ren, Y.; Feng, C.L.; Liu, X.N. mtDNA D-loop Region Structure and Genetic Diversity of Musk Deer in Shaanxi Province, China. Acta Ecol. Sin. 2014, 34, 5887–5895. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Lu, G.; Gao, Y.; Yan, L.; Li, M.; Hu, D.; Zhang, D. mtDNA CR Evidence Indicates High Genetic Diversity of Captive Forest Musk Deer in Shaanxi Province, China. Animals 2023, 13, 2191. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical; Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to Account for Spatial Autocorrelation in the Analysis of Species Distributional Data: A Review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Boomsma, A. Regression Diagnostics with R; University of Groningen: Groningen, The Netherlands, 2014. [Google Scholar]
- Bates, J.E.; Dhakal, S.; Mazloom, A.; Constine, L.S. The Benefit of Adjuvant Radiotherapy in High-Grade Nonmetastatic Retroperitoneal Soft Tissue Sarcoma: A SEER Analysis. Am. J. Clin. Oncol. 2018, 41, 274. [Google Scholar] [CrossRef] [PubMed]
- Fieberg, J.; Matthiopoulos, J.; Hebblewhite, M.; Boyce, M.S.; Frair, J.L. Correlation and Studies of Habitat Selection: Problem, Red Herring or Opportunity? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2233–2244. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.7.2. 2012. Available online: http://CRAN.R-project.org/package=MuMIn (accessed on 17 October 2024).
- Feizabadi, H.A.; Ashrafi, S.; Hemami, M.R.; Ahmadi, M.; Naderi, M. Mesocarnivores Den Site Selection in Arid Ecosystems; A Case Study of Rüppell’s Fox and Sand Cat in Central Iran. Glob. Ecol. Conserv. 2024, 49, e02793. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. Glmm.Hp: An R Package for Computing Individual Effect of Predictors in Generalized Linear Mixed Models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Mykytowycz, R.; Hesterman, E.R.; Gambale, S.; Dudziński, M.L. A Comparison of the Effectiveness of the Odors of Rabbits, Oryctolagus cuniculus, in Enhancing Territorial Confidence. J. Chem. Ecol. 1976, 2, 13–24. [Google Scholar] [CrossRef]
- Jiang, G.; Ma, J.; Zhang, M.; Stott, P. Assessing Microhabitat Use by Roe Deer and Moose in China. Wildl. Res. 2009, 36, 134. [Google Scholar] [CrossRef]
- Khadka, K.K.; James, D.A. Habitat Selection by Endangered Himalayan Musk Deer (Moschus chrysogaster) and Impacts of Livestock Grazing in Nepal Himalaya: Implications for Conservation. J. Nat. Conserv. 2016, 31, 38–42. [Google Scholar] [CrossRef]
- Sheng, H.; Liu, Z.X. China’s Musk Deer Species; Shanghai Scientific and Technical Publishing House: Shanghai, China, 2007; ISBN 978-7-5323-8794-6. [Google Scholar]
- Hu, Z.J.; Wang, Y.; Xue, W.J.; Jiang, H.R.; Xu, H.F. Winter Habitat Selection of Forest Musk Deer in Ziba Mountain Nature Reserve. Henan Univ. J. Nat. Sci. 2006, 1, 70–74. (In Chinese) [Google Scholar] [CrossRef]
- Yang, C.; Ma, G.; Meng, X.X.; Xu, H.F. Summer Habitat Characteristics of Forest Musk Deer in Liangshan Mountain Range. Ecol. J. 2011, 30, 18–23. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, H.R.; Xue, W.J.; Wang, Y.; Hu, Z.J.; Xiao, Y.; Xu, H.F. Preliminary Analysis of Spring Habitat of Musk Deer in Feng County, Shaanxi. Sichuan J. Anim. 2008, 1, 115–119. (In Chinese) [Google Scholar]
- Tian, X.M.; Ji, X.Y.; Wang, X.X.; Zhang, Z.D.; Chen, H.; Liu, X.H.; Liu, L.; Wang, Q.F.; Ma, Y.H.; Qian, X.Y.; et al. Habitat Suitability and Activity Rhythm of Roe Deer in the Arctic Village National Nature Reserve. Acta Ecol. Sin. 2024, 44, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Qi, Y.Z.; Huang, B.X.; Zhai, P.H.; Li, Y.; Bao, H.; Jiang, G.S. Effects of Fire Disturbance on Population Density and Habitat Use of Co-occurring Cervid Species in the Greater Khingan Mountains. Acta Ecol. Sin. 2024, 23, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Srivastava, T.; Kumar, A.; Kumar, V.; Umapathy, G. Diet Drives Differences in Reproductive Synchrony in Two Sympatric Mountain Ungulates in the Himalaya. Front. Ecol. Evol. 2021, 9, 647465. [Google Scholar] [CrossRef]
- Pei, C.; Li, Y.X.; Luo, Z.W.; Hai, L.Y.; Lan, X.N.; Hu, D.F. Spring Dietary and Ecological Separation Between Forest Musk Deer and Roe Deer in the Lüliang Mountain Range. Ecol. J. 2024, 1–16. Available online: http://kns.cnki.net/kcms/detail/21.1148.Q.20241023.0948.002.html (accessed on 17 October 2024).
- Khadka, K.K.; Singh, N.; Magar, K.T.; James, D.A. Dietary Composition, Breadth, and Overlap between Seasonally Sympatric Himalayan Musk Deer and Livestock: Conservation Implications. J. Nat. Conserv. 2017, 38, 30–36. [Google Scholar] [CrossRef]
- Teng, L.W.; Liu, Z.S.; Zhang, E.D.; Ma, J.Z. Winter Bedsite Selection of Roe Deer in the Southern Lesser Khingan Mountains. Ecol. J. 2007, 213–218. (In Chinese) [Google Scholar] [CrossRef]
- Argunov, A.; Stepanova, V. Diet Structure of the Siberian Roe Deer in Yakutia. Russ. J. Ecol. 2011, 42, 161–164. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, R.; He, L.; Liu, S.; Zhou, J.; Qi, L.; Li, L.; Hu, D. The Progress in Nutrition Research of Musk Deer: Implication for Conservation. Appl. Anim. Behav. Sci. 2015, 172, 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.H.; Hu, Y.M.; Xiong, Z.P.; Wu, W.; Li, Y. Habitat Selection by Roe Deer (Capreolus pygargus) over Winter in the Tieli Forestry Bureau of the Lesser Xing’an Mountains. Biodivers. Sci. 2017, 25, 401–408. (In Chinese) [Google Scholar] [CrossRef]

| Habitat Variable | Data Description |
|---|---|
| Elevation (m) (E) | The elevation of the center of the 10 × 10 m plot |
| Slope (°) (S) | The slope of the 10 × 10 m plot |
| Aspect (A) | The aspect of the 10 × 10 m plot |
| Slope Position (SP) | The slope position of the 10 × 10 m plot |
| Covertness (m) (C) | A 1-m pole was placed at the quadrat center. Researchers walked in four cardinal directions until the pole was no longer visible, measured the distances, and calculated the average. |
| Tree Coverage (T_C) | The percentage of ground covered by the upper canopy of vegetation in the 10 × 10 m plot |
| Tree Diversity (T_DI) | The number of tree species in the 10 × 10 m plot |
| Tree Density (T_DE) | The total number of trees in the 10 × 10 m plot |
| Average Tree Height (m) (ATH) | The average height of trees in the 10 × 10 m plot |
| Average diameter at breast height of trees (cm) (DBH) | The average diameter at breast height (DBH) of trees in the 10 × 10 m plot |
| Diameter at Breast Height: greater than 30 cm (Dbhg30) | The number of trees with a DBH greater than 30 cm in the 10 × 10 m plot |
| Shrub Diversity (S_DI) | The number of shrub species in the 5 × 5 m plot |
| Shrub Density (S_DE) | The total number of shrubs in the 5 × 5 m plot |
| Shrub Canopy (S_C) | The percentage of ground area covered by the projection of shrub canopies in the 5 × 5 m plot |
| Average Shrub Height (m) (ASH) | The average height of shrubs in the 5 × 5 m plot |
| Herbal Diversity (HD) | The number of herbaceous species across four 1 × 1 m plots |
| Average Herbaceous Cover (AHC) | The average cover of herbaceous plants across four 1 × 1 m plots |
| Average Herb Height (m) (AHH) | The average height of herbaceous plants across four 1 × 1 m plots |
| Models | Variables | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|---|
| 1 | T_DI + S + E + ASH + AHC | 7 | −122.5114 | 259.5342 | 0.0000 | 0.0579 |
| 2 | T_DI + ATH + S + E + ASH +AHC | 8 | −121.5188 | 259.6981 | 0.1639 | 0.0533 |
| 3 | T_DI + ATH + S+ E + ASH + AHC+ AHH | 9 | −120.5267 | 259.8829 | 0.3487 | 0.0486 |
| 4 | T_DI + S + E + ASH + AHC + AHH | 8 | −121.6952 | 260.0510 | 0.5168 | 0.0447 |
| 5 | T_DI + A+ S + E + ASH + AHC | 8 | −121.7049 | 260.0704 | 0.5363 | 0.0443 |
| 6 | T_DI + S+ E + AHC | 6 | −123.8530 | 260.0879 | 0.5537 | 0.0439 |
| 7 | T_DI + A + S + E + ASH + AHC + AHH | 9 | −120.7451 | 260.3196 | 0.7855 | 0.0391 |
| Variables | Estimate | Std. Error | Z Value | Pr (>|z|) | I.perc (%) |
|---|---|---|---|---|---|
| (Intercept) | 6.575242 | 2.818556 | 2.333 | 0.01966 * | |
| S | 0.048079 | 0.017765 | 2.706 | 0.00680 ** | 15.79 |
| A | 0.674537 | 0.591218 | 1.141 | 0.25390 | 1.38 |
| E | −0.005199 | 0.002095 | −2.482 | 0.01308 * | 4.26 |
| T_DI | 0.529648 | 0.186624 | 2.838 | 0.00454 ** | 15.64 |
| ATH | −0.088258 | 0.071005 | −1.243 | 0.21387 | 3.85 |
| ASH | −0.470024 | 0.242346 | −1.939 | 0.05244 | 5.41 |
| AHC | −2.556200 | 1.148427 | −2.226 | 0.02603 * | 19.93 |
| AHH | −4.005994 | 2.800626 | −1.430 | 0.15260 | 33.73 |
| Models | Variables | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|---|
| 1 | E + AHC | 4 | −120.1152 | 248.4106 | 0.0000 | 0.0944 |
| 2 | E + AHC + C | 5 | −119.2310 | 248.7334 | 0.3228 | 0.0804 |
| 3 | E + S_DI + AHC | 5 | −119.3063 | 248.8841 | 0.4735 | 0.0745 |
| 4 | ATH +E + AHC | 5 | −119.3694 | 249.0103 | 0.5997 | 0.0700 |
| 5 | SP + E + AHC + C | 6 | −118.4382 | 249.2582 | 0.8476 | 0.0618 |
| Variables | Estimate | Std. Error | Z Value | Pr (>|z|) | I.perc (%) |
|---|---|---|---|---|---|
| (Intercept) | −9.530762 | 2.957765 | −3.222 | 0.001272 ** | |
| SP | −0.313664 | 0.208598 | −1.504 | 0.132665 | 10.42 |
| E | 0.008789 | 0.002306 | 3.812 | 0.000138 *** | 54.63 |
| ATH | −0.051760 | 0.062103 | −0.833 | 0.404586 | 1.78 |
| S_DI | 0.100891 | 0.104420 | 0.966 | 0.333940 | 0.62 |
| C | −0.016903 | 0.019613 | −0.862 | 0.388787 | 3.24 |
| AHC | −2.806144 | 0.917925 | −3.057 | 0.002235 ** | 29.31 |
| Variables | Mean ± SD | Statistic | p | ||
|---|---|---|---|---|---|
| Total (n = 227) | Forest Musk Deer (n = 100) | Siberian Roe Deer (n = 71) | |||
| T_DE | 9.55 ± 4.60 | 10.38 ± 3.73 | 9.73 ± 4.41 | t = 1.04 | 0.301 |
| E (m) | 1194.09 ± 147.03 | 1179.99 ± 141.24 | 1265.38 ± 134.64 | Z = −3.63 | <0.001 *** |
| A | 0.47 ± 0.34 | 0.47 ± 0.34 | 0.45 ± 0.36 | Z = −0.06 | 0.955 |
| S (°) | 24.11 ± 13.36 | 26.46 ± 13.15 | 27.37 ± 12.05 | Z = −0.71 | 0.476 |
| C (m) | 15.72 ± 13.06 | 13.98 ± 9.41 | 14.40 ± 9.65 | Z = −0.17 | 0.868 |
| T_DI | 2.75 ± 1.35 | 3.18 ± 1.28 | 2.69 ± 1.14 | Z = −2.33 | 0.020 * |
| TC | 0.68 ± 0.25 | 0.73 ± 0.18 | 0.69 ± 0.23 | Z = −0.84 | 0.403 |
| ATH (m) | 11.62 ± 3.97 | 12.77 ± 2.73 | 11.68 ± 3.26 | Z = −2.31 | 0.021 * |
| DBH (cm) | 22.70 ± 7.55 | 24.43 ± 5.37 | 23.46 ± 6.02 | Z = −0.59 | 0.556 |
| DBHG30 | 1.68 ± 1.68 | 1.84 ± 1.78 | 1.66 ± 1.64 | Z = −0.56 | 0.573 |
| S_DI | 5.21 ± 2.31 | 4.97 ± 1.79 | 4.97 ± 1.76 | Z = −0.18 | 0.857 |
| S_DE | 48.81 ± 47.29 | 43.73 ± 36.42 | 46.68 ± 38.93 | Z = −0.31 | 0.760 |
| SC | 0.37 ± 0.27 | 0.34 ± 0.25 | 0.36 ± 0.23 | Z = −0.64 | 0.520 |
| ASH (m) | 2.24 ± 0.84 | 2.14 ± 0.82 | 2.31 ± 0.78 | Z = −0.74 | 0.459 |
| HD | 7.31 ± 5.41 | 6.51 ± 3.82 | 5.17 ± 4.18 | Z = −2.84 | 0.005 ** |
| AHC | 0.29 ± 0.31 | 0.20 ± 0.20 | 0.17 ± 0.19 | Z = −1.47 | 0.141 |
| AHH (m) | 0.18 ± 0.15 | 0.14 ± 0.07 | 0.14 ± 0.09 | Z = −0.15 | 0.880 |
| SP | 2.47 ± 1.17 | 2.53 ± 1.04 | 2.87 ± 1.26 | Z = −2.00 | 0.046 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hai, L.; Luo, P.; Zheng, W.; Jin, X.; Liu, J.; Wang, H.; Hu, D. Defecation Site Preferences and Spatial Ecological Segregation of Forest Musk Deer and Siberian Roe Deer in North China. Animals 2025, 15, 61. https://doi.org/10.3390/ani15010061
Li Y, Hai L, Luo P, Zheng W, Jin X, Liu J, Wang H, Hu D. Defecation Site Preferences and Spatial Ecological Segregation of Forest Musk Deer and Siberian Roe Deer in North China. Animals. 2025; 15(1):61. https://doi.org/10.3390/ani15010061
Chicago/Turabian StyleLi, Yixin, Luyao Hai, Pengfei Luo, Wangshan Zheng, Xuelin Jin, Jiangcheng Liu, Haiyan Wang, and Defu Hu. 2025. "Defecation Site Preferences and Spatial Ecological Segregation of Forest Musk Deer and Siberian Roe Deer in North China" Animals 15, no. 1: 61. https://doi.org/10.3390/ani15010061
APA StyleLi, Y., Hai, L., Luo, P., Zheng, W., Jin, X., Liu, J., Wang, H., & Hu, D. (2025). Defecation Site Preferences and Spatial Ecological Segregation of Forest Musk Deer and Siberian Roe Deer in North China. Animals, 15(1), 61. https://doi.org/10.3390/ani15010061




