Development of Serum-Free Culture Systems for an Immortalized Porcine Kidney-Derived Macrophage Cell Line
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures for Cell Culture and Media Preparation
2.2. Preparation of pCSF1 and pCSF2 Recombinant Proteins
2.3. Evaluation of Cytokines and Polymer Compounds on Cell Proliferation
2.4. Cell Proliferation Assay
2.5. RT-PCR
2.6. Immunocytochemistry
2.7. Chromosome Number Analysis
2.8. Statistical Analysis
3. Results
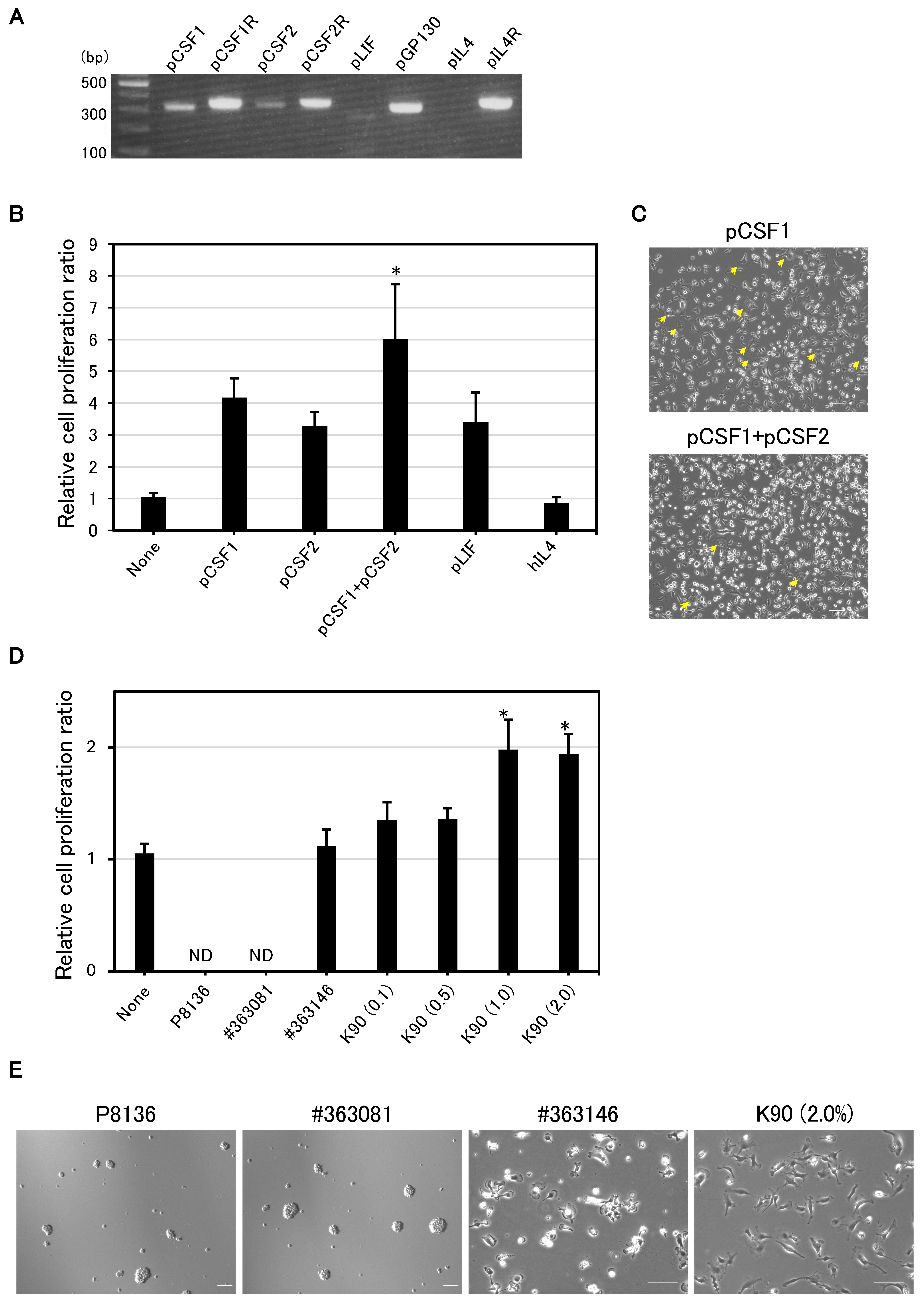
3.1. pCSF1 and pCSF2 Support the Proliferation of IPKM Cells
3.2. PVP K90 Addition Improves Cell Growth and Adhesion
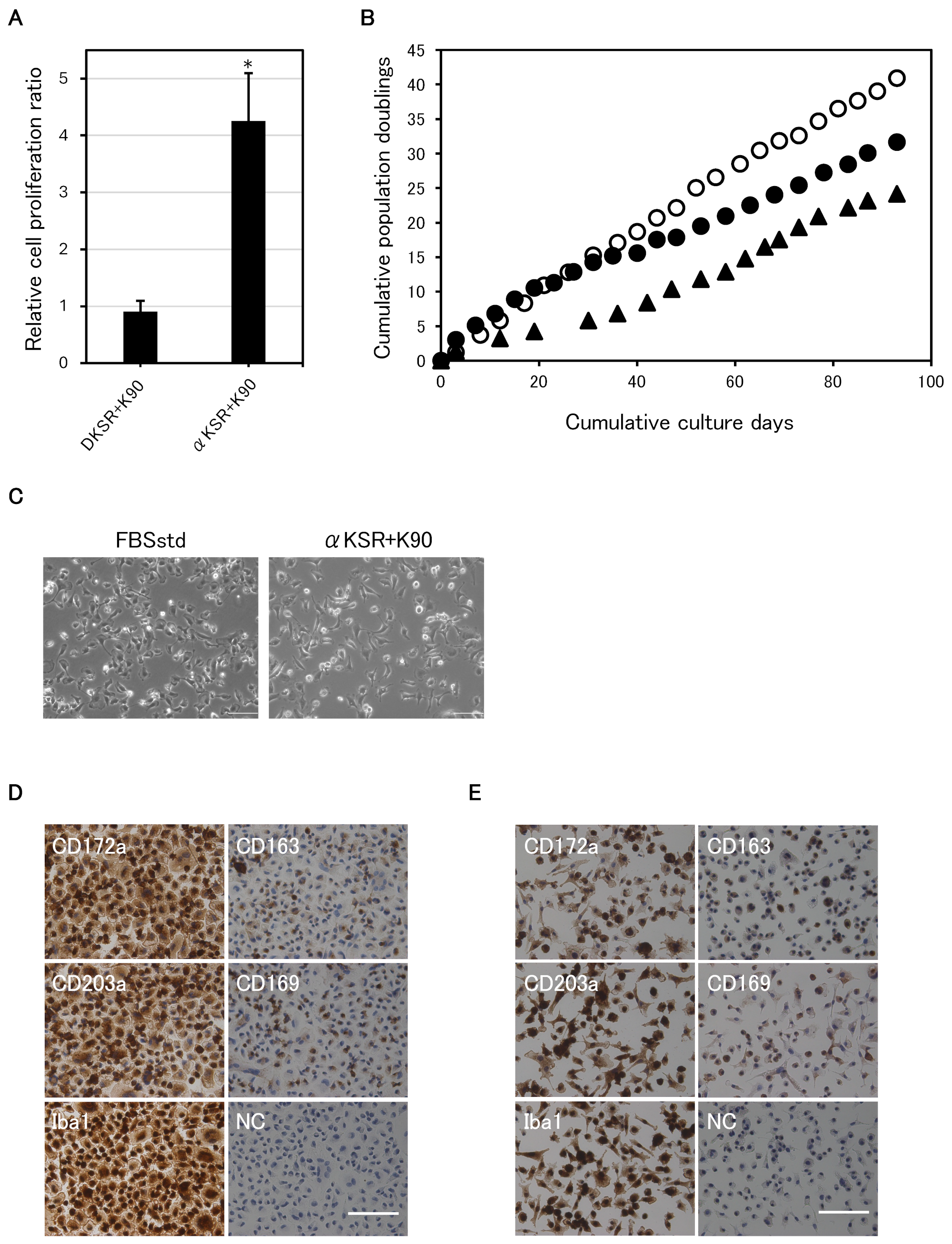
3.3. α-MEM Is Superior to DMEM for the Serum-Free Culture of IPKM Cells
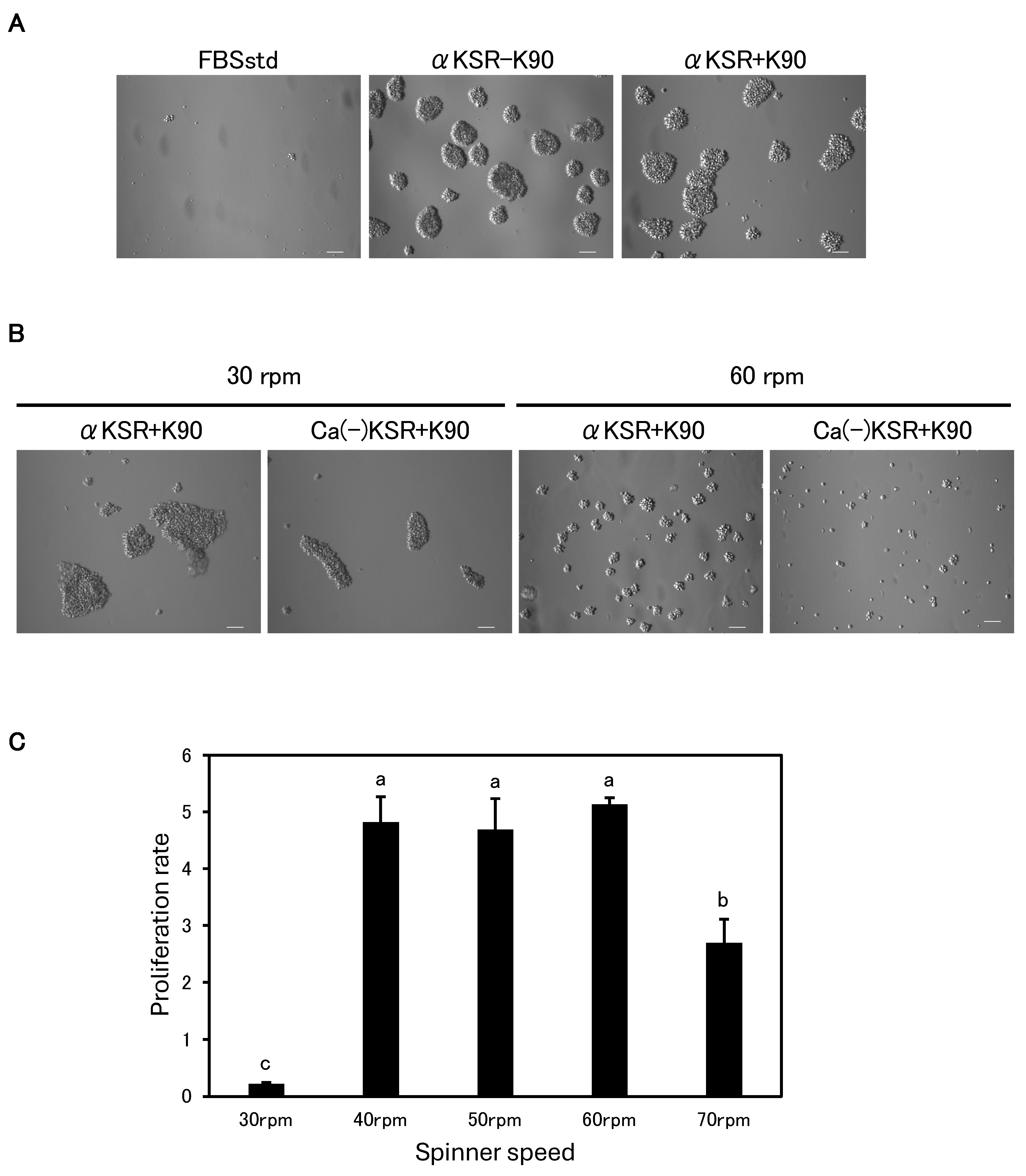
3.4. Successful Long-Term Serum-Free Adherent Subculture
3.5. Successful Long-Term Serum-Free Suspension Subculture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2013, 173, 122–130. [Google Scholar] [PubMed]
- Sánchez-Torres, C.; Gómez-Puertas, P.; Gómez-del-Moral, M.; Alonso, F.; Escribano, J.M.; Ezquerra, A.; Domínguez, J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch. Virol. 2003, 148, 2307–2323. [Google Scholar] [CrossRef]
- Zhu, Z.; Mao, R.; Liu, B.; Liu, H.; Shi, Z.; Zhang, K.; Liu, H.; Zhang, D.; Liu, J.; Zhao, Z.; et al. Single-cell profiling of African swine fever virus disease in the pig spleen reveals viral and host dynamics. Proc. Natl. Acad. Sci. USA 2024, 121, e2312150121. [Google Scholar] [CrossRef]
- Takenouchi, T.; Kitani, H.; Suzuki, S.; Nakai, M.; Fuchimoto, D.I.; Tsukimoto, M.; Shinkai, H.; Sato, M.; Uenishi, H. Immortalization and Characterization of Porcine Macrophages That Had Been Transduced with Lentiviral Vectors Encoding the SV40 Large T Antigen and Porcine Telomerase Reverse Transcriptase. Front. Vet. Sci. 2017, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Rai, A.; Espinoza, N.; Ramirez-Medina, E.; Spinard, E.; Velazquez-Salinas, L.; Valladares, A.; Silva, E.; Burton, L.; Meyers, A.; et al. African Swine Fever Vaccine Candidate ASFV-G-ΔI177L Produced in the Swine Macrophage-Derived Cell Line IPKM Remains Genetically Stable and Protective against Homologous Virulent Challenge. Viruses 2023, 15, 2064. [Google Scholar] [CrossRef] [PubMed]
- Mehinagic, K.; Liniger, M.; Samoilenko, M.; Soltermann, N.; Gerber, M.; Ruggli, N. A sensitive luciferase reporter assay for the detection of infectious African swine fever virus. J. Virol. Methods 2024, 323, 114854. [Google Scholar] [CrossRef] [PubMed]
- Masujin, K.; Kitamura, T.; Kameyama, K.; Okadera, K.; Nishi, T.; Takenouchi, T.; Kitani, H.; Kokuho, T. An immortalized porcine macrophage cell line competent for the isolation of African swine fever virus. Sci. Rep. 2021, 11, 4759. [Google Scholar] [CrossRef]
- Kameyama, K.; Kitamura, T.; Okadera, K.; Ikezawa, M.; Masujin, K.; Kokuho, T. Usability of Immortalized Porcine Kidney Macrophage Cultures for the Isolation of ASFV without Affecting Virulence. Viruses 2022, 14, 1794. [Google Scholar] [CrossRef]
- Kitamura, T.; Masujin, K.; Yamazoe, R.; Kameyama, K.; Watanabe, M.; Ikezawa, M.; Yamada, M.; Kokuho, T. A Spontaneously Occurring African Swine Fever Virus with 11 Gene Deletions Partially Protects Pigs Challenged with the Parental Strain. Viruses 2023, 15, 311. [Google Scholar] [CrossRef]
- Ramirez-Medina, E.; Rai, A.; Espinoza, N.; Spinard, E.; Silva, E.; Burton, L.; Clark, J.; Meyers, A.; Valladares, A.; Velazquez-Salinas, L.; et al. Recombinant Vaccine Strain ASFV-G-Δ9GL/ΔUK Produced in the IPKM Cell Line Is Genetically Stable and Efficacious in Inducing Protection in Pigs Challenged with the Virulent African Swine Fever Virus Field Isolate Georgia 2010. Pathogens 2024, 13, 319. [Google Scholar] [CrossRef]
- Segeritz, C.P.; Vallier, L. Cell culture: Growing cells as model systems in vitro. In Basic Science Methods for Clinical Researchers; Academic Press: Cambridge, MA, USA, 2017; pp. 151–172. [Google Scholar] [CrossRef]
- Genzel, Y.; Fischer, M.; Reichl, U. Serum-free influenza virus production avoiding washing steps and medium exchange in large-scale microcarrier culture. Vaccine 2006, 24, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.Y.; Tseng, Y.F.; Weng, T.C.; Liao, C.C.; Wu, J.; Chou, A.H.; Chao, H.J.; Gu, A.; Chen, J.; Lin, S.C.; et al. Production of inactivated influenza H5N1 vaccines from MDCK cells in serum-free medium. PLoS ONE 2011, 6, e14578. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Peng, W.J.; Ye, Q.; Liu, X.P.; Zhao, L.; Fan, L.; Xia-Hou, K.; Jia, H.J.; Luo, J.; Zhou, L.T.; et al. Serum-Free Suspension Culture of MDCK Cells for Production of Influenza H1N1 Vaccines. PLoS ONE 2015, 10, e0141686. [Google Scholar] [CrossRef] [PubMed]
- Chia, M.Y.; Lin, C.Y.; Chen, P.L.; Lai, C.C.; Weng, T.C.; Sung, W.C.; Hu, A.Y.; Lee, M.S. Characterization and Immunogenicity of Influenza H7N9 Vaccine Antigens Produced Using a Serum-Free Suspension MDCK Cell-Based Platform. Viruses 2022, 14, 1937. [Google Scholar] [CrossRef]
- Dill, V.; Hoffmann, B.; Zimmer, A.; Beer, M.; Eschbaumer, M. Influence of cell type and cell culture media on the propagation of foot-and-mouth disease virus with regard to vaccine quality. Virol. J. 2018, 15, 46. [Google Scholar] [CrossRef]
- Dill, V.; Zimmer, A.; Beer, M.; Eschbaumer, M. Investigation of cell culture conditions for optimal foot-and-mouth disease virus production. BMC Biotechnol. 2019, 19, 33. [Google Scholar] [CrossRef]
- Castro, R.; Fernandes, P.; Laske, T.; Sousa, M.F.; Genzel, Y.; Scharfenberg, K.; Alves, P.M.; Coroadinha, A.S. Production of canine adenovirus type 2 in serum-free suspension cultures of MDCK cells. Appl. Microbiol. Biotechnol. 2015, 99, 7059–7068. [Google Scholar] [CrossRef]
- Yaoi, Y.; Onoda, T.; TakahashiI, H. Inhibition of Mitosis in Suspension Culture of Chick Embryo Cells. Nature 1972, 237, 285–286. [Google Scholar] [CrossRef]
- Haraguchi, S.; Kikuchi, K.; Nakai, M.; Tokunaga, T. Establishment of self-renewing porcine embryonic stem cell-like cells by signal inhibition. J. Reprod. Dev. 2012, 58, 707–716. [Google Scholar] [CrossRef]
- Folkman, J.; Moscona, A. Role of cell shape in growth control. Nature 1978, 273, 345–349. [Google Scholar] [CrossRef]
- Dang-Nguyen, T.Q.; Haraguchi, S.; Kikuchi, K.; Somfai, T.; Bodó, S.; Nagai, T. Leukemia inhibitory factor promotes porcine oocyte maturation and is accompanied by activation of signal transducer and activator of transcription 3. Mol. Reprod. Dev. 2014, 81, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef]
- Wiles, M.V.; Johansson, B.M. Embryonic stem cell development in a chemically defined medium. Exp. Cell Res. 1999, 247, 241–248. [Google Scholar] [CrossRef]
- Keskintepe, L.; Brackett, B.G. In vitro developmental competence of in vitro-matured bovine oocytes fertilized and cultured in completely defined media. Biol. Reprod. 1996, 55, 333–339. [Google Scholar] [CrossRef]
- Yoshioka, K.; Suzuki, C.; Tanaka, A.; Anas, I.M.K.; Iwamura, S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol. Reprod. 2002, 66, 112–119. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Ishida, R.; Kikuchi, M.; Sudo, K.; Morita, M.; Crisostomo, R.V.; Yamamoto, R.; Loh, K.M.; Nakamura, Y.; Watanabe, M.; et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 2019, 571, 117–121. [Google Scholar] [CrossRef]
- Mizumachi, S.; Aritomi, T.; Sasaki, K.; Matsubara, K.; Hirao, Y. Macromolecular crowded conditions strengthen contacts between mouse oocytes and companion granulosa cells during in vitro growth. J. Reprod. Dev. 2018, 64, 153–160. [Google Scholar] [CrossRef]
- Hirao, Y.; Itoh, T.; Shimizu, M.; Iga, K.; Aoyagi, K.; Kobayashi, M.; Kacchi, M.; Hoshi, H.; Takenouchi, N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: Effects of high polyvinylpyrrolidone concentration in culture medium. Biol. Reprod. 2004, 70, 83–91. [Google Scholar] [CrossRef]
- Tsiapalis, D.; Zeugolis, D.I. It is time to crowd your cell culture media—Physicochemical considerations with biological consequences. Biomaterials 2021, 275, 120943. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, M.; Zeugolis, D.I. Transforming eukaryotic cell culture with macromolecular crowding. Trends Biochem. Sci. 2021, 46, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Papoutsakis, E.T. Media additives for protecting freely suspended animal cells against agitation and aeration damage. Trends Biotechnol. 1991, 9, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Michaels, J.D.; Nowak, J.E.; Mallik, A.K.; Koczo, K.; Wasan, D.T.; Papoutsakis, E.T. Interfacial protection of cell culture media with cell-protecting additives. Biotechnol. Bioeng. 1995, 47, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Mather, J.P. Laboratory Scaleup of Cell Cultures. Methods Cell Biol. 1998, 57, 219–227. [Google Scholar]

| Composition | FBSstd | DKSR + K90 | αKSR + K90 | Ca(−)KSR + K90 |
|---|---|---|---|---|
| Medium | DMEM | DMEM | αMEM | Ca(−)DMEM |
| Insulin | 10 μg/mL | – | – | – |
| FBS | 10% | – | – | – |
| MTG | 25 μM | 25 μM | 25 μM | 25 μM |
| Anti-Anti | 1% | 1% | 1% | 1% |
| KSR | – | 15% | 15% | 20% |
| pCSF1 | – | 0.25% | 0.25% | 0.25% |
| pCSF2 | – | 0.25% | 0.25% | 0.25% |
| PVP K-90 | – | 2% (w/v) | 2% (w/v) | 2% (w/v) |
| GlutaMAX | – | – | – | 1% |
| Pyruvate | – | – | – | 1 mM |
| Nucleosides | – | – | – | 1% |
| Media | Total Passage No. | No. (%) of 2n Chromosomes | ||
|---|---|---|---|---|
| <38 | 38 | 38< | ||
| FBSstd | P22 | 14 (28.6) | 32 (65.3) | 3 (6.1) |
| αKSR + K90 | P21 | 16 (28.6) | 36 (64.3) | 4 (7.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haraguchi, S.; Takenouchi, T.; Masujin, K.; Suzuki, S.; Kokuho, T.; Uenishi, H. Development of Serum-Free Culture Systems for an Immortalized Porcine Kidney-Derived Macrophage Cell Line. Animals 2025, 15, 558. https://doi.org/10.3390/ani15040558
Haraguchi S, Takenouchi T, Masujin K, Suzuki S, Kokuho T, Uenishi H. Development of Serum-Free Culture Systems for an Immortalized Porcine Kidney-Derived Macrophage Cell Line. Animals. 2025; 15(4):558. https://doi.org/10.3390/ani15040558
Chicago/Turabian StyleHaraguchi, Seiki, Takato Takenouchi, Kentaro Masujin, Shunichi Suzuki, Takehiro Kokuho, and Hirohide Uenishi. 2025. "Development of Serum-Free Culture Systems for an Immortalized Porcine Kidney-Derived Macrophage Cell Line" Animals 15, no. 4: 558. https://doi.org/10.3390/ani15040558
APA StyleHaraguchi, S., Takenouchi, T., Masujin, K., Suzuki, S., Kokuho, T., & Uenishi, H. (2025). Development of Serum-Free Culture Systems for an Immortalized Porcine Kidney-Derived Macrophage Cell Line. Animals, 15(4), 558. https://doi.org/10.3390/ani15040558






