Terpinen-4-ol Improves the Intestinal Barrier Function of the Colon in Immune-Stressed Weaning Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals and Experimental Design
2.3. Sample Collection
2.4. Histological Study
2.5. Goblet Cell Number Analysis
2.6. Immunohistochemical Analysis
2.7. Determination of the Levels of Antioxidant Indicators
2.8. Cytokine Level Analysis by ELISA
2.9. mRNA Expression Analysis by Real-Time PCR
2.10. Analysis of the 16S rDNA Microbiota in the Colon Contents
2.11. Statistical Analysis
3. Results
- Study Number of goblet cells and expression of MUC2
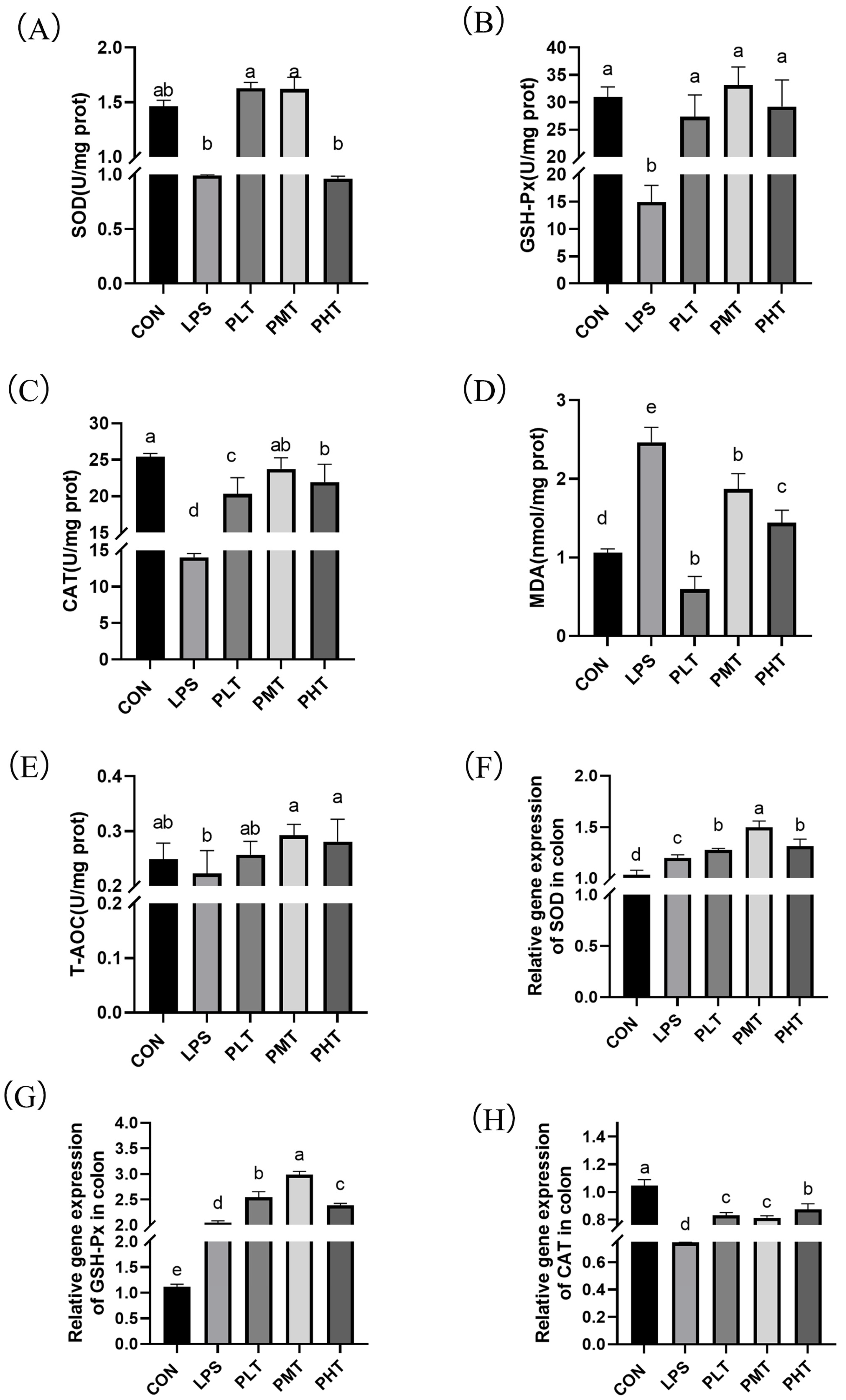
- Gene expression and antioxidant enzyme activity
- Cytokine content and gene expression
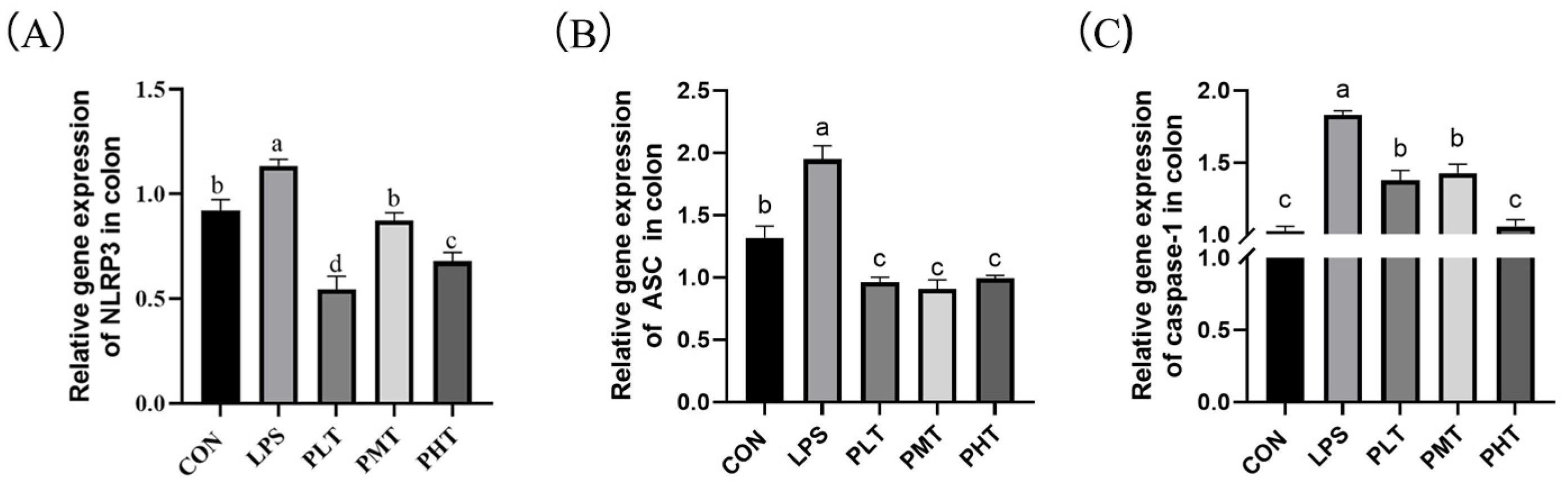
- NLRP3 expression in the colon
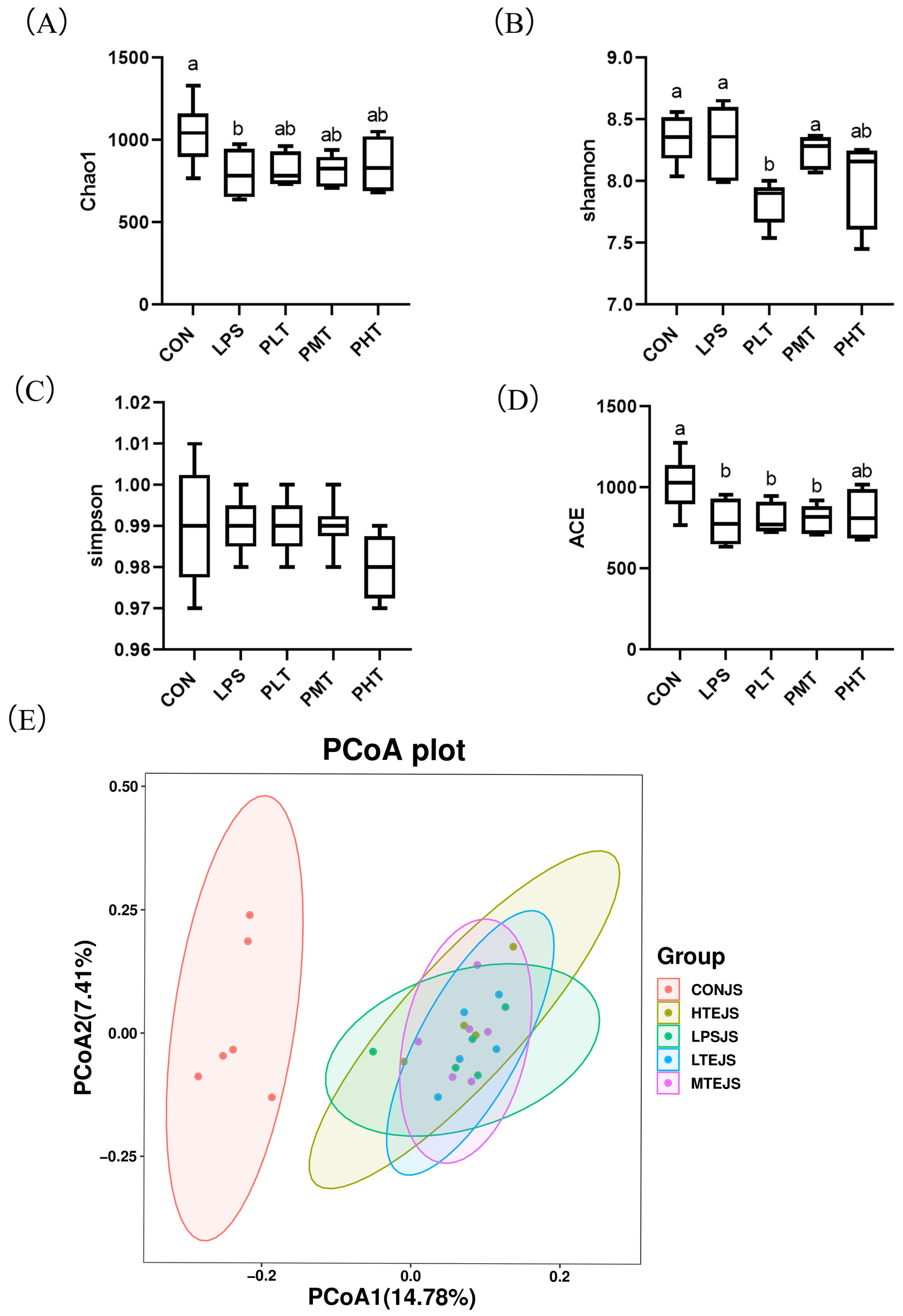
- Alpha and beta diversity analysis
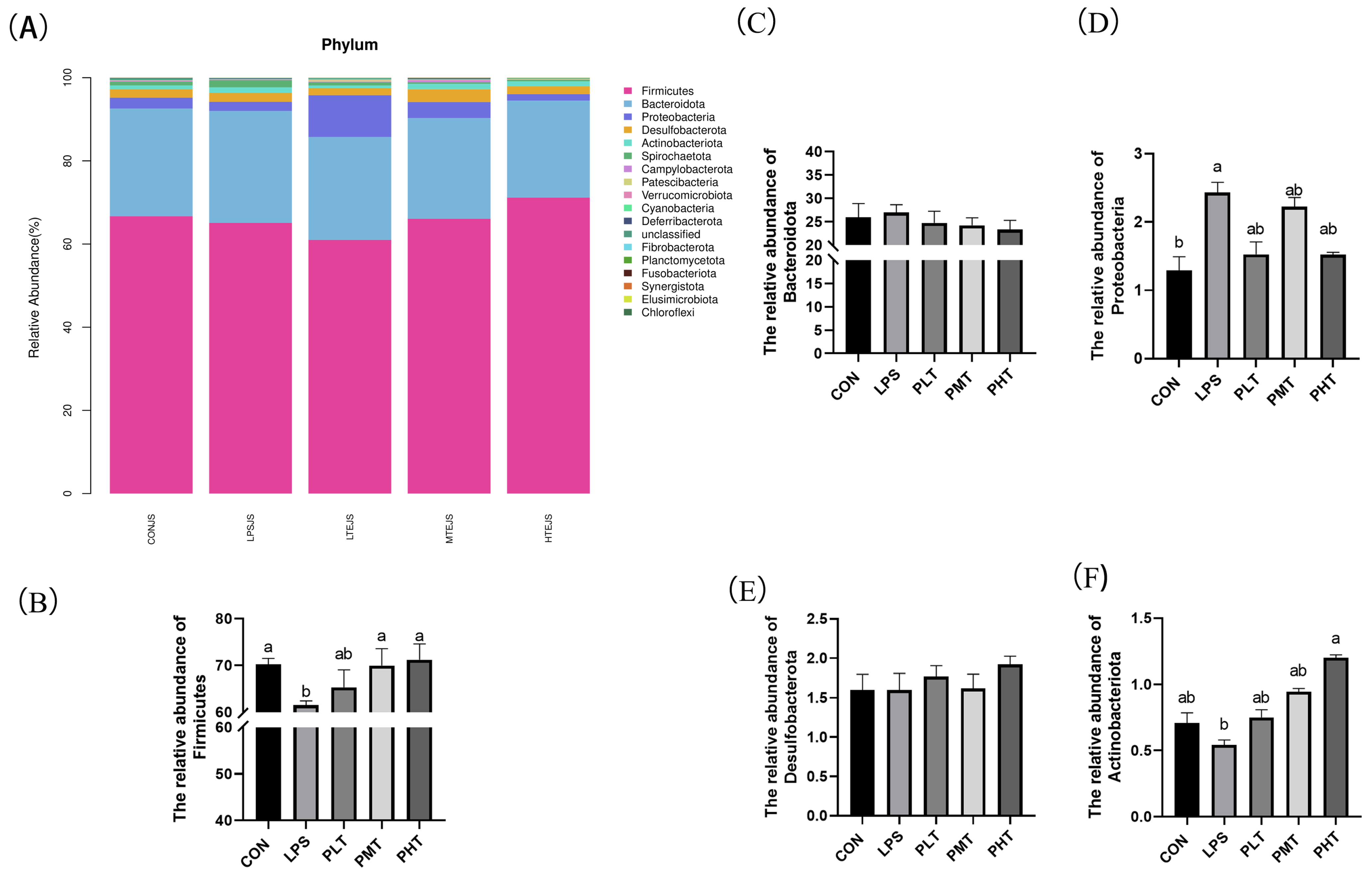
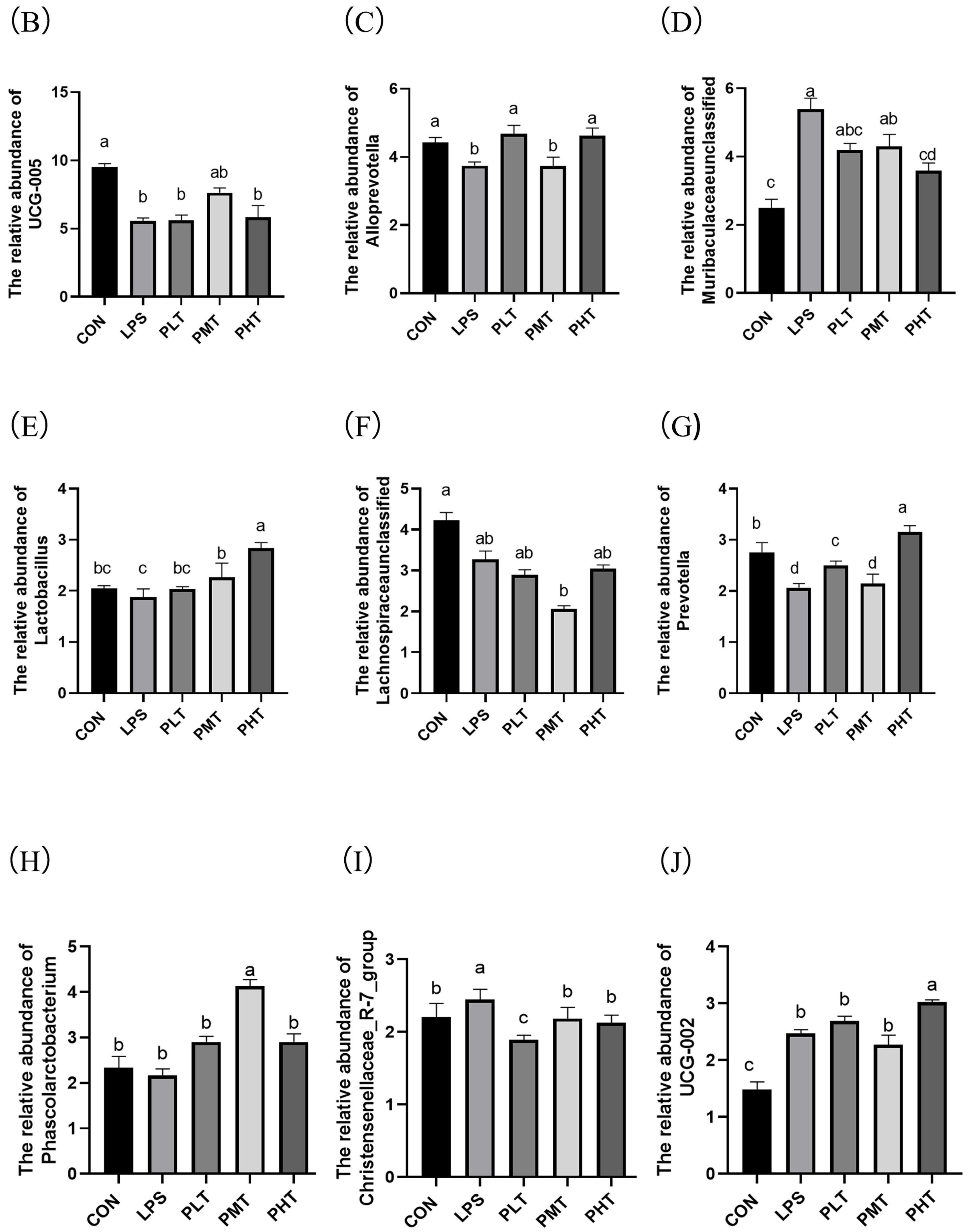
- Bacterial abundance in the colonic mucosa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, W.; Zhao, Z.; Yang, Y.; Ding, L.; Yao, W. The synbiotic mixture of lactulose and Bacillus coagulans protects intestinal barrier dysfunction and apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.X.; He, J.; Wu, S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar] [CrossRef]
- Yang, L.; Wu, G.; Wu, Q.; Peng, L.; Yuan, L. METTL3 overexpression aggravates LPS-induced cellular inflammation in mouse intestinal epithelial cells and DSS-induced IBD in mice. Cell Death Discov. 2022, 8, 62. [Google Scholar] [CrossRef]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The effect of low or high molecular weight oat beta-glucans on the inflammatory and oxidative stress status in the colon of rats with LPS-induced enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef]
- Li, H.M. Regulating Effects of Terpinen-4-ol on Growth Performance and Intestinal Damage of Weaned Piglets with Oxidative Stress. Master’s Thesis, Yangzhou University, Yangzhou, China, 2024. [Google Scholar]
- Yang, J. The Protective Effects of Resveratrol on the Intestinal Health in Piglets Under Deoxynivalenol Exposure. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Yu, Q.H.; Yang, Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol. Int. 2009, 33, 78–82. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, C.; Yu, S.; Zhu, L.; Wang, Y.; Hu, H.; Deng, J. Progress in Mycotoxins Affecting Intestinal Mucosal Barrier Function. Int. J. Mol. Sci. 2019, 20, 2777. [Google Scholar] [CrossRef]
- Xu, K.J.; Li, L.J. Gut normal flora and intestinal immune system. Int. J. Epidemiol. Infect. Dis. 2005, 32, 181–183. (In Chinese) [Google Scholar]
- Xing, L.K. Study on Intestinal Mucosal Barrier Function and Microecology in Patients with Hepatitis B Cirrhosis. Master’s Thesis, Lanzhou University, Lanzhou, China, 2020. [Google Scholar]
- Yong, Y.; Fang, B.; Huang, Y.; Li, J.; Yu, T.; Wu, L.; Hu, C.; Liu, X.; Yu, Z.; Ma, X.; et al. Tea Tree Oil Terpinen-4-ol Protects Gut Barrier Integrity by Upregulation of Tight Junction Proteins via the ERK1/2-Signaling Pathway. Front. Nutr. 2022, 8, 805612. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Piao, X.; Guo, L.; Liu, S.; Chai, F.; Gao, L. Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 2016, 13, 4779–4785. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, B.; Wang, Z.; Chen, Y.; Dong, Y. The Effect Mechanism of N6-adenosine Methylation (m6A) in Melatonin Regulated LPS-induced Colon Inflammation. Int. J. Biol. Sci. 2024, 20, 2491–2506. [Google Scholar] [CrossRef]
- Zang, Z.; Li, L.; Yang, M.; Zhang, H.; Naeem, A.; Wu, Z.; Zheng, Q.; Song, Y.; Tao, L.; Wan, Z.; et al. Study on the ameliorative effect of honeysuckle on DSS-induced ulcerative colitis in mice. J. Ethnopharmacol. 2024, 325, 117776. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Mao, J.; Peng, Z.; Zhong, Z.; Wang, H.; Dong, L. Dietary Palygorskite Clay-Adsorbed Nano-ZnO Supplementation Improves the Intestinal Barrier Function of Weanling Pigs. Front. Nutr. 2022, 9, 857898. [Google Scholar] [CrossRef]
- Dong, L.; Li, H.M.; Wang, S.N.; Wang, T.L.; Yu, L.H.; Wang, H.R. Meishan neonatal piglets tend to have higher intestinal barrier function than crossbred neonatal piglets. Anim. Int. J. Anim. Biosci. 2021, 15, 100037. [Google Scholar] [CrossRef]
- Lv, X.; Chen, L.; Zhou, C.; Guo, Y.; Zhang, G.; Kang, J.; Tan, Z.; Tang, S.; Liu, Z. Dietary tea tree (Melaleuca alternifolia) oil supplementation enhances the expressions of amino acid transporters in goat ileal mucosa and improves intestinal immunity. Food Sci. Nutr. 2022, 10, 3749–3758. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Gabler, N.K.; Spurlock, M.E. Integrating the immune system with the regulation of growth and efficiency. J. Anim. Sci. 2008, 86 (Suppl. 14), E64–E74. [Google Scholar] [CrossRef]
- López-Colom, P.; Yu, K.; Barba-Vidal, E.; Saco, Y.; Martín-Orúe, S.M.; Castillejos, L.; Solà-Oriol, D.; Bassols, A. I-FABP, Pig-MAP and TNF-α as biomarkers for monitoring gut-wall integrity in front of Salmonella Typhimurium and ETEC K88 infection in a weaned piglet model. Res. Vet. Sci. 2019, 124, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, X.; Wang, X.; Tan, B.; Li, T.; Yin, Y. Effects of Weaning on Intestinal Upper Villus Epithelial Cells of Piglets. PLoS ONE 2016, 11, e0150216. [Google Scholar] [CrossRef] [PubMed]
- Pang, W. The Protective Effect and Mechanism of Compound SLL-1-43 on DSS Induced Acute and Chronic Ulcerative Colitis in Mice. Master’s Thesis, Liaoning University, Shenyang, China, 2023. [Google Scholar]
- Ru, M. The Alleviating Effect of the Extract from the Leaves of Geophyllum on DSS Induced Colitis in Mice. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2023. [Google Scholar]
- Wu, J.; Wang, J.; Lin, Z.; Liu, C.; Zhang, Y.; Zhang, S.; Zhou, M.; Zhao, J.; Liu, H.; Ma, X. Clostridium butyricum alleviates weaned stress of piglets by improving intestinal immune function and gut microbiota. Food Chem. 2023, 405 Pt B, 135014. [Google Scholar] [CrossRef] [PubMed]
- Van Klinken, B.J.; Dekker, J.; Büller, H.A.; Einerhand, A.W. Mucin gene structure and expression: Protection vs. adhesion. Am. J. Physiol. 1995, 269 Pt 1, G613–G627. [Google Scholar] [CrossRef]
- Tytgat, K.M.; Büller, H.A.; Opdam, F.J.; Kim, Y.S.; Einerhand, A.W.; Dekker, J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology 1994, 107, 1352–1363. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Bengtsson, R.J.; MacIntyre, N.; Guthrie, J.; Wilson, A.D.; Finlayson, H.; Matika, O.; Pong-Wong, R.; Smith, S.H.; Archibald, A.L.; Ait-Ali, T. Lawsonia intracellularis infection of intestinal crypt cells is associated with specific depletion of secreted MUC2 in goblet cells. Vet. Immunol. Immunopathol. 2015, 168, 61–67. [Google Scholar] [CrossRef]
- Li, Y.; Lan, T.; Qiu, L.; Lin, L.; Dai, A.; Guo, H. Effects of Ginkgo biloba compound on the morphology of small intestinal mucosa and the number of intraepithelial lymphocytes and goblet cells in weaned piglets under LPS stress. Chin. J. Anim. Sci. 2018, 12, 109–113. [Google Scholar] [CrossRef]
- Yang, W. Study on the Effect of Jujube Polyphenol Extract on DSS Induced Ulcerative Colitis in Mice Based on Intestinal Flora Interaction. Master’s Thesis, Ningxia University, Ningxia, China, 2024. [Google Scholar]
- Dong, L.; Yuan, Q.; Qiu, G.; Zhang, Y.; Wang, H.; Yu, L. Protective Effects of Tea Tree Oil on Inflammatory Injury of Porcine Intestinal Epithelial Cells Induced by Lipopolysaccharide In Vitro. Animals 2024, 14, 2577. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional Intervention for the Intestinal Development and Health of Weaned Pigs. Front. Vet. Sci. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Hussain, T.; Tan, B.; Liu, Y.; Ji, P.; Yin, Y. The Evaluation of Antioxidant and Anti-Inflammatory Effects of Eucommia ulmoides Flavones Using Diquat-Challenged Piglet Models. Oxidative Med. Cell. Longev. 2017, 2017, 8140962. [Google Scholar] [CrossRef] [PubMed]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. J. Can. Chir. 2011, 54, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Paunkov, A.; Chartoumpekis, D.V.; Ziros, P.G.; Sykiotis, G.P. A Bibliometric Review of the Keap1/Nrf2 Pathway and its Related Antioxidant Compounds. Antioxidants 2019, 8, 353. [Google Scholar] [CrossRef]
- Cox, S.D.; Gustafson, J.E.; Mann, C.M.; Markham, J.L.; Liew, Y.C.; Hartland, R.P.; Bell, H.C.; Warmington, J.R.; Wyllie, S.G. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 355–358. [Google Scholar] [CrossRef]
- Hilal, B.; Khan, M.M.; Fariduddin, Q. Recent advancements in deciphering the therapeutic properties of plant secondary metabolites: Phenolics, terpenes, and alkaloids. Plant Physiol. Biochem. 2024, 211, 108674. [Google Scholar] [CrossRef]
- Sadi, G.; Bozan, D.; Yildiz, H.B. Redox regulation of antioxidant enzymes: Post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver. Mol. Cell. Biochem. 2014, 393, 111–122. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Liu, J.; Mao, J.; Peng, Z.; Huo, Y.; Yu, L. Effect of tea tree oil on antioxidant indexes of serum, liver and intestinal mucosa of weaned piglets. J. Anim. Nutr. 2018, 4, 1440–1446. [Google Scholar]
- Mak’Anyengo, R.; Duewell, P.; Reichl, C.; Hörth, C.; Lehr, H.A.; Fischer, S.; Clavel, T.; Denk, G.; Hohenester, S.; Kobold, S.; et al. Nlrp3-dependent IL-1β inhibits CD103+ dendritic cell differentiation in the gut. JCI Insight 2018, 3, e96322. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Miller, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14, 1590–1604. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Castejón-Vega, B.; Cordero, M.D. Stress-Induced NLRP3 Inflammasome in Human Diseases. Adv. Protein Chem. Struct. Biol. 2017, 108, 127–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, X.; Yu, D.; Liu, G. Suppression of NLRP3 and NF-κB signaling pathways by α-Cyperone via activating SIRT1 contributes to attenuation of LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2019, 76, 105886. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Q.; Wang, Y.; Liang, T.; Li, X.; Wang, D.; Wang, X.; Zhu, H.; Xiao, K. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. 2021, 12, 62. [Google Scholar] [CrossRef]
- Yin, P.; Zou, W.; Li, J.; Jin, N.; Gao, Q.; Liu, F. Using high-throughput sequencing to explore the anti-inflammatory effects of α-mangostin. Sci. Rep. 2019, 9, 15626. [Google Scholar] [CrossRef]
- Zhang, Y.S. The Regulatory Effect of Tea Tree Oil on Immune Stress Induced by Lipopolysaccharide in Porcine Ileal Epithelial Cells. Master’s Thesis, Yangzhou University, Yangzhou, China, 2020. [Google Scholar]
- Nogueira, M.N.; Aquino, S.G.; Rossa Junior, C.; Spolidorio, D.M. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2014, 63, 769–778. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, P.; Lu, X.; Li, Y.; Liu, J.; Liu, B.; Fu, Y.; Cao, Y.; Zhang, N. In Vivo and In Vitro Study on the Efficacy of Terpinen-4-ol in Dextran Sulfate Sodium-Induced Mice Experimental Colitis. Front. Immunol. 2017, 8, 558. [Google Scholar] [CrossRef]
- Gan, Z.; Wei, W.; Li, Y.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Curcumin and Resveratrol Regulate Intestinal Bacteria and Alleviate Intestinal Inflammation in Weaned Piglets. Molecules 2019, 24, 1220. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, H.; Shen, Z.; Zhu, G.; Hao, L.; Chen, H.; Xu, H.; Zhou, X. Long-lasting anti-bacterial activity and bacteriostatic mechanism of tea tree oil adsorbed on the amino-functionalized mesoporous silica-coated by PAA. Colloids Surf. B Biointerfaces 2020, 188, 110784. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef]
- Wen, X.; Wan, F.; Wu, Y.; Liu, L.; Liu, Y.; Zhong, R.; Chen, L.; Zhang, H. Caffeic acid supplementation ameliorates intestinal injury by modulating intestinal microbiota in LPS-challenged piglets. Food Funct. 2023, 14, 7705–7717. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Meng, T.; He, W.; Huang, H.; Liu, C.; Fu, X.; He, J.; Yin, Y.; Xiao, D. Dietary Chito-oligosaccharides Improve Intestinal Immunity via Regulating Microbiota and Th17/Treg Balance-Related Immune Signaling in Piglets Challenged by Enterotoxigenic, E. coli. J. Agric. Food Chem. 2021, 69, 15195–15207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Su, W.; Li, W.; Wen, C.; Du, S.; He, H.; Zhang, Y.; Gong, T.; Wang, X.; Wang, Y.; et al. Bacillus amyloliquefaciens 40 regulates piglet performance, antioxidant capacity, immune status and gut microbiota. Anim. Nutr. 2022, 12, 116–127. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, L.; Sun, Q.; Chen, Q.; Hua, W.; Zhang, J. Effects of Acremonium terricola Culture on Lactation Performance, Immune Function, Antioxidant Capacity, and Intestinal Flora of Sows. Antioxidants 2024, 13, 970. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]



| Ingredient | Content |
|---|---|
| Raw materials, % | |
| Corn | 10.00 |
| Fish meal Puffed corn Soybean oil Fermented soybean meal Soybean preconcentrated protein Whole soybeans Whey powder Flour Rice husk powder Glucose | 5.83 27.67 1.67 8.00 2.50 6.50 15.00 10.00 0.33 2.50 |
| Carrier rice husk powder | 2.31 |
| Calcium hydrogen phosphate Lysine | 0.15 0.43 |
| Methionine | 0.30 |
| Choline chloride Salt Premix (1) Total 100.00 | 0.12 0.30 1.39 100.00 |
| Nutritional levels (2) | |
| Digestible energy (kcal/kg) | 3.33 |
| Crude protein, % | 18.83 |
| Calcium, % | 0.43 |
| Total phosphorus, % | 0.52 |
| Lysine, % | 1.44 |
| Methionine, % | 0.62 |
| Threonine, % | 1.00 |
| Tryptophan, % | 0.35 |
| Genes | Accession No. | Primer Sequence (5′ to 3′) |
|---|---|---|
| β-Actin | DQ845171.1 | F AGGCCAACCGTGAGAAGATG |
| R CATGACAATGCCAGTGGTGC | ||
| CAT | NM 214301.2 | F CTGTAAGGCTAGTCGGACACC |
| R ATATCAGGTTTCTGCGCGGC | ||
| SOD1 | NM 001190422.1 | F GTGCAGGGCACCATCTACTTC |
| R GATCACCTTCAGCCAGTCCTT | ||
| Gpx1 | NM 001206359.1 | F CTAGCAGTGCCTAGAGTGCC |
| R CGCCCATCTCAGGGGATTTT | ||
| NLRP3 | NM001256770.2 | F TGTATTGAGAACTGTCGCCATGTGG |
| R CTCCTCTTCCTCCTCCTCCTCTTTG | ||
| ASC | XM003124468.5 | F GAAGGTGCTGACGGAAGAGC |
| R TCCTTGCAGGTCAGGTTCCA | ||
| Caspase-1 | NM214162.1 | F CCAGTTAAGCCTGCGTCTTCAGAG |
| R GGCGTGTGCGAATTGATTTTCCC | ||
| IL-1β | NM001302388.2 | F AAGAGGGACATGGAGAAGCGATTTG |
| R TTGTTCTGCTTGAGAGGTGCTGATG | ||
| IL-18 | NM213997.1 | F AGACCTGGAATCGGATTACTTTGGC |
| R ACGGCTTGATGTCCCTGGTTAATG | ||
| TNF-α | NM214022 | F CCACCACGCTCTTCTGCCTAC |
| R TTGAGACGATGATCTGAGTCCTTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Qiu, G.; Yu, X.; Zhao, J.; Liu, J.; Wang, H.; Dong, L. Terpinen-4-ol Improves the Intestinal Barrier Function of the Colon in Immune-Stressed Weaning Piglets. Animals 2025, 15, 9. https://doi.org/10.3390/ani15010009
Yu L, Qiu G, Yu X, Zhao J, Liu J, Wang H, Dong L. Terpinen-4-ol Improves the Intestinal Barrier Function of the Colon in Immune-Stressed Weaning Piglets. Animals. 2025; 15(1):9. https://doi.org/10.3390/ani15010009
Chicago/Turabian StyleYu, Lihuai, Guangzhi Qiu, Xiaomu Yu, Jianwei Zhao, Jun Liu, Hongrong Wang, and Li Dong. 2025. "Terpinen-4-ol Improves the Intestinal Barrier Function of the Colon in Immune-Stressed Weaning Piglets" Animals 15, no. 1: 9. https://doi.org/10.3390/ani15010009
APA StyleYu, L., Qiu, G., Yu, X., Zhao, J., Liu, J., Wang, H., & Dong, L. (2025). Terpinen-4-ol Improves the Intestinal Barrier Function of the Colon in Immune-Stressed Weaning Piglets. Animals, 15(1), 9. https://doi.org/10.3390/ani15010009






